Bacterial Epidemiology and Antimicrobial Resistance in the Surgery Wards of a Large Teaching Hospital in Southern Italy
Silvano Esposito1, Renato Gioia1, Giuseppe De Simone1, Silvana Noviello1, Domenico Lombardi2, Vincenzo Giuseppe Di Crescenzo3, Amelia Filippelli4, Maria Rosaria Rega5, Angelo Massari5, Maria Giovanna Elberti6, Lucilla Grisi6, Giovanni Boccia7, Francesco De Caro7 and Sebastiano Leone1
1 Division of Infectious Diseases, University of Salerno, Italy
2 Division of Surgery, University of Salerno, Italy
3 Division of Thoracic Surgery, University of Salerno, Italy
4 Pharmacology Unit, University of Salerno, Italy
5 Microbiology Unit,“San Giovanni di Dio e Ruggi d’Aragona Hospital”, Salerno, Italy
6 Pharmacy Unit,“San Giovanni di Dio e Ruggi d’Aragona Hospital”, Salerno, Italy
7 Hygiene Unit, University of Salerno, Italy
Corresponding author: Silvano
Esposito MD, Division of Infectious Diseases, Azienda Ospedaliera
Universitaria San Giovanni di Dio e Ruggi d'Aragona, Largo Città di
Ippocrate, 84131 Salerno, Italy. Phone: +39089960898, Fax:
+39089960812,
silvanoesposito@libero.it OR
Sebastiano Leone MD. Division of Infectious Diseases, Azienda
Ospedaliera Universitaria San Giovanni di Dio e Ruggi d'Aragona, Largo
Città di Ippocrate, 84131 Salerno, Italy. Phone: +39089960899, Fax:
+39089960812,
sebastianoleone@yahoo.it
Published: June 1, 2015
Received: February 27, 2015
Accepted: May 17, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015040, DOI
10.4084/MJHID.2015.040
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Objectives: Surgical infections
represent an increasingly important problem for the National Health
System. In this study we retrospectively evaluated the bacterial
epidemiology and antimicrobial susceptibility of the microorganisms
concerned as well as the utilization of antibiotics in the General and
Emergency Surgery wards of a large teaching hospital in southern Italy
in the period 2011-2013.
Methods: Data concerning non-duplicate
bacterial isolates and antimicrobial susceptibility were retrieved from
the Vitek 2 database. The pharmacy provided data about the consumption
of antibiotics in the above-reported wards. Chi-square or Fisher’s
exact test were used.
Results: In all, 94 Gram-negative
were isolated in 2011, 77 in 2012, and 125 in 2013, Escherichia coli,
Acinetobacter baumannii and Pseudomonas aeruginosa always being the
most frequently isolated microorganisms. A. baumannii showed high rates
of resistance to carbapenems (with values of 100% in 2011 and 2012) and
low rates of resistance to tigecycline, colistin, and amikacin. In the
same years, there were respectively 105, 93, and 165 Gram-positive
isolated. The rate of MRSA isolates ranged from 66% to 75% during the
study period.
Conclusions: Our results show no significant
increase in antimicrobial resistance over the period in question, and a
higher rate of both MRSA isolates and resistance to carbapenems in A.
baumannii compared with other European data.
|
Introduction
The considerable progress that occurred in the last century in
treating post-operative infections led many authors to underestimate
the problem of antibiotic resistance in the surgical field. This caused
the re-emergence of the problem in the late 1990s and at the beginning
of the new millennium, with a growing demand for multicenter studies
that would contextualize the problem of either the epidemiology of
these infections and the increasing antibiotic resistance or the
increasingly innovative mechanisms put in place by pathogens to evade
the action of antibiotics.[1] Currently, the main
problems related to antibiotic resistance consist in the increasing
prevalence of extended-spectrum beta-lactamases (ESBLs) producing Enterobacteriaceae, and in the increasing intra-hospital presence of methicillin-resistant Staphylococcus aureus
(MRSA) and vancomycin-resistant enterococci (VRE), even though the
spread of multi-drug resistant (MDR) bacteria greatly differs according
to the geographic areas.[2-5] Knowledge of the local
epidemiology of antibiotic resistance may help to develop therapeutic
strategies and stewardship of more effective measures to optimize
therapeutic outcomes and reduce the length of hospital stay.[6-9]
In this study we retrospectively examined the bacterial epidemiology of
the major Gram-negative and Gram-positive pathogens isolated in the
Departments of General Surgery and Emergency Surgery of the Salerno
University Hospital “San Giovanni di Dio e Ruggi d'Aragona” in the
period 2011-2013, paying special attention to the antibacterial
susceptibility profile of Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii and Staphylococcus aureus.
Materials and methods
Data concerning non-duplicate Gram-positive and Gram-negative
microorganisms isolated from different clinical specimens of patients
hospitalized in the period 2011-2013 at the General Surgery and
Emergency Surgery of "San Giovanni di Dio e Ruggi d’Aragona", a
university hospital having 900 bed capacity with major programs in a
broad range of clinical specialties located in Salerno (Italy), were
retrieved from the database of the Laboratory of Bacteriology of
the same hospital. Overall, the number and type of admission to the
hospital did not change over time in the study period. In these wards,
mainly patients undergoing major elective and
urgent/emergency abdominal procedures are admitted. Microbial isolates
are labelled as clinically relevant (both community- and
healthcare-acquired) according to the information as detailed in the
laboratory request form. We also focused on the evaluation of any
change over time of the main expressions of resistance, with particular
regard to the microorganisms considered to be the most difficult to
treat in surgical wards, i.e., K. pneumoniae, A. baumannii, P. aeruginosa and S. aureus.
In particular, we analyzed rates of resistance to carbapenems
(imipenem, meropenem, and ertapenem), amikacin, tigecycline, and
colistin in the Gram-negative and of methicillin-resistance in S. aureus.
Microbial identification and antibiotic susceptibility testing were
performed by the fully automated system VITEK 2 (bioMérieux, Marcy
l'Etoile, France). Colleagues at the Department of Pharmacy provided
data on consumption, in the years 2011-2013, of antibiotics with a
spectrum of activity mainly directed against Gram-negative
microorganisms, i.e.,
imipenem, meropenem, ertapenem, amikacin, tigecycline, and colistin.
Antibiotic usage was evaluated using defined daily doses (DDDs).
Indeed, in our study, we defined the consumption of drugs as DDD/100
bed days.[10] Chi-square or Fisher’s exact test
were used to analyze possible significant differences in isolation
rate. Statistical significance was established at a two-tailed level of
<5%.
Results
Overall,
296 Gram-negative bacteria were isolated in the two departments
considered in the period 2011-2013: 94 in 2011, 77 in 2012, and 125 in
2013. The three most represented pathogens were, in descending order, E. coli, A. baumannii, and P. aeruginosa. This order was constantly maintained throughout the three years, as well as the presence of other microorganisms such as K. pneumoniae and Proteus spp (Table 1). A total of 363 Gram-positive bacteria were isolated in the three-year period: 105 in 2011, 93 in 2012, and 165 in 2013. Table 2
shows the frequencies of isolation of Gram-positive microorganisms
isolated from 2011 to 2013, and the trend did not significantly change
over the study period. The resistance rates of the main Gram-negative
microorganisms in the Departments of General Surgery and Emergency
Surgery during the period 2011-2013 are reported in Table 3. A. baumannii
showed high rates of resistance to carbapenems (with values of 100% in
2011 and 2012) and low rates of resistance to tigecycline, colistin,
and amikacin. P. aeruginosa
showed variable resistance rates, between 11 and 60%, to carbapenems
(imipenem and meropenem) and amikacin, whereas all isolates proved to
be susceptible to colistin. K. pneumoniae
showed variable resistance rates to carbapenems and tigecycline,
whereas all isolates were susceptible to colistin and 2 of the through
2011 were resistant to amikacin. Of note, the prevalence of isolation
of Klebsiella producing
carbapenemase was higher in intensive care units than surgical wards in
the study period. Resistance rates of the main Gram-positive
microorganisms are described in Table 4. The rate of MRSA isolates ranged from 66% to 75% during the study period. Moreover, no S. aureus isolate proved to be resistant to daptomycin, linezolid, tigecycline, and glycopeptides. Table 5 shows the antibiotics usage rates of surgical wards expressed by DDD/100 bed days.
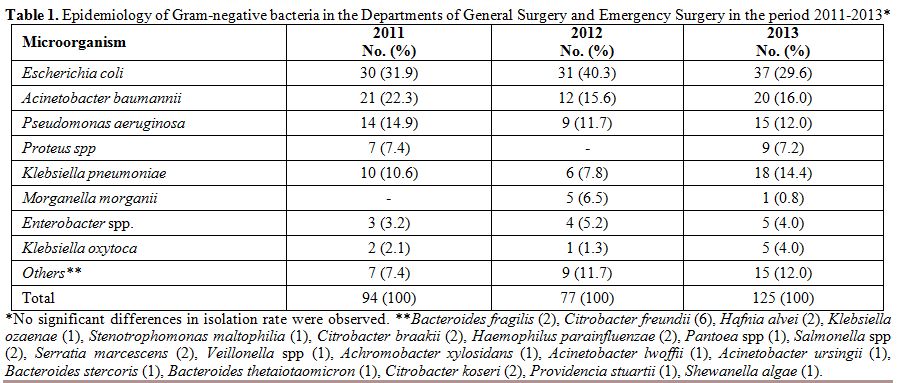 |
Table 1. Epidemiology of Gram-negative
bacteria in the Departments of General Surgery and Emergency Surgery in
the period 2011-2013* |
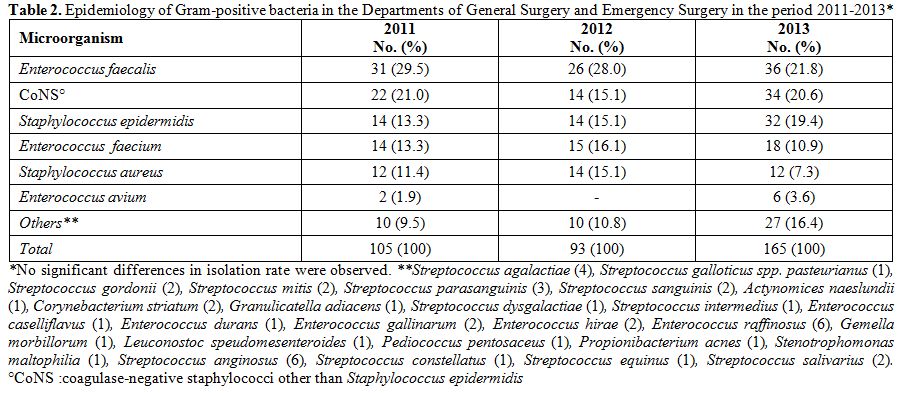 |
Table 2. Epidemiology of Gram-positive
bacteria in the Departments of General Surgery and Emergency Surgery in
the period 2011-2013*. |
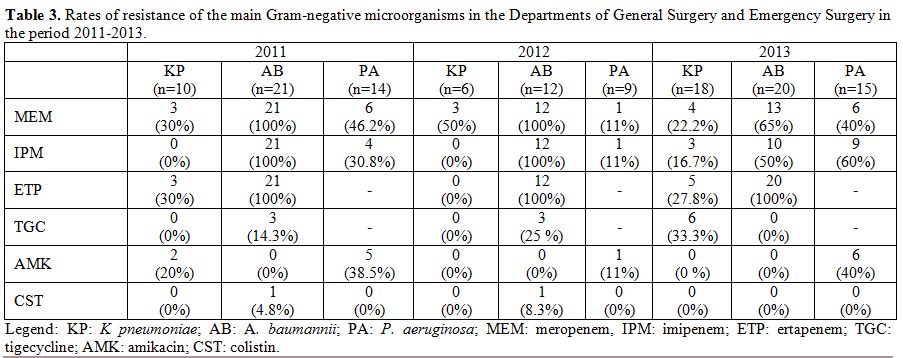 |
Table 3. Rates of resistance of the main
Gram-negative microorganisms in the Departments of General Surgery and
Emergency Surgery in the period 2011-2013. |
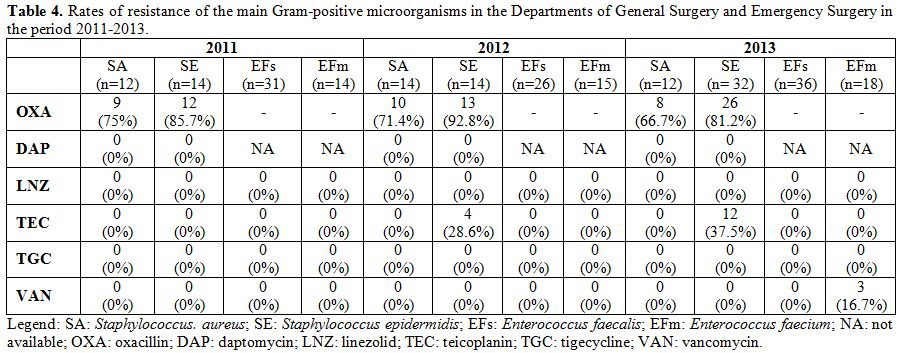 |
Table 4. Rates of resistance of the main
Gram-positive microorganisms in the Departments of General Surgery and
Emergency Surgery in the period 2011-2013. |
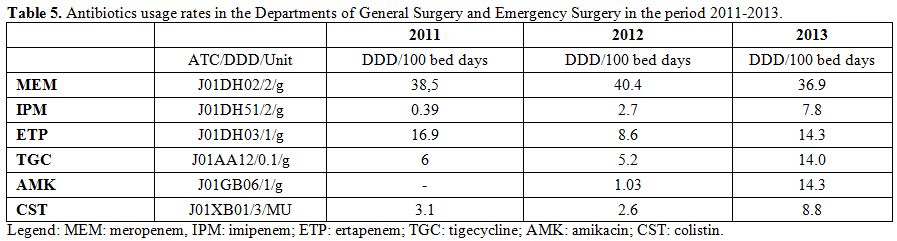 |
Table 5. Antibiotics usage rates in the Departments of General Surgery and Emergency Surgery in the period 2011-2013. |
Discussion
Bacterial infections in surgical wards are an increasing problem.[11-13]
It has been estimated that in Italy surgical infections represent about
14% of all nosocomial infections, a rate lower than in Europe where the
incidence is around 20%.[14] In recent years,
considerable importance has also been given to their impact on health
care costs. For example, a patient with a post-surgical infection can
cost the National Health System up to €325 for a single day’s
hospitalization, with a high risk of re-hospitalization when the
antibiotic therapy is inappropriate.[3] The largest
Italian study published in 2009 primarily aimed to investigate the
epidemiology of the microorganisms responsible for infections in
surgical wards, and the modifiable risk factors showed that E. coli, E. faecalis, and S. aureus are the most common pathogens responsible for surgical site infections (SSIs).[3]
In particular, an increased rate of SSIs due to MRSA was reported, with
a mortality rate of 5%. Data concerning the increasing rate of SSIs
sustained by MRSA were in agreement with those of the study by Manian
that relates such an increase to a number of factors: age over 70
years, surgery duration over 4 hours, and hospital stay longer than 3
days.[6] In the present study, we report a high rate of MRSA (60-80% of all S. aureus isolates) in the three years. These values are in agreement with those reported by Shree (60% of MRSA isolates).[15]
Methicillin resistance was only based on phenotypic resistance (Vitek
2) and was not confirmed either by detection of PBP2a by latex
agglutination tests nor by molecular detection of the mecA
gene. While this can represent a limit of our study, it must be said
that in a study by Nonhoff et al., Vitek 2 system detected oxacillin
resistance with a sensitivity/specificity of 99/96%. In
Europe, the MYSTIC (Meropenem Yearly Susceptibility Test Information
Collection) study has turned its attention to ESBL-producing bacteria.
In particular, it was observed that the expression of ESBL differs
according to the region considered, with higher values being observed
in Italy, Poland, and Russia. Another interesting consideration comes
from MYSTIC data concerning SSIs sustained by P. aeruginosa: isolation of P. aeruginosa
in the course of SSIs does not necessarily involve its etiological
role. The excessive use of carbapenems also employed for their
anti-pseudomonas spectrum might have resulted in an increase in the
resistance of these pathogens to this class of antibiotics.[17] These same data are confirmed by the results of our study where the resistance of P. aeruginosa
to carbapenems lies between 40 and 60%. This datum is in agreement
with those of the European surveillance program of infections EARS-Net
(Annual Report of the European Antimicrobial Resistance Surveillance
Network) which reports, regard to Italy, a P. aeruginosa resistance to these antibiotics more than 50% in 2012.[18] In
Asia, however, the SMART study showed, between 2008 and 2009, a
positive trend in the isolation of ESBL-producing enterobacteria in the
intra-abdominal infections (IAIs), with rates of 10% in Australia,
Japan, Korea and Singapore, and 50% in India, China and Thailand.[19]
The same study also showed that ESBL production is closely related to
increased mortality in the IAIs in agreement with data reported
previously by Esposito.[20] The rise in mortality
from surgical infections was attributed to inadequate antimicrobial
treatment defined as the lack of administration of antibiotics for an
infection or administration of antibiotics to which the microorganism
responsible for the infection is resistant.[20] In our study, we focused on the following Gram-negative pathogens: A. baumannii, P. aeruginosa, which, along with E. faecium and S. aureus, mainly "escape" the effects of antibiotics due to MDR mechanisms.[21-25] Our results related to A. baumannii
are in agreement with those of the study on the SENTRY Antimicrobial
Surveillance Program (2006-2009) reported by Gales with the exception
of a higher resistance to colistin (values between 4.7 and 8.3% versus
0.9% in the SENTRY study).[26] The resistance
rate to tigecycline for this microorganism was not tested in the SENTRY
study. However, our data (15-25%) are in agreement with the
findings (24%) of Fernandez-Cuenca.[27] Concerning the antibiotic resistance pattern of P. aeruginosa and K. pneumoniae, we compared our data with those from the EARS-Net study. The resistance of P. aeruginosa
to carbapenems detected in our study (30-60%) was similar to that
provided by EARS-Net (25-50%), whereas resistance to amikacin (38-40%)
was much greater than that reported in the above study (10-25%).[18] Resistance
to colistin was not tested in the EARS-Net study; however, our data (0%
resistance) differ from those reported by the CANWARD study (6.9%
resistance).[28] The resistance of K. pneumoniae
to carbapenems that we detected (16-50%) is in agreement with that
provided by EARS-Net (25-50%). While the resistance to colistin and
amikacin was not tested in the EARS-Net study. Our data (0% resistance
to both) are similar to those reported by the CANWARD study (0%
resistance to colistin and amikacin at 0.4%).[28] Our data, albeit limited due to the small number of K. pneumoniae
isolates, are in agreement with findings elsewhere that report
increasing antibiotic resistance of this microorganism to carbapenems
and colistin.[29,30] During our observation period,
carbapenems (especially meropenem and imipenem) were found to be the
most frequently used antibiotics. In addition, we observed an
increasing use over the years of tigecycline that can be explained by
the rise of surgical infections sustained by strains of ESBL-producing
bacteria, for which tigecycline proves to be an effective antibiotic.[17]
The same considerations are also appropriate for amikacin and colistin
whose increasing use, according to Shree, could be explained by their
effectiveness for the treatment of infections caused by ESBL-producing
strains.[15] In conclusion, it may be stated that present findings, together with a previous one,[31-34]
have significant clinical implications in fighting infections caused by
MDR bacteria in general surgery and emergency surgery wards. Indeed,
through better understanding of the local bacterial epidemiology, it is
possible to obtain useful information to administer an appropriate
empiric therapy and prevent the spread of antibiotic-resistant
organisms.
References
- Ramcharan AA, den Heijer CD, Smeets EE, Rouflart
MM, van Tiel FH, Bruggeman CA, Breukink SO, Tordoir JH, Baeten CG,
Stobberingh EE. Microbiology of surgical site infections after
gastrointestinal surgery in the south region of The Netherlands. Future
Microbiol. 2014;9:291-8. http://dx.doi.org/10.2217/fmb.13.169 PMid:24762304
 .
. - Montravers
P, Lepape A, Dubreuil L, Gauzit R, Pean Y, Benchimol D, Dupont H.
Clinical and microbiological profiles of community-acquired and
nosocomial intra-abdominal infections: results of the French
prospective, observational EBIIA study. J Antimicrob Chemother.
2009;63:785-94. http://dx.doi.org/10.1093/jac/dkp005 PMid:19196742
 .
. - De Werra C, Schiavone D, Di Micco R, Triassi M. Surgical site infections in Italy. Infez Med. 2009;17:205-18. PMid:20046101
 .
. - Stefani S. Evolution in the antibiotic susceptibility and resistance. Infez Med. 2009;17(Suppl 3):5-12. PMid:19838094
 .
. - Esposito
S, Capuano A, Noviello S, Mazzeo F, Ianniello F, Filippelli A, Rossi F,
Leone S. Modification of patients' endogenous bacterial flora during
hospitalization in a large teaching hospital in Naples. J Chemother.
2003;15:568-73. http://dx.doi.org/10.1179/joc.2003.15.6.568 PMid:14998082
 .
. - Manian
FA, Meyer PL, Setzer J, Senkel D. Surgical site infections associated
with methicillin-resistant Staphylococcus aureus: do postoperative
factors play a role? Clin Infect Dis. 2003;36:863-68. http://dx.doi.org/10.1086/368195 PMid:12652387
 .
. - Esposito
S, Ianniello F, Leone S, Noviello S, Marvaso A, Iannantuoni N, Esposito
E, Imperato L, Aiello D, Aloisio T, Maio P, Acierno D, Romano G,
Patrelli G. Multicentre survey of post-surgical infections in Campania
(Italy). Infez Med. 2003;11:146-52. PMid:14985647
 .
. - Drago
L. Epidemiology and mechanisms of resistance: clinical and
environmental impact. Infez Med. 2007;15(Suppl 2):6-12. PMid:17940407
 .
. - Camporese
A, Santini G. Surveillance of antibiotic-resistant microorganisms for
the rational use of antimicrobial drugs. Infez Med. 1999;7:172-76.
PMid:12736554
 .
. - WHO Collaborating Centre for Drug Statistics Methodology. http://www.whocc.no/atc_ddd_index/ (accessed April 19, 2015)
- Owens
CD, Stoessel K. Surgical site infections: epidemiology, microbiology
and prevention. J Hosp Infect. 2008;70(Suppl 2):3-10. http://dx.doi.org/10.1016/S0195-6701(08)60017-1
 .
. - Leaper DJ. Risk factors for and epidemiology of surgical site infections. Surg Infect. 2010;11:283-87. http://dx.doi.org/10.1089/sur.2010.022 PMid:20528147
 .
. - Young
MH, Washer L, Malani PN. Surgical site infections in older adults:
epidemiology and management strategies. Drugs Aging. 2008;25:399-414. http://dx.doi.org/10.2165/00002512-200825050-00004 PMid:18447404
 .
. - Esposito
S, Leone S, Noviello S, Lanniello F, Fiore M. Antibiotic resistance in
long-term care facilities. New Microbiol. 2007;30:326-31. PMid:17802920
 .
. - Shree
N, Arora BS, Mohil RS, Kasana D, Biswal I. Bacterial profile and
patterns of antimicrobial drug resistance in intra-abdominal
infections: current experience in a teaching hospital. Indian J Pathol
Microbiol. 2013;56:388-92. http://dx.doi.org/10.4103/0377-4929.125321 PMid:24441227
 .
. - Nonhoff
C, Rottiers S, Struelens MJ. Evaluation of the Vitek 2 system for
identification and antimicrobial susceptibility testing of
Staphylococcus spp. Clin Microbiol Infect 2005;11:150-153. http://dx.doi.org/10.1111/j.1469-0691.2004.01047.x PMid:15679491
 .
. - Nicoletti
G, Nicolosi D, Rossolini GM, Stefani S. Etiology, epidemiology and
microbiological diagnosis of intra-abdominal infections. Infez Med.
2008;16(Suppl 1):8-18. PMid:18382147
 .
. - European
Centre for Disease Prevention and Control. Antimicrobial resistance
surveillance in Europe 2011. Annual Report of the European
Antimicrobial Resistance Surveillance Network (EARS-Net). 2012.
- Sheng
WH, Badal RE, Hsueh PR. SMART Program. Distribution of
extended-spectrum ß-lactamases, AmpC ß-lactamases, and carbapenemases
among Enterobacteriaceae isolates causing intra-abdominal infections in
the Asia-Pacific region: results of the study for Monitoring
Antimicrobial Resistance Trends (SMART). Antimicrob Agents Chemother.
2013;57:2981-88. http://dx.doi.org/10.1128/AAC.00971-12 PMid:23587958 PMCid:PMC3697370
 .
. - Esposito
S, Leone S, Carosi G. Analysis of current guidelines for
intra-abdominal infections. J Chemother. 2009;21(Suppl 1):30-35. http://dx.doi.org/10.1179/joc.2009.21.Supplement-1.30 PMid:19622448
 .
. - Deveci
Ö, Dal T, Tekin R, Bozkurt F, Tekin A, Dayan S. Carbapenem resistance
in Acinetobacter baumannii: where is it heading? Infez Med.
2013;21:211-15. PMid:24008854
 .
. - Bassetti
M. Strategies for management of difficult to treat Gram-negative
infections: focus on Pseudomonas aeruginosa. Infez Med. 2007;15(Suppl
2):20-26. PMid:17940409
 .
. - Ece
G, Samlioglu P, Atalay S, Kose S. Evaluation of the in vitro colistin
susceptibility of Pseudomonas aeruginosa and Acinetobacter baumannii
strains at a tertiary care centre in Western Turkey. Infez Med.
2014;22:36-40. PMid:24651089
 .
. - Rice
LB. Federal funding for the study of antimicrobial resistance in
nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197:1079-81. http://dx.doi.org/10.1086/533452 PMid:18419525
 .
. - Ippolito
G, Leone S, Lauria FN, Nicastri E, Wenzel RP. Methicillin-resistant
Staphylococcus aureus: the superbug. Int J Infect Dis. 2010;14 (Suppl
4):7-11. http://dx.doi.org/10.1016/j.ijid.2010.05.003 PMid:20851011
 .
. - Gales
AC, Jones RN, Sader HS. Contemporary activity of colistin and polymyxin
B against a worldwide collection of Gram-negative pathogens: results
from the SENTRY Antimicrobial Surveillance Program (2006-09). J
Antimicrob Chemother. 2011;66:2070-74. http://dx.doi.org/10.1093/jac/dkr239 PMid:21715434
 .
. - Fernández-Cuenca
F, Tomás-Carmona M, Caballero-Moyano F, Bou G, Martínez-Martínez L,
Vila J, Pachón J, Cisneros JM, Rodríguez-Ba-o J, Pascual A. In vitro
activity of 18 antimicrobial agents against clinical isolates of
Acinetobacter spp.: multicenter national study GEIH-REIPI-Ab 2010.
Enferm Infecc Microbiol Clin. 2013;31:4-9. http://dx.doi.org/10.1016/j.eimc.2012.06.010 PMid:22939566
 .
. - Karlowsky
JA, Adam HJ, Baxter MR, Lagacé-Wiens PR, Walkty AJ, Hoban DJ, Zhanel
GG. Antimicrobial susceptibility of 22746 pathogens from Canadian
hospitals: results of the CANWARD 2007-11 study. J Antimicrob
Chemother. 2013;68(Suppl 1):17-22.
 .
. - Morrissey
I, Hackel M, Badal R, Bouchillon S, Hawser S, Biedenbach D. A Review of
Ten Years of the Study for Monitoring Antimicrobial Resistance Trends
(SMART) from 2002 to 2011. Pharmaceuticals (Basel). 2013;6:1335-46. http://dx.doi.org/10.3390/ph6111335 PMid:24287460 PMCid:PMC3854014
 .
. - Castanheira
M, Mendes RE, Woosley LN, Jones RN. Trends in carbapenemase-producing
Escherichia coli and Klebsiella spp. from Europe and the Americas:
report from the SENTRY antimicrobial surveillance programme (2007-09).
J Antimicrob Chemother. 2011;66:1409-11. http://dx.doi.org/10.1093/jac/dkr081 PMid:21421581
 .
. - Manfredi
R, Nanetti A. An active microbiological surveillance project at an
Italian teaching hospital: microbial isolates, recent epidemiological
trends, major clinical concerns, and antimicrobial susceptibility rates
during a four-year period. Infez Med. 2009;17:219-27. PMid:20046102
 .
. - Leone
S, Stefani S, Venditti M, Grossi P, Colizza S, De Gasperi A, Scaglione
F, Sganga G, Esposito S. Intra-abdominal infections: model of
antibiotic stewardship in an era with limited antimicrobial options.
Int J Antimicrob Agents. 2011;38:271-2. http://dx.doi.org/10.1016/j.ijantimicag.2011.06.003 PMid:21782394
 .
. - Esposito
S, Leone S, Noviello S, Ianniello F. Management of severe bacterial
infections and role of the infectious disease specialist: results of an
interview-based survey. Infez Med 2004;12:90-100. PMid:15316294
 .
. - Esposito S, Leone S, Noviello S. Management of severe bacterial infections. Expert Rev Anti Infect Ther. 2005;3:593-600. http://dx.doi.org/10.1586/14787210.3.4.593 PMid:16107198 .
[TOP]







 .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.