Received: January 18, 2015
Accepted: March 13, 2014
Mediterr J Hematol Infect Dis 2015, 7(1): e2015029, DOI 10.4084/MJHID.2015.029
This article is available on PDF format at:

Fahad A S Al-Eidan
College of Medicine, King Saud bin Abdulaziz University for Health Sciences, Kingdom of Saudi Arabia

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Background. The increasing
trend of using low-molecular-weight-heparin (LMWH) versus
unfractionated heparin (UFH) in hospitalized adult patients is raising
concerns about the incidence of heparin-induced thrombocytopenia (HIT). Method. A retrospective study analyzed the requests for heparin-induced antibodies by enzyme-linked immunosorbent assay (ELISA) among adult hospitalized patients during the period from January 2011 to December 2013. These patients received either UFH or LMWH for prevention or therapeutic indications. Those with positive immune-mediated HIT were identified and considered as case patients. Result. The usage of LMWH and UFH and development of HIT was determined during the study period. The incidence of HIT in patients receiving UFH and those receiving LMWH was 4.09 per thousand patients and 0.48 per thousand patients, respectively, (p<0.0001) with an overall incidence of 2.49 per thousand patients. Conclusion. The increased trend of using LMWH over UFH among hospitalized adult patients was observed and can be said to contribute to the diminished overall incidence of HIT. |
Introduction
Venous thromboembolism (VTE) is the most common preventable cause of
hospital morbidity and mortality. A preventative pharmacological agent
is recommended for all hospitalized patients at risk of developing VTE.
Low-molecular-weight-heparin (LMWH) and unfractionated heparin (UFH)
are widely used and cost effective VTE prevention agents.[1,2]
However, heparin-induced thrombocytopenia (HIT) is an immune-mediated,
potentially life-threatening adverse effect of heparin therapy.[3,4]
Heparin can induce immunoglobulin G (IgG) antibodies production
against itself and platelet factor 4 (PF4); the antibodies stimulate
platelets and endothelial cells, resulting in an excess production of
thrombin, inducing thrombocytopenia and thromboembolic events.[5-7] HIT occurs in approximately 3% of patients who receive UFH and approximately 0.2% of patients who receive LMWH.[4,8-11]
HIT is clinically diagnosed by a drop in platelet count to less than 100X109/L or a 50% decrease in platelets after the initiation of heparin therapy with no apparent explanation other than HIT.[12]
A positive laboratory test for HIT antibodies supports this clinical
diagnosis. The development of HIT can be either; delayed-onset,
typically 5 to14 days after the initial administering of heparin, or
rapid-onset, occurring soon after the re-administering of heparin to a
patient with prior heparin exposure and HIT antibodies.[7,13,14]
Heparin
exposure has a unique HIT complication that is characterized by a
defined thrombocytopenia and immune-mediated platelet activation that
can lead to thrombin over-production and increase the chance of
developing VTE in the majority of patients. This can lead to
life-threatening complications.[15,16]
A
retrospective database analysis was performed on the annual incidence
of HIT at a single teaching center. We assessed the effect of
prescribing UFH and LMWH, with additional analysis of the annual lab
requests for HIT antibodies and confirmed positive HIT tests
Methods
Data from The King Abdulaziz Medical City, (KAMC) Riyadh, Saudi
Arabia, were used to conduct a retrospective study. The following
characteristic data were extracted from patient’s medical record: age,
gender, admitting services, indication of heparin administration, and
type of heparin.
All patients aged at least 18 years who were
admitted to the hospital between January 1, 2011, and December 31,
2013, and who received LMWH or UFH at prevention or therapeutic doses
during admission, were reviewed. Enoxaparin was the only LMWH on KAMC
Formulary during the study period. First admission in which the
diagnosis of HIT occurred was only considered in patients with multiple
readmissions. The main clinical suspicion parameter was the platelet
count, determined as follows: pretreatment platelet count at baseline,
and then every 2 to 3 days from commencing UFH or LMWH administration
for first two weeks.
Thrombocytopenia was defined as a platelet
count fall of ≥50% from a baseline that was apparent by HIT diagnosis
based on clinical probability which routinely evaluated according to
the 4Ts score system by the hematology services. The origin of
thrombocytopenia was confirmed by the detection of serum
heparin-induced antibodies, using a commercial enzyme-linked
immunosorbent assay (ELISA) for IgG, IgA, and IgM class antibodies
(Asserachrom HPIA, Diagnostica Stago, Asniere sur Seine, France).[1,24,25]
All HIT positive patients were diagnosed and labeled by hematology
services. ELISA was performed in the KAMC hematology laboratory
according to manufacturer's procedures. HIT results expressed in
optical density (OD) units and a value of >0.4 was considered to be
positive according to the manufacturer's range. Exclusion criteria were
as follows: (1) patients who developed HIT or thrombocytopenia before
the study period and still in the hospital during the study period. (2)
Heparin or its derivatives given after the thrombocytopenia occurred.
(3) Thrombocytopenia explained by other conditions such as a
chemotherapeutic agent being administered. From this data, the annual
number of patients who received UFH or LMWH for prevention or
therapeutic indications and the total number of heparin-induced
antibody assays performed over the study period was determined.
Identified
HIT patients were divided into three groups: (1) patients receiving
LMWH; (2) patients receiving UFH; and (3) total number of patients
receiving LMWH and UFH. The incidence of HIT was determined for each
group, and the HIT incidence trend was also determined over the study
period.The relative risk was calculated by comparing patients exposed
to UFH and those exposed to LMWH for prevention or therapeutic
indications.
Statistical analysis.
Data was summarized as means (S.D) or proportions. Cumulative incidence
rate and 95% confidence interval (CI) was calculated per one thousand
patients. Comparison between incidence rates was conducted using a
chi-square test. All tests were two-sided and a P value < 0.05 was
considered significant. The STATA statistical software (STATACORP, TX,
USA, version 11) was used to carry out the statistical analysis.
Results
The main clinical, demographic characteristics of the 116 patients who developed HIT including sex, age, indication, admitting hospital department, and laboratory results are summarized in Table 1.
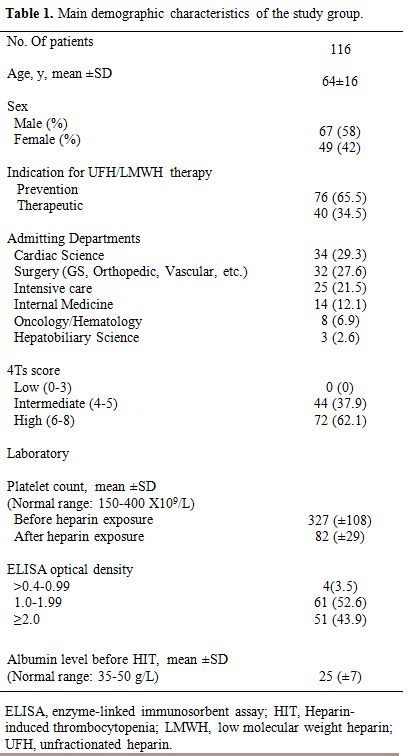 |
Table 1. Main demographic characteristics of the study group. |
Sixty-seven patients were male, and 49 were female. The mean
(±SD) age was 64 (±16). UFH for prevention indication (65.5%) was used
more frequently than LMWH. The mean (±SD) platelet nadir was 82 (±18).
The majority of HIT patients (62.1%) had a high clinical the 4Ts score.
ELISA assays were reported and classified by OD values. HIT patients
were associated with a decreased albumin level (Mean ±SD; 25±7).
Table 2
describes the annual development of HIT in our data from January 2011
to December 2013. In patients receiving UFH and those receiving LMWH,
the annual incidence rate of HIT per one thousand patients was 4.42 and
0.46 in 2011 (P<0.0001); 4.19 and 0.47 in 2012 (P<0.0001); 3.48
and 0.50 in 2013 (P<0.0001), respectively, with an over 3-year
incidence of 4.09 and 0.48 (P<0.0001) respectively. The patients who
received UFH were 8.5 times more likely to develop HIT than those who
received LMWH.
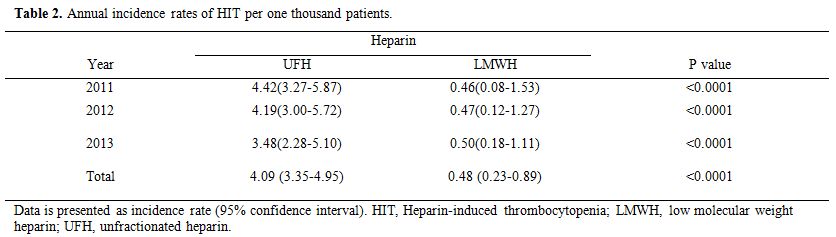 |
Table 2. Annual incidence rates of HIT per one thousand patients. |
A decrease in the total annual incidence rate of HIT, UFH and LMWH, was observed: 3.24 in 2011, 2.62 in 2012 and 1.72 in 2013 (Figure 1).The difference in the incidence of HIT between 2011 and 2012 was not statistically significant (difference=0.63, 95%CI -0.67 to 1.92, P=0.32). Similarly the difference between 2012 and 2013 was not statistically significant (difference=0.90, 95%CI -0.19 to 1.99, P=0.08). However the difference between the incidence in 2011 and 2013 was statistically significant (difference=1.53, 95%CI 0.36 to 2.71, P=0.006).
| Figure 1. Trends in the incidence rates of HIT per 1000 patients from 2011 to 2013 and the total over a three-year study period incidence rate. Each bar represents incidence rate and confidence intervals. |
The annual number of hospitalized patients who received heparin (UFH and LMWH) and the number of HIT assays performed with HIT test results are summarized in Table 3. Briefly, the number of patients who received UFH decreased from 10,175 patients (70%) in 2011 to 6,890 (40%) in 2014, while the number of patients who received LMWH increased from 4,309 patients (30%) in 2011 to 9,989 (59) in 2014. The total number of patients who received heparin (UFH or LMWH) increased from 14,484 patients in 2011 to 16,879 in 2014. However, the total annual HIT assays performed decreased by 48 % from 953 tests in 2011 to 462 in 2014. The annual number of patients receiving LMWH inversely correlated with annual number of HIT assays performed (Figure 2A), while the annual number of patients receiving UFH correlated very closely with the annual numbers of HIT assays performed (Figure 2B).
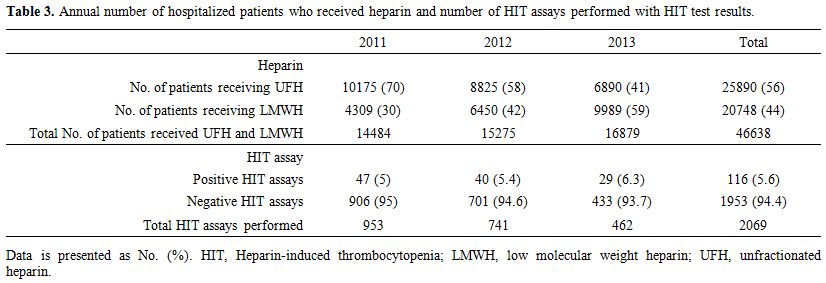 |
Table 3. Annual number of hospitalized patients who received heparin and number of HIT assays performed with HIT test results. |
| Figure 2. Graph ‘A’ shows a correlation between the annual number of patients receiving low molecular weight heparin (LMWH) and annual number of heparin-induced thrombocytopenia (HIT) assays performed. ‘B’ shows the correlation between annual number of patients receiving unfractionated heparin (UFH) and annual numbers of HIT assays. |
Discussion
.
Conclusion
In this three-year study period, we identified a decreasing incidence rate of HIT in hospitalized adult patients that may be attributed to the increasing use of LMWH over UFH.
References
 .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
. [TOP]