New Insight on Epidemiology and Management of Bacterial Bloodstream Infection in Patients with Hematological Malignancies
Sara Lo Menzo*, Giulia la Martire*, Giancarlo Ceccarelli and Mario Venditti
Department of Public Health and Infectious Diseases. University of Rome “Sapienza”, Rome (Italy)
* These authors contributed equally to this paper
Corresponding author: Mario Venditti MD, Ph.D. Department of
Public Health and Infectious Diseases, University of Rome “Sapienza”,
Viale del Policlinico 155, (00161) Rome, Italy. Tel. +39-06-49970313,
Fax +39-06-49972625. E-mail:
mario.venditti@uniroma1.it
Published: July 01, 2015,
Received: April 28, 2015
Accepted: June 08, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015044, DOI
10.4084/MJHID.2015.044
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Bloodstream infections (BSI) are a
significant cause of morbidity and mortality in onco-hematologic
patients. The Gram-negative bacteria were the main responsible for the
febrile neutropenia in the sixties; their impact declined due to the
use of fluoroquinolone prophylaxis. This situation was followed
by the gradual emergence of Gram-positive bacteria also following the
increased use of intravascular devices and the introduction of new
chemotherapeutic strategies. In the last decade, the Gram-negative
etiology is raising again because of the emergence of resistant strains
that make questionable the usefulness of current strategies for
prophylaxis and empirical treatment. Gram-negative BSI attributable
mortality is relevant, and the appropriate empirical treatment
significantly improves the prognosis; on the other hand the adequate
delayed treatment of Gram-positive BSI does not seem to have a high
impact on survival. The clinician has to be aware of the epidemiology
of his institution and colonizations of his patients to choose the most
appropriate empiric therapy. In a setting of high endemicity of
multidrug-resistant infections also the choice of targeted therapy can
be a challenge, often requiring strategies based on off-label
prescriptions and low grade evidence.
In this review, we
summarize the current evidence for the best targeted therapies for
difficult to treat bacteria BSIs and future perspectives in this topic.
We also provide a flow chart for a rational approach to the empirical
treatment of febrile neutropenia in a multidrug resistant, high
prevalence setting.
|
Emerging Bacterial Infection in Hematological Neutropenic Patients
Although in the last decades noteworthy improvements have been
achieved in the management of hematologic cancer patients, infections
persist as leading cause of morbidity and mortality particularly
during the cytotoxic neutropenia, defined as a neutrophil count
< 500/mmc.[1,2] Respiratory tract infections occur very often,
followed by bloodstream infections (BSI), urinary tract infections,
skin/skin structure infections and oro-pharynx/gastrointestinal tract
infections.[2] In this paper, we shall focus only on BSI.
These
infections, mostly caused by bacteria, range from 11 to 38% mortality
in neutropenic patients,[3,4] with an unknown origin in most cases
(oropharyngeal and gastrointestinal tract are assumed as probable
sources). As shown in figure 1,
the etiology of BSI has changed through the years. Since 1960, the
importance of Gram-negative bacilli in BSI began to be clearly
recognized and in the following two decades these organisms represented
the most frequent etiological agents. During the nineties,
Gram-positive bacteria and emerged as a leading cause of BSI. This
increased prevalence has been analyzed by several authors,[5-7] factors
such as the large use of central venous catheters (CVC),
fluoroquinolones (FQ) and antifungal prophylaxis, gut decolonization
strategies, use of high cytarabine doses, use of protonic pump
inhibitors have been highlighted as possible causative factors. In the
last few years, many papers report a turnaround in BSI etiology, with
an increasing role of gram negative bacteria,[2,5,8] becoming the first
cause of BSI in some settings.[7]
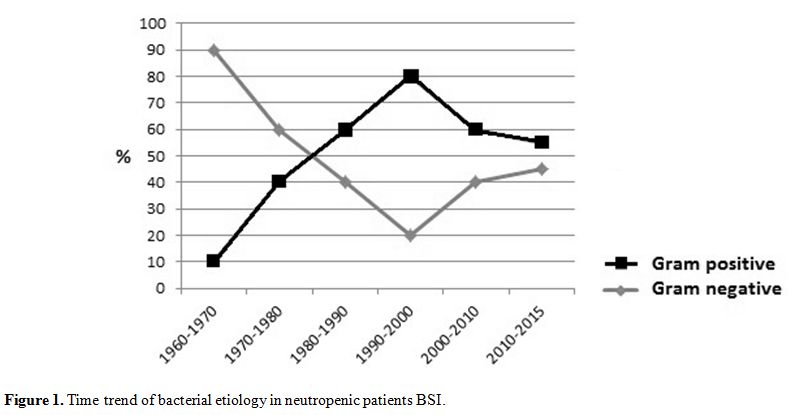 |
Figure
1. Time trend of bacterial etiology in neutropenic patients BSI |
Moreover, the widespread of antimicrobial resistance,
especially among Gram-negative bacilli as extended spectrum
beta-lactamase (ESBL) producing Enterobacteriaceae or carbapenem
resistant Gram- negative bacteria (Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa),
makes the correct setting of empirical therapy becoming a challenge,
since alternative regimes are very few and often present some
management issues.[1]
The aim of this paper is to review the
current BSI epidemiology among neutropenic onco-hematologic patients,
as well as to highlight the most important clinical features and
therapeutic management issues.
Gram-positive BSI in Hematologic Cancer Patients
Gram-positive bacteria BSIs in neutropenic patients became a major
concern during the nineties because of their growing prevalence. The
emergence of staphylococcal infections in relation to the increased use
of CVC and FQ prophylaxis led to a significant reduction in the
proportion of Gram-negative bacteria.[5] A large prospective
multicenter study by Cordonnier et al[6] established the Gram-positive
risk index based on four major factors represented by the use of high
cytarabine doses, proton pump inhibitors, decolonization strategies
with colimycin without aminoglycosides and the presence of chills at
the onset of fever.
Other authors also outlined the importance of
high-grade mucositis and toxic enterocolitis in the development of
streptococcal and enterococcal bacteremia during neutropenia.[9-10]
Nowadays Gram-positive bacteria still reaches 50% of BSI in neutropenic
patients,[8-7] being coagulase negative staphylococci (CoNS) the most
frequent, followed by streptococci, S. aureus, enterococci, and occasionally Corynebacterium spp or other rare Gram-positive bacteria.
Coagulase Negative Staphylococci (CoNS)
CoNS normally colonize mammalian skin and mucosa. In the past, they
were almost universally considered as blood cultures contaminants. S.
epidermidis has been recognized as the single most frequently isolated
species from BSI. S. haemoliticus, S. lugdunensis, S. saprophiticus, S. capitis, S. auricularis
have been isolated less frequently. In general they have a low grade
virulence with a poor propensity to invade; however they have a
peculiar ability to form a biofilm on biomaterials[11] and often
carry resistance genes.
CoNS are a major cause of BSI in
neutropenic patients reaching 25% (5-60%) of all cases.[8] As
previously outlined, their incidence in this population seem to be
related to the use of FQ prophylaxis. Gudiol et al. observed a
significant reduction of Gram-positive BSI since FQ prophylaxis was
abandoned in their center. A significant part of CoNS’s bacteremias
seems to be related to mucosal more than commensal skin
bacteria.[12-13] This could explain their important role in neutropenic
patients in which mucosal disruption is very frequent due to the
cytotoxic treatment.
Even if they are the first BSI etiologic
agent in neutropenic patients, their clinical relevance is
questionable. Their attributable mortality is low,[14] as for
immune-competent patients in the absence of specific risk factors (such
as prosthetic heart valves, joints, and other prosthetic materials).
CoNS blood isolates are usually methicillin resistant, achieving an 80% rate in the last reports[15] except S. lugdunensis or S. capitis that are almost always susceptible to oxacillin.[16]
Concerning glycopeptides, growing resistance to teicoplanin has been observed,[15] in particular in S. haemoliticus where it can reach 20% of clinical isolates.[14] On the other hand, resistance to vancomycin is still very low, except for S. schlefferi.[16] More recently an alarming emergence of linezolid resistant S. epidermidis
has been described in Greece.[17] Resistance to linezolid has been
associated with higher virulence and higher attributable mortality
compared to linezolid susceptible staphylococci[18] but they have not
been described yet among neutropenic patients. Resistance to daptomycin
is still anecdotic.[19]
Staphylococcus aureus
S. aureus is a common cause
of both hospital and community acquired BSIs[11] and it handles 6%
(0-20%) of BSIs in onco-hematologic patients.[8] The clinical
management of S. aureus BSI
(SAB) changes in case of complicated or uncomplicated presentation,[20]
in terms of duration of treatment, indication to perform an
echocardiogram and metastatic foci research.
Surprisingly,
compared to non neutropenic patients, S. aureus BSI during neutropenia
seems to be associated with lower attributable mortality and low
incidence of metastatic events or endocarditis (Table 1).[21] Two explanations have been proposed for this phenomenon. Firstly, in neutropenia even few cells of S. aureus
could be able to gain access to bloodstream trough altered mucosal and
skin barrier and evade phagocytosis; thus an altered bacterial
clearance could be responsible for positive blood cultures even with
very low inoculum bacteremia. On the other hand, the absence of severe
sepsis and septic shock could be related to the inability of these
patients to produce the highly orchestrated inflammatory response (that
include neutrophils and macrophages).[21]
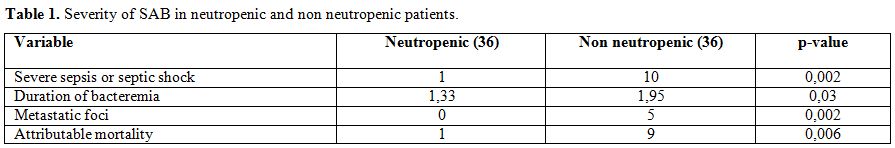 |
Table 1. Severity of SAB in neutropenic and non neutropenic patients |
Methicillin resistance among S. aureus
isolates reported in Europe in 2013 was 18% with percentages ranging
from 0 to 64% depending on the country.[22] Neutropenic patients are at
high risk to become MRSA carriers. In fact the use of FQ, recommended
as prophylaxis in all cases of prolonged neutropenia,[1] can represent
an important risk factor for the emergence of MRSA.[23]
Vancomycin
resistance is a marginal problem but high vancomycin MIC (between 1 and
2 mg/L), is associated with risk of failure.[24] Interestingly
vancomycin MIC >1 mg/L seem to be independently associated with the
worst outcome also in methicillin susceptible S. aureus (MSSA) infected patients.[25]
Linezolid resistant S. aureus
are still rarely isolated, but several reports in the last few
years[26,27] highlight this emerging problem that is not yet described
in neutropenic population. Daptomycin resistance is also very rare and
described mainly in case reports.
Because of low attributable
morbidity and mortality of methicillin resistant strains, empirical
treatment with glycopeptides is not required in neutropenic patients as
demonstrated in two recent meta-analysis, where it was outlined that a
appropriate delayed treatment had no impact on prognosis.[28-29]
Considering
the high rate of gastrointestinal origin of staphylococcal BSI and the
management problems in onco-hematologic population (piastrinopenia,
chemotherapies needing a central line) the indication for the removal
of CVC has to be considered for each single case. However the
ascertained S. aureus etiology of a catheter related BSI is an absolute indication for the removal of the catheter.[30]
The
antibiogram guided therapy for MRSA has to take into account that a
vancomycin MIC >1 mg/L could lead to a failure when treated with
vancomycin. Daptomycin should be preferred in these cases unless in the
presence of pneumonia. The possible use of clindamycin, cotrimoxazole
and aminoglicosides needs to be evaluated in each case, due to the
variable susceptibility of these antibiotics in MRSA.
The use of newer drugs need further evaluations but should be considered for cases difficult to treat. In Table 2 are reported the newest anti-staphylococcal drugs that are already or will be soon available.
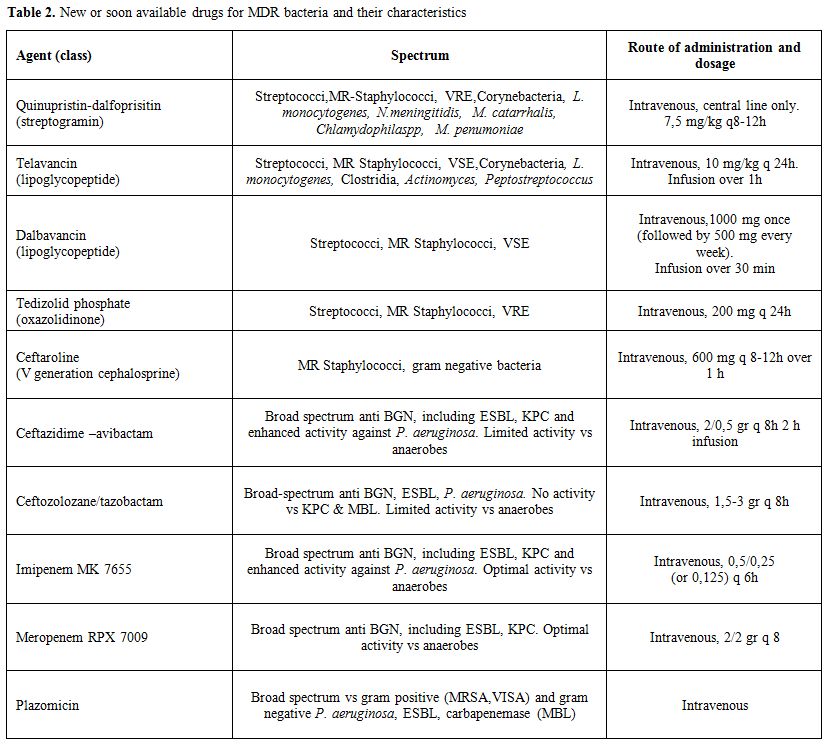 |
Table 2. New or soon available drugs for MDR bacteria and their characteristics |
Enterococci
Enterococci
reach only 5% (0-38%) of BSIs (E-BSI) in neutropenic patients, while
the higher rates are observed in hematopoietic stem cell
transplantation (HSCT) recipients, in particular in the first 10 days
after transplantation.[8] Mikulska and collaborators[10] identified
risk factors associated with E-BSI in this category of patients: donors
other than HLA identical, pharyngeal enterococcal colonization, high
grade mucositis, Karnofsky score <50, previous use of third
generation cephalosporines. E. faecalis and E. faecium are the most frequent isolated species, but E. faecalis/E. faecium ratio
of isolation has changed during the last 20 years. It was approximately
10:1 in the eighties[31] and it is almost 20:1 in more recent
reports;[10,32] this is probably due to E. faecium resistance profile. The clinical significance of their isolation (poor clinical condition marker versus “true infection maker”) should be established in each cause of BSI during neutropenia. Enterococci
intrinsic virulence is low but they are intrinsically resistant to
aminoglicosides, cotrimoxazole (in vivo), cephalosporines.[11]
Ampicillin resistance is very prevalent for E. faecium and rare for E. faecalis. High level aminoglycosides resistance is very prevalent for both E. faecalis and E. faecium. Resistance to vancomycin is mainly present in E. faecium.
The most frequent genes being Van A and Van B that codify for modified
cell wall proteins. In Van B strains teicoplanin is active but not in
Van A strains. Vancomycin resistance is more frequent in Eastern
Europe, UK and USA.[22] Factors associated with vancomycin resistant
enterococci BSI (VRE-BSI) among E-BSI are the recent use of vancomycin
or glucocorticosteroids or severity of illness.[33] In HSCT recipients,
previous VRE colonization and Graft versus Host Disease (GVHD) were
also associated with VRE-BSI.[34] VRE
seem to a have a peculiar clinical behavior compared to VSE (Vancomycin
Susceptible Enterococci). Diaz Granados and collaborators performed a
meta-analysis including 1614 E-BSI cases, and highlighted an increased
mortality in VRE-BSI, compared to VSE-BSI (OR 2.51).[35] The authors
could not conclude if this observation was an effect of a delay of
appropriate therapy or of an increased virulence of VRE (that are E. faecium in most cases). Empirical
treatment for E-BSI is not recommended. No benefit was observed even in
HSCT recipients colonized with VRE receiving empirical linezolid.[36] Even
if randomized controlled trials comparing linezolid and daptomycin in
VRE-BSI are lacking, the available evidence suggests a superiority of
linezolid in terms of mortality and treatment failure.[37-38] This
observation has also been highlighted in neutropenic patients.[39] .Viridans Streptococci
Viridans
streptococci are an important part of the normal microbial flora. They
are indigenous to the upper respiratory tract, the female genital
tract, and all regions of the gastrointestinal tract but are most
prevalent in the oral cavity.[11] They normally have a low virulence
and tendency to invade. However not surprisingly they are an important
cause (5%) of BSI in neutropenic patients.[8] In
the previously mentioned paper, Cordonnier established a score of risk
for the development of viridians streptococcal BSI in neutropenic
patients. This score included the use of high dose cytarabine
during induction therapy, oral colimycin without aminoglycosides as
decontamination, prophylaxis with antifungal drugs and the presence of
diarrhea.[6] Oral mucositis appeared associated with this infection
only in univariate analysis. Another possible association has been seen
with periodontitis at the time of the onset of neutropenia.[40] Viridans
streptococci BSIs (VS-BSI) during neutropenia carry substantial
morbidity and mortality. Attributable mortality is ranging from 6 to
12%.[11] Severe cases, presenting ARDS or shock or both were associated
with allogenic bone marrow transplantation, presence of severe oral
mucositis (grade 3 or 4) and high dose therapy with cyclophosphamide
reaching 11% of the streptococcal bacteremias in Marron et al
series.[41] Since the end of the eighties, reduced susceptibility (MIC
> 0.12mg/L) and resistance to penicillin (> 0.25 mg/L) have been
described in viridians streptococci,[41-43] including those isolated in
onco-hematologic patients.[11] Poor susceptibility of streptococci to
ceftazidime[41] should suggest not using this agent as an empirical
treatment of febrile neutropenia in institutions, and should preclude
its use in patients at high risk of streptococcal BSI.[41]
Corynebacterium spp. and other Rare Gram-Positive Etiologies
“Other
Gram-positive” etiologies reach 6% (0-21%) of BSI in neutropenic
patients.[8] They include Corynebacteria (usually represented by
multidrug resistant (MDR) isolates with a spectrum of antibiotic
resistances similar to that of MRSA,[9] beta haemolytic streptococci
and several organisms that colonize the skin such as Aerococcus spp., Bacillus spp., Micrococcus spp. and S. pneumonia are also relevant. Organisms, such as Listeria monocytogenes, Rhodococcus equi, and vancomycin-resistant bacteria, such as Lactobacillus spp., Leuconostoc spp. and Pediococcus, are occasionally encountered (Figure 2).[44,45]
Both linezolid and daptomycin demonstrated good in vitro activity
against all Gram-positive isolates in cancer patients.[44]
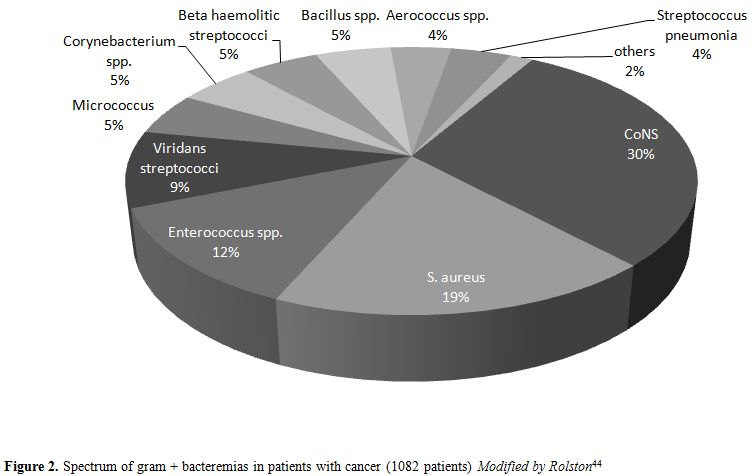 |
Figure 2. Spectrum of gram + bacteremias in patients with cancer (1082 patients) Modified by Rolston[44] |
Gram-negative BSI in Hematologic Cancer Patients
We
already mentioned in the introduction the turnaround in BSI etiology in
neutropenic patients. In a recent Italian multicenter study,[7]
Gram-negative bacteria were the most frequent isolates in patients with
hematologic malignancy. Infections caused by these microorganisms have
been identified as an independent predictor of death in patients
with malignancies and bloodstream infection: BSI alone reach 12%-42% of
mortality.[46,47] The
distribution of Gram-negative bacilli from BSI remained stable over
time but the emergence of multi drug resistant (non susceptible to more
than 1 agent in 3 or more antimicrobial categories, MDR), extremely
drug resistant (non susceptible to more than 1 agent in all but 2 or
less antimicrobial categories, XDR) and pandrug resistant (non
susceptible to all antimicrobial active agents, PDR) isolates represent
today the main challenge in managing of Gram-negative BSI.[48]
Escherichia coli
Due to its ability to colonize the human gastrointestinal tract, E. coli
is the most common bacterial species found in human fecal flora. Thus,
it is not surprising that it also represents the more frequent
cause of Gram-negative BSI in neutropenic patients, reaching almost one
quarter of all isolates.[8,44,49,50] In this patient population, the
morbidity and mortality due to E. coli
BSI might be due to several factors, including antibiotic
resistance, FQ resistance and ESBL production, which are the most
represented, can be present in almost one third of all isolates and
both are favoured by the widespread use of FQ prophylaxis.[5,51-54]Since
inadequate initial antimicrobial therapy has been associated with
poorer outcomes, in recent years carbapenems have been employed
increasingly as agents of choice against ESBL E. coli
and for the empirical treatment of BSI in neutropenic
patients.[50,55] However, the concomitant emergence of carbapenems
resistance GNB and the observation that carbapenem restriction might be
associated with lower rates of carbapenem-resistances have led
researchers to consider carbapenem-spearing antibiotic strategies.[56] Among
the potential alternative therapies explored, the role of
piperacillin-tazobactam, has been reassessed: in fact patients treated
with this combination and those treated with carbapenem against
β-lactam-β-lactamase inhibitor BLBLI susceptible E. coli presented a similar therapeutic outcome.[51,57] Therefore,
considered these assumptions, in a setting of high ESBL prevalence, a
possible antimicrobial stewardship program could be based on simply
model of de-escalation strategy. Indeed, in case of proven
susceptibility, the change of treatment from carbapenem to
piperacillin/tazobactam could be safe and prevent the risk of
carbapenemase induction.Otherwise in epidemiologic settings characterized by a high prevalence of infection due to piperacillin-tazobactam resistant E. coli, empirical therapy with a combination piperacillin-tazobactam and tigecyclin could be another suitable option.[58]
Klebsiella pneumoniae
K. pneumoniae
is the primary species of genus Klebsiella associated with illness in
human beings. It is found in the gastrointestinal tract and is
frequently involved in health-care and intensive care unit (ICU)
associated infections.[59] Infections with K. pneumoniae are usually
hospital-acquired, sustained by MDR strains and occur primarily in
patients with impaired host defenses.As described for E. coli, K. pneumoniae is often represented by FQ and third generation cephalosporin resistant strains; thus it shares with E. coli
all the therapeutic challenges deriving from these types of resistance.
Moreover, several mechanisms have been identified as responsible for
carbapenem resistance among Enterobacteriaceae: Ambler class A
beta-lactamases are enzymes that can be either plasmid encoded (blaKPC and, less frequent, blaIMI-2, blaGES) or chromosomally encoded (blaNMC, blaSME, blaIMI-1, blaSFC-1).
The class B metallo-β-lactamases includes (MBLs) Verona
integron-encoded metallo-β-lactamase (blaVIM), blaIMP, and the New
Delhi metallo-β-lactamase (blaNDM). BlaOXA-48 carbapenemases belong to
Ambler class D. Finally resistance to carbapenems can also be caused by
hyperexpression of AmpC gene or to decreased permeability of the outer
membrane because of porin loss in combination with the expression of
AmpC enzymes or ESBLs.[60,61]From
an epidemiological point of view, K. pneumoniae represents the third
leading cause of GNB BSI in neutropenic patients population reaching
almost 12.5% in a recent Italian multicenter study.[7] Since 2008 an
increasing number of reports described the spread of carbapenem
resistant strains mainly in the Mediterranean and Southern European
countries with a rapid spread in Israel and Greece.[62] The spread of
carbapenem resistant Enterobacteriaceae (CRE) has dramatically
increased also in Italy rising from 15.2% in 2010 to 34.3% in
2013.[7,63,64]The
high incidence of these MDR strains in immune-compromised populations
was confirmed by recent multicenter studies reporting that a KPC
producing K. pneumonia (KPC-Kp) rectal colonization was common in
onco-hematological patients. In this cohort the colonization was
followed by an infection in 39.2% cases of allogeneic Stem Cell
Transplantation Recipients (allo-SCT)[65] and 45% of cases of neutropenic
patients.[66] Observed mortality rate attributable to KPC-Kp BSI was of
57.6% in adult inpatients,[64] while it was of 64.4% in allo-STC
recipients.[65] Therefore considering these aspects, it is crucial to
recognize the KPC-Kp carriers and consider this information in febrile
neutropenic patients at risk of BSI.Although
empirical treatments against KPC-Kp are not recommended by current
IDSA-ECIL guidelines, due to their potential toxicity and off label
usage (Figure 3), we believe that these therapies are justified in this
setting, according to the evidence of the literature. The different
scenarios potentially met by the clinician are analyzed in the figure
3, which provides a diagnostic and therapeutic algorithm that might be
useful in this setting. In any case, it is essential to stress that
neutropenic patients should be routinely screened on rectal swab
cultures to identify patients with KPC-Kp gut colonization.
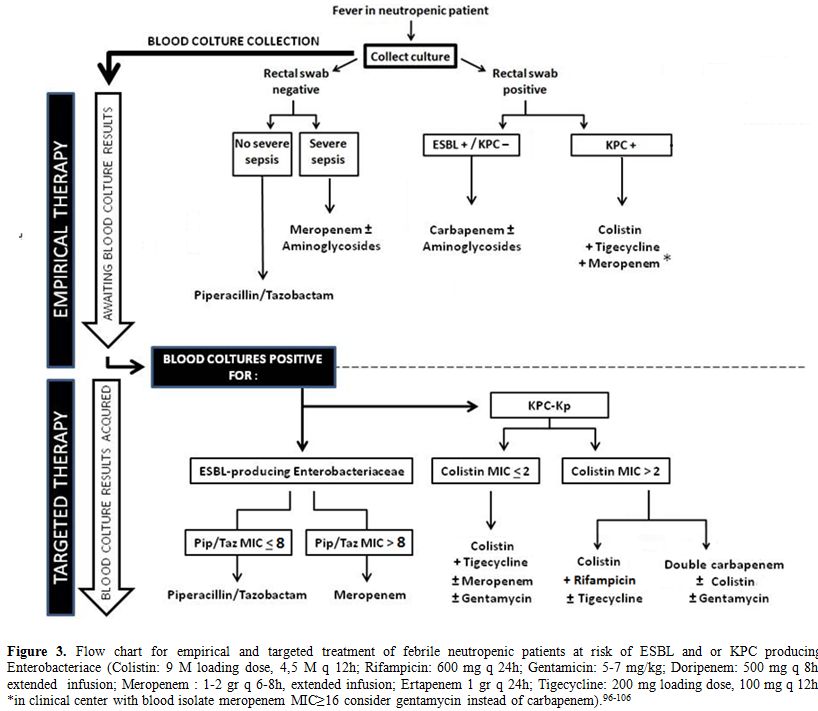 |
Figure 3. Flow chart for empirical and
targeted treatment of febrile neutropenic patients at risk of ESBL and
or KPC producing Enterobacteriace (Colistin: 9 M loading dose,
4,5 M q 12h; Rifampicin: 600 mg q 24h; Gentamicin: 5-7 mg/kg;
Doripenem: 500 mg q 8h, extended infusion; Meropenem : 1-2 gr q
6-8h, extended infusion; Ertapenem 1 gr q 24h; Tigecycline: 200 mg
loading dose, 100 mg q 12h; *in clinical center with blood isolate
meropenem MIC≥16 consider gentamycin instead of carbapenem).[95-105]
|
Awaiting
new drugs showed in Table 2 potentially active against KPC-Kp, at the
moment colistin represents the back-bone of therapeutic regimes against
KPC-KP;[64,67-70] its use is possible in the case of infections due to
both colistin susceptible and resistant strains, as showed in figure 3.Synergistic
activity have been reported with therapeutic strategies combining
colistin with rifampicin[71] and ertapenem with meropenem +/-colistin.[72]
This last strategy, so called “double carbapenem” therapy, could be
employed in cases of severe infection not responsive to previous
treatment. The activity of combination is justified from in vivo
studies[73-74] that seems to corroborate in vitro experiments performed
by Bulik et al., who recently postulated that the enhanced
efficacy of this therapy against KPC-Kp may be related to the KPC
enzyme's preferential affinity for ertapenem.[75] Finally,
it is worth to remember that rectal swab surveillance is recommended as
a component of infection prevention programs and of antimicrobial
stewardship that can reduce the rate of CRE infections, including
BSI.
Pseudomonas aeruginosa and other non Fermentative Gram Negative Bacilli (NFGNB)
P. aeruginosa is an ubiquitary Gram-negative invasive pathogen, responsible for severe infections in immune-compromised hosts. Since 1960 P. aeruginosa
BSI has been highlighted as an important and frequent cause of
morbidity and mortality in neutropenic patients. With the introduction
of FQ prophylaxis P. aeruginosa
prevalence progressively declined, but nevertheless it is still
responsible for 18% to 27% of BSI in this population[2,5,7] with a
mortality rate of 40%.[76] However,
at the moment the benefit of FQ prophylaxis, despite its historical
value, is a matter of concern for its association with the
emergence of antibiotic resistance, especially in those countries where
MDR and XDR strains reached 50% of isolates.[63] Furthermore the
problem of the emergence of MDR strains is related to the clinical
outcome. In fact, an increased invasive capacity of MDR P. aeruginosa
was evidenced by the observation that the patients colonized with MDR
strains are at higher risk of BSI compared to those with a no-MDR
colonization.[77] Concerning
therapeutic resources, first line recommended therapy of ECIL and IDSA
guidelines for the management of fever in neutropenic patients ensure
coverage for susceptible P. aeruginosa. The addition of aminoglycosides could be effective in cases of severe sepsis or septic shock.Regimes
based on colistin, in association with rifampicin +/- antipseudomonal
carbapenem, have been suggested to treat MDR/XDR strains due to their
possible synergistic effect.[78,79] Among soon available drugs,
ceftolozane-tazobactam, seems to be the most promising in the treatment
of such infections.[80] NFGNB only account for less than 3% of BSI in neutropenic patients. In this group, Stenotrophomonas maltophilia and Acinetobacter baumannii are the most represented bacteria (see Table 3).
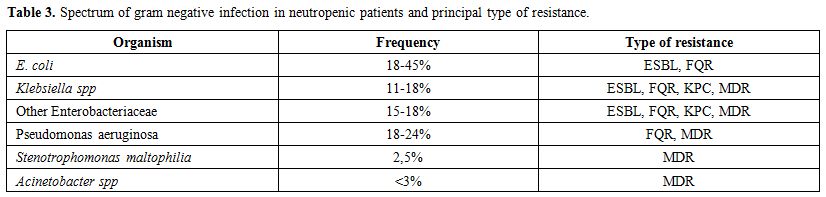 |
Table 3. Spectrum of gram negative infection in neutropenic patients and principal type of resistance. |
Ecthyma gangrenosum (EG) is a well-recognized cutaneous infection classically associated with S. maltophilia and P. aeruginosa bacteremia.
EG usually occurs in patients who are critically ill and
immunocompromised; it is almost always a sign of pseudomonal or
stenotrophomonal sepsis.[81,82] Intrinsic resistance profile of S. maltophilia
is a therapeutic hitch worthy of consideration:
trimethroprim-sulfametoxazole is the drug of choice, but also
levofloxacine and moxifloxacine were usually active.[83,84] Moreover
recently i.v. minocycline demonstrated an exellent in vitro
activity.[85] Acinetobacter spp BSI accounts for only 1% of neutropenic patients. Therapies versus A. baumannii are
based on carbapenem or aminoglycosides when the strains are susceptible
to these drugs and colistin, eventually in association with rifampicin
and ortigecyclin, ampicillin-sulbactam or carbapenem, when the stains
are XDR.[86-91] In
conclusion, the current epidemiology of BSI in onco-hematologic
patients is characterized by the emergence of MDR pathogens. This
observation has several implications both in the institution of
empirical and targeted treatment and in the need of containment
strategies. We proposed here some possible regimens for empirical and
focused treatment based on current evidence to help the clinician who
is going to treat febrile neutropenia in the MDR bugs era. We believe
that every effort has to be made for the containment of the spread of
these pathogens. For this purpose, shared antibiotic stewardship
strategies need to be implemented. Concepts like antibiotic
de-escalation, availability of the antibiograms, isolation of the
colonized patients, and careful limitation of carbapenem use are
cornerstones of resistance containment both in neutropenic and
non-neutropenic patients. Some
special considerations should be made on neutropenic patients. First of
all, FQ prophylaxis has been highlighted as one of the most important
causative factors for the emergence of ESBL enterobateriaceae and MRSA.
Therefore, its use need probably to be systematically re-evaluated at
least in selected epidemiological settings (i.e. in relation to FQ
resistance prevalence among E. coli
isolates). Secondly, around 70% of fevers in neutropenia are classified
as fever of unknown origin (FUO)[92] in which antibiotic therapy could
be unneeded. Expert opinion[93] and recent evidence[94] support
early discontinuation of antibiotic therapy in FUOs. Finally,
approaches to reduce the antibiotic exposure with the adoption of short
antibiotic treatments for specific infections should be evaluated. As
an example, a five days course of daptomycin for CoNS BSIs promptly
responding to CVC removal might prove efficacious.The
knowledge of the general and local epidemiology and resistance profiles
are of a paramount importance in the correct management of febrile
neutropenia. Frequent, up to dated, reports about trends in etiology
and emerging resistances need to be implemented. .
References
- Averbuch D, Orasch C, Cordonnier C, Livermore DM,
Mikulska M, Viscoli C, Gyssens IC, Kern WV, Klyasova G, Marchetti O,
Engelhard D, Akova M e ECIL4, a joint venture of EBMT, EORTC, ICHS,
ESGICH/ESCMID and ELN. European guidelines for empirical antibacterial
therapy for febrile neutropenic patients in the era of growing
resistance: summary of the 2011 4th european conference on infections
in leukemia. Haematologica. 2013, Vol. 98, 1826-35.
http://dx.doi.org/10.3324/haematol.2013.091025 PMid:24323983
PMCid:PMC3856957
 .
. - Nesher L, Rolston KV.
The current spectrum of infection in cancer patients with chemotherapy
related neutropenia. Infection. 2014; 42, 5-13.
http://dx.doi.org/10.1007/s15010-013-0525-9 PMid:23975584
 .
.
- Klastersky J, Ameye L, Maertens J,
Georgala A, Muanza F, Aoun M, Ferrant A, Rapoport B, Rolston K,
Paesmans M. Bacteraemia in febrile neutropenic cancer patients. Int J
Antimicrob Agents. 2007; 30 S, 51-9.
 .
. - Gaytán-Martínez
J1, Mateos-García E, Sánchez-Cortés E, González-Llaven J,
Casanova-Cardiel LJ, Fuentes-Allen JL. Microbiological findings in
febrile neutropenia. Arch Med Res. 2000; 31, 388-92.
PMid:11068081
 .
. - Gudiol
C1, Bodro M, Simonetti A, Tubau F, González-Barca E, Cisnal M,
Domingo-Domenech E, Jiménez L, Carratalà J. Changing aetiology,
clinical features, antimicrobial resistance, and outcomes of
bloodstream infection in neutropenic cancer patients. Clin Microbiol
Infect. 2012; 19, 474-9. PMid:22524597
 .
. - Cordonnier
C1, Buzyn A, Leverger G, Herbrecht R, Hunault M, Leclercq R,
Bastuji-Garin S e Onco-Hématologie., Club de Réflexion sur les
Infections en. Epidemiology and Risk Factors for Gram-Positive Coccal
Infections in Neutropenia: Toward a More Targeted Antibiotic Strategy.
Clin Infect dis. 2003; 36, 149-58. http://dx.doi.org/10.1086/345435
PMid:12522746
 .
. - Trecarichi
EM, Pagano L, Candoni A, Pastore D, Cattaneo C, Fanci R, Nosari A,
Caira M, Spadea A, Busca A, Vianelli N, Tumbarello M. Current
epidemiology and antimicrobial resistance data for bacterial
bloodstream infections in patients with hematologic malignancies: an
Italian multicentre prospective survey. Clin Microbiol Infect. 2014;
pii: S1198-743X(14)00108-6.
 .
. - Mikulska M,
Viscoli C, Orasch C, Livermore DM, Averbuch D, Cordonnier C, Akova M e
Fourth European Conference on Infections in Leukemia Group (ECIL-4), a
joint venture of EBMT, EORTC, ICHS, ELN and ESGICH/ESCMID. Aetiology
and resistance in bacteraemias among adult and paediatric haematology
and cancer patients. J Infect. 2013; 68, 321-31.
http://dx.doi.org/10.1016/j.jinf.2013.12.006
PMid:24370562
 .
. - Zinner
SH. Changing epidemiology of infections in patients with neutropenia
and cancer: emphasis on gram-positive and resistant bacteria. Clin
Infect Dis. 1999; 29, 490-4. http://dx.doi.org/10.1086/598620
PMid:10530434
 .
. - Mikulska
M, Del Bono V, Prinapori R, Boni L, Raiola AM, Gualandi F, Van Lint MT,
Dominietto A, Lamparelli T, Cappellano P, Bacigalupo A, Viscoli C. Risk
factors for enterococcal bacteremia in allogeneic hematopoietic stem
cell transplant recipients. Transpl Infect Dis. 2010; 6, 505-12.
http://dx.doi.org/10.1111/j.1399-3062.2010.00544.x
PMid:20636482
 .
. - Mandell
G L, Bennett J E, Dolin R.Mandell, Douglas, and Bennett's principles
and practice of infectious diseases. 7th ed. Philadelphia : Churcill
Livingstone Elsevier, 2010.
- Costa SF,
Barone AA, Miceli MH, van der Heijden IM, Soares RE, Levin AS, Anaissie
EJ. Colonization and molecular epidemiology of coagulase-negative
Staphylococcal bacteremia in cancer patients: a pilot study. Am J
Infect Control. 2006; 34, 36-40.
http://dx.doi.org/10.1016/j.ajic.2005.10.007
PMid:16443091
 .
. - Costa
SF, Miceli MH, Anaissie EJ. Mucosa or skin as source of
coagulase-negative staphylococcal bacteraemia? Lancet Infect Dis. 2004;
4, 278-86. http://dx.doi.org/10.1016/S1473-3099(04)01003-5

- Falcone
M, Micozzi A, Pompeo ME, Baiocchi P, Fabi F, Penni A, Martino P,
Venditti M. Methicillin-resistant staphylococcal bacteremia in patients
with hematologic malignancies: clinical and microbiological
retrospective comparative analysis of S. haemolyticus, S. epidermidis
and S. aureus. J Chemother. 2004; 16, 540-8.
http://dx.doi.org/10.1179/joc.2004.16.6.540
PMid:15700845
 .
. - Mendes RE, Hogan
PA, Streit JM, Jones RN, Flamm RK. Zyvox® Annual Appraisal of Potency
and Spectrum (ZAAPS) program: report of linezolid activity over 9 years
(2004-12). J Antimicrob Chemother. 2014; 69, 1582-8.
http://dx.doi.org/10.1093/jac/dkt541
PMid:24468866
 .
. - Stuart JI, John
MA, Milburn S, Diagre D, Wilson B, Hussain Z. Susceptibility patterns
of coagulase-negative staphylococci to several newer antimicrobial
agents in comparison with vancomycin and oxacillin. Int J Antimicrob
Agents. 2011; 37, 248-52.
http://dx.doi.org/10.1016/j.ijantimicag.2010.11.020
PMid:21295951
 .
. - Karavasilis V,
Zarkotou O, Panopoulou M, Kachrimanidou M, Themeli-Digalaki K,
Stylianakis A, Gennimata V, Ntokou E, Stathopoulos C, Tsakris A,
Pournaras S e Resistance., Greek Study Group on Staphylococcal
Linezolid. Wide dissemination of linezolid-resistant Staphylococcus
epidermidis in Greece is associated with a linezolid-dependent ST22
clone. J Antimicrob Chemother. 2015, Epub ahead of print.
- Russo
A, Campanile F, Falcone M, Tascini C, Bassetti M, Goldoni P,
Trancassini M, Della Siega P, Menichetti F, Stefani S, Venditti M.
Linezolid-resistant staphylococcal bacteraemia: A multicentre
case-case-control study in Italy. Int J Antimicrob Agents. 2015; 45,
255-61. http://dx.doi.org/10.1016/j.ijantimicag.2014.12.008
PMid:25600893
 .
. - Sader HS, Farrell
DJ, Flamm RK, Jones RN. Daptomycin activity tested against 164457
bacterial isolates from hospitalised patients: summary of 8 years of a
Worldwide Surveillance Programme (2005-2012). Int J Antimicrob Agents.
2014; 43, 465-9. http://dx.doi.org/10.1016/j.ijantimicag.2014.01.018
PMid:24636430
 .
. - Thwaites GE,
Edgeworth JD, Gkrania-Klotsas E, Kirby A, Tilley R, Török ME, Walker S,
Wertheim HF, Wilson P, Llewelyn MJ e Group., UK Clinical Infection
Research. Clinical management of Staphylococcus aureus bacteraemia.
Lancet Infect Dis. 2011; 11, 208-22.
http://dx.doi.org/10.1016/S1473-3099(10)70285-1
 .
. - Venditti
M, Falcone M, Micozzi A, Carfagna P, Taglietti F, Serra PF, Martino P.
Staphylococcus aureus bacteremia in patients with hematologic
malignancies: a retrospective case-control study. Haematologica. 2004;
88, 923-30.
 .
. - European Antimicrobial Resistance
Surveillance Network (EARS-Net). European Centre for Disease Prevention
and Control. Antimicrobial resistance surveillance in Europe 2013.
Annual Report of the European Antimicrobial Resistance Surveillance
Network (EARS-Net). Stockholm : ECDC, 2014.
- Tacconelli
E, De Angelis G, Cataldo MA, Pozzi E, Cauda R. Does antibiotic exposure
increase the risk of methicillin-resistant Staphylococcus aureus (MRSA)
isolation? A systematic review and meta-analysis. J Antimicrob
Chemother. 2008; 61, 26-38. http://dx.doi.org/10.1093/jac/dkm416
PMid:17986491
 .
. - Soriano A, Marco
F, Martínez JA, Pisos E, Almela M, Dimova VP, Alamo D, Ortega M, Lopez
J, Mensa J. Influence of vancomycin minimum inhibitory concentration on
the treatment of methicillin-resistant Staphylococcus aureus
bacteremia. Clin Infect Dis. 2008; 46, 193-200.
http://dx.doi.org/10.1086/524667 PMid:18171250
 .
. - Cervera
C, Casta-eda X, de la Maria CG, del Rio A, Moreno A, Soy D, Pericas JM,
Falces C, Armero Y, Almela M, Ninot S, Pare JC, Mestres CA, Gatell JM,
Marco F, Miro JM e Group., Hospital Clinic Endocarditis Study. Effect
of vancomycin minimal inhibitory concentration on the outcome of
methicillin-susceptible Staphylococcus aureus endocarditis. Clin Infect
Dis. 2014; 58, 1668-75. http://dx.doi.org/10.1093/cid/ciu183
PMid:24647021
 .
. - Sánchez García M,
De la Torre MA, Morales G, Peláez B, Tolón MJ, Domingo S, Candel FJ,
Andrade R, Arribi A, García N, Martínez Sagasti F, Fereres J, Picazo J.
Clinical outbreak of linezolid-resistant Staphylococcus aureus in an
intensive care unit. JAMA. 2010; 303, 2260-4.
http://dx.doi.org/10.1001/jama.2010.757
PMid:20530779
 .
. - Endimiani A,
Blackford M, Dasenbrook EC, Reed MD, Bajaksouszian S, Hujer AM, Rudin
SD, Hujer KM, Perreten V, Rice LB, Jacobs MR, Konstan MW, Bonomo RA.
Emergence of linezolid-resistant Staphylococcus aureus after prolonged
treatment of cystic fibrosis patients in Cleveland, Ohio. Antimicrob
Agents Chemother. 2011; 55, 1684-92.
http://dx.doi.org/10.1128/AAC.01308-10 PMid:21263048 PMCid:PMC3067150
 .
. - Paul
M, Dickstein Y, Borok S, Vidal L, Leibovici L. Empirical antibiotics
targeting Gram-positive bacteria for the treatment of febrile
neutropenic patients with cancer. Cochrane Database Syst Rev. 2014 Jan
14;1:CD003914. http://dx.doi.org/10.1002/14651858.cd003914.pub3
 .
. - Vardakas
KZ, Samonis G, Chrysanthopoulou SA, Bliziotis IA, Falagas ME. Role of
glycopeptides as part of initial empirical treatment of febrile
neutropenic patients: a meta-analysis of randomised controlled trials.
Lancet Infect Dis. 2005; 5, 431-9.
http://dx.doi.org/10.1016/S1473-3099(05)70164-X
 .
. - Mermel
LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders
BJ, Sherertz RJ, Warren DK. Clinical practice guidelines for the
diagnosis and management of intravascular catheter-related infection:
2009 Update by the Infectious Diseases Society of America. Clin Infect
Dis. 2009; 49, 1-45. http://dx.doi.org/10.1086/599376 PMid:19489710
PMCid:PMC4039170
 .
. - Venditti M, Tarasi A, Visco Comandini
U, Gentile G, Girmenia C, Micozzi A, Martino P. Enterococcal septicemia
in patients with hematological malignancies. Eur J Clin Microbiol
Infect Dis. 1993; 12, 241-7. http://dx.doi.org/10.1007/BF01967253
PMid:8513811
 .
. - Todeschini G,
Tecchio C, Borghero C, D'Emilio A, Pegoraro E, de Lalla F, Benedetti P,
Spolaore P, Pellizzer G. Association between Enterococcus bacteraemia
and death in neutropenic patients with haematological malignancies. J
Infect. 2006; 53, 266-73. http://dx.doi.org/10.1016/j.jinf.2005.11.012
PMid:16388852
 .
. - Vergis EN, Hayden
MK, Chow JW, Snydman DR, Zervos MJ, Linden PK, Wagener MM, Schmitt B,
Muder RR. Determinants of vancomycin resistance and mortality rates in
enterococcal bacteremia. a prospective multicenter study. Ann Intern
Med. 2001; 135, 484-92.
http://dx.doi.org/10.7326/0003-4819-135-7-200110020-00007
PMid:11578151
 .
. - Vydra J, Shanley
RM, George I, Ustun C, Smith AR, Weisdorf DJ, Young JA. Enterococcal
bacteremia is associated with increased risk of mortality in recipients
of allogeneic hematopoietic stem cell transplantation. Clin Infect Dis.
2012; 55, 764-70. http://dx.doi.org/10.1093/cid/cis550 PMid:22693346
PMCid:PMC3657510
 .
. - DiazGranados CA, Zimmer SM, Klein M,
Jernigan JA. Comparison of mortality associated with
vancomycin-resistant and vancomycin-susceptible enterococcal
bloodstream infections: a meta-analysis. Clin Infect Dis. 2005; 41,
327-33. http://dx.doi.org/10.1086/430909
PMid:16007529
 .
. - Lisboa LF,
Miranda BG, Vieira MB, Dulley FL, Fonseca GG, Guimarães T, Levin AS,
Shikanai-Yasuda MA, Costa SF. Empiric use of linezolid in febrile
hematology and hematopoietic stem cell transplantation patients
colonized with vancomycin-resistant Enterococcus spp. Int J Infect Dis.
2015; 33, 171-76. http://dx.doi.org/10.1016/j.ijid.2015.02.001
PMid:25660090
 .
. - Balli EP, Venetis
CA, Miyakis S. Systematic review and meta-analysis of linezolid versus
daptomycin for treatment of vancomycin-resistant enterococcal
bacteremia. Antimicrob Agents Chemother. 2014; 58, 734-9.
http://dx.doi.org/10.1128/AAC.01289-13 PMid:24247127 PMCid:PMC3910884
 .
. - Chuang
YC, Wang JT, Lin HY, Chang SC. Daptomycin versus linezolid for
treatment of vancomycin-resistant enterococcal bacteremia: systematic
review and meta-analysis. BMC Infect Dis. 2014; 14, 687.
http://dx.doi.org/10.1186/s12879-014-0687-9 PMid:25495779
PMCid:PMC4269951
 .
. - McKinnell JA, Patel M, Shirley RM,
Kunz DF, Moser SA, Baddley JW. Observational study of the epidemiology
and outcomes of vancomycin-resistant Enterococcus bacteraemia treated
with newer antimicrobial agents. Epidemiol Infect. 2011; 139, 1342-50.
http://dx.doi.org/10.1017/S0950268810002475 PMid:21073764
PMCid:PMC3879115
 .
. - Raber-Durlacher JE, Laheij AM, Epstein
JB, Epstein M, Geerligs GM, Wolffe GN, Blijlevens NM, Donnelly JP.
Periodontal status and bacteremia with oral viridans streptococci and
coagulase negative staphylococci in allogeneic hematopoietic stem cell
transplantation recipients: a prospective observational study. Support
Care Cancer. 2013; 21, 1621-7.
http://dx.doi.org/10.1007/s00520-012-1706-2
PMid:23288398
 .
. - Marron A,
Carratalà J, González-Barca E, Fernández-Sevilla A, Alcaide F, Gudiol
F. Serious complications of bacteremia caused by Viridans streptococci
in neutropenic patients with cancer. Clin Infect Dis. 2000; 31,
1126-30. http://dx.doi.org/10.1086/317460
PMid:11073739
 .
. - Venditti M,
Baiocchi P, Santini C, Brandimarte C, Serra P, Gentile G, Girmenia C,
Martino P. Antimicrobial susceptibilities of Streptococcus species that
cause septicemia in neutropenic patients. Antimicrob Agents Chemother.
1989; 33, 580-2. http://dx.doi.org/10.1128/AAC.33.4.580 PMid:2729950
PMCid:PMC172484
 .
. - Carratalá J, Alcaide F,
Fernández-Sevilla A, Corbella X, Linares J, Gudiol F. Bacteremia due to
viridans streptococci that are highly resistant to penicillin: increase
among neutropenic patients with cancer. Clin Infect Dis. 1995; 20,
1169-73. http://dx.doi.org/10.1093/clinids/20.5.1169
PMid:7619995
 .
. - Rolston KV,
Kapadia M, Tarrand J, Coyle E, Prince RA. Spectrum of gram-positive
bacteraemia and in vitro activities of daptomycin, linezolid and
vancomycin against organisms isolated from cancer patients. Int J
Antimicrob Agents. 2013; 41, 516-21.
http://dx.doi.org/10.1016/j.ijantimicag.2013.01.014
PMid:23481658
 .
. - Rozdzinski E,
Kern W, Schmeiser T, Kurrle E. Corynebacterium jeikeium bacteremia at a
tertiary care center. Infection. 1991; 19, 201-4.
http://dx.doi.org/10.1007/BF01644945
PMid:1917029
 .
. - Tumbarello M,
Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B, Citton
R, D'Inzeo T, Fadda G, Cauda R, Spanu T. Predictors of mortality in
patients with bloodstream infections caused by
extended-spectrum-beta-lactamase-producing Enterobacteriaceae:
importance of inadequate initial antimicrobial treatment. Antimicrob
Agents Chemother. 2007; 51, 1987-94.
http://dx.doi.org/10.1128/AAC.01509-06 PMid:17387156 PMCid:PMC1891412
 .
. - Tumbarello
M, Spanu T, Caira M, Trecarichi EM, Laurenti L, Montuori E, Fianchi L,
Leone F, Fadda G, Cauda R, Pagano L. Factors associated with mortality
in bacteremic patients with hematologic malignancies. Diagn Microbiol
Infect Dis. 2009; 64, 320-6.
http://dx.doi.org/10.1016/j.diagmicrobio.2009.02.008
PMid:19345033
 .
. - Magiorakos AP,
Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S,
Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB,
Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL.
Multidrug-resistant, extensively drug-resistant and pandrug-resistant
bacteria: an international expert proposal for interim standard
definitions for acquired resistance. Clin Microbiol Infect. 2011; 18,
268-81. http://dx.doi.org/10.1111/j.1469-0691.2011.03570.x
PMid:21793988
 .
. - Cattaneo C,
Quaresmini G, Casari S, Capucci MA, Micheletti M, Borlenghi E,
Signorini L, Re A, Carosi G, Rossi G. Recent changes in bacterial
epidemiology and the emergence of fluoroquinolone-resistant Escherichia
coli among patients with haematological malignancies: results of a
prospective study on 823 patients at a single institution. J Antimicrob
Chemother. 2008; 61, 721-8. http://dx.doi.org/10.1093/jac/dkm514
PMid:18218645
 .
. - Trecarichi EM,
Tumbarello M, Spanu T, Caira M, Fianchi L, Chiusolo P, Fadda G, Leone
G, Cauda R, Pagano L. Incidence and clinical impact of
extended-spectrum-beta-lactamase (ESBL) production and fluoroquinolone
resistance in bloodstream infections caused by Escherichia coli in
patients with hematological malignancies. J Infect. 2009; 58, 299-307.
http://dx.doi.org/10.1016/j.jinf.2009.02.002
PMid:19272650
 .
. - Kim SH, Kwon JC,
Choi SM, Lee DG, Park SH, Choi JH, Yoo JH, Cho BS, Eom KS, Kim YJ, Kim
HJ, Lee S, Min CK, Cho SG, Kim DW, Lee JW, Min WS. Escherichia coli and
Klebsiella pneumoniae bacteremia in patients with neutropenic fever:
factors associated with extended-spectrum ß-lactamase production and
its impact on outcome. Ann Hematol. . 2013; 92, 533-41.
http://dx.doi.org/10.1007/s00277-012-1631-y
PMid:23161391
 .
. - EJ, Bow.
Fluoroquinolones, antimicrobial resistance and neutropenic cancer
patients. Curr Opin Infect Dis. 2011; 24, 545-53.
http://dx.doi.org/10.1097/QCO.0b013e32834cf054
PMid:22001945
 .
. - Lingaratnam S,
Thursky KA, Slavin MA. Fluoroquinolone prophylaxis: a word of caution.
Leuk Lymphoma. 2011; 52, 5-6.
http://dx.doi.org/10.3109/10428194.2010.527408
PMid:21067448
 .
. - Kang CI, Chung
DR, Ko KS, Peck KR, Song JH e Diseases., Korean Network for Study of
Infectious. Risk factors for infection and treatment outcome of
extended-spectrum ß-lactamase-producing Escherichia coli and Klebsiella
pneumoniae bacteremia in patients with hematologic malignancy. Ann
Hematol. 2012; 91, 115-21. http://dx.doi.org/10.1007/s00277-011-1247-7
PMid:21556875
 .
. - Pitout JD.
Infections with extended-spectrum beta-lactamase-producing
enterobacteriaceae: changing epidemiology and drug treatment choices.
Drugs. 2010; 70, 313-33.
http://dx.doi.org/10.2165/11533040-000000000-00000
PMid:20166768
 .
. - Pakyz AL, Oinonen
M, Polk RE. Relationship of carbapenem restriction in 22 university
teaching hospitals to carbapenem use and carbapenem-resistant
Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009; 53(5):
1983-6. http://dx.doi.org/10.1128/AAC.01535-08 PMid:19273670
PMCid:PMC2681502
 .
. - Rodríguez-Ba-o J, Navarro MD, Retamar
P, Picón E, Pascual Á e Group, Extended-Spectrum Beta-Lactamases–Red
Espa-ola de Investigación en Patología Infecciosa/Grupo de Estudio de
Infección Hospitalaria.ß-Lactam/ß-lactam inhibitor combinations for the
treatment of bacteremia due to extended-spectrum ß-lactamase-producing
Escherichia coli: a post hoc analysis of prospective cohorts. Clin
Infect Dis. 2012; 54, 167-74. http://dx.doi.org/10.1093/cid/cir790
PMid:22057701
 .
. - Bucaneve G,
Micozzi A, Picardi M, Ballanti S, Cascavilla N, Salutari P, Specchia G,
Fanci R, Luppi M, Cudillo L, Cantaffa R, Milone G, Bocchia M,
Martinelli G, Offidani M, Chierichini A, Fabbiano F, Quarta G, Primon
V, Martino B, Manna A, Zuffa E, Ferrar.Results of a multicenter,
controlled, randomized clinical trial evaluating the combination of
piperacillin/tazobactam and tigecycline in high-risk hematologic
patients with cancer with febrile neutropenia. J Clin Oncol. 2014; 32,
1463-71. http://dx.doi.org/10.1200/JCO.2013.51.6963
PMid:24733807
 .
. - Girometti N,
Lewis RE, Giannella M, Ambretti S, Bartoletti M, Tedeschi S, Tumietto
F, Cristini F, Trapani F, Gaibani P, Viale P. Klebsiella pneumoniae
bloodstream infection: epidemiology and impact of inappropriate
empirical therapy. Medicine (Baltimore). 2014; 93, 298-309.
http://dx.doi.org/10.1097/MD.0000000000000111
PMid:25398065
 .
. - K, Bush. Alarming
ß-lactamase-mediated resistance in multidrug-resistant
Enterobacteriaceae. Curr Opin Microbiol. 2010; 13, 558-64.
http://dx.doi.org/10.1016/j.mib.2010.09.006
PMid:20920882
 .
. - García-Fernández
A, Villa L, Carta C, Venditti C, Giordano A, Venditti M, Mancini C,
Carattoli A. Klebsiella pneumoniae ST258 producing KPC-3 identified in
italy carries novel plasmids and OmpK36/OmpK35 porin variants.
Antimicrob Agents Chemother. 2012; 56, 2143-5.
http://dx.doi.org/10.1128/AAC.05308-11 PMid:22252815 PMCid:PMC3318348
 .
. - Tsakris
A, Kristo I, Poulou A, Markou F, Ikonomidis A, Pournaras S. First
occurrence of KPC-2-possessing Klebsiella pneumoniae in a Greek
hospital and recommendation for detection with boronic acid disc tests.
J Antimicrob Chemother. 2008; 62(6) :1257-60.
http://dx.doi.org/10.1093/jac/dkn364
PMid:18772158
 .
. - European Centre
for Disease Prevention and Control. Antimicrobial resistance
surveillance in Europe 2013. Annual Report of the European
Antimicrobial Resistance Surveillance Network (EARS-Net). ECDC,
Stockholm 2014
- Tumbarello M, Viale P, Viscoli C,
Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio
F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. Predictors
of mortality in bloodstream infections caused by Klebsiella pneumoniae
carbapenemase-producing K. pneumoniae: importance of combination
therapy. Clin Infect Dis. 2012; 55, 943-50.
http://dx.doi.org/10.1093/cid/cis588
PMid:22752516
 .
. - Girmenia C,
Rossolini GM, Piciocchi A, Bertaina A, Pisapia G, Pastore D, Sica S,
Severino A, Cudillo L, Ciceri F, Scimè R, Lombardini L, Viscoli C,
Rambaldi A, (GITMO), Gruppo Italiano Trapianto Midollo Osseo e GITMO.,
Gruppo Italiano Trapianto Midollo Osseo.Infections by
carbapenem-resistant Klebsiella pneumoniae in SCT recipients: a
nationwide retrospective survey from Italy. Bone Marrow Transplant.
2015; 50, 282-8. http://dx.doi.org/10.1038/bmt.2014.231
PMid:25310302
 .
. - Giannella M,
Trecarichi EM, De Rosa FG, Del Bono V, Bassetti M, Lewis RE, Losito AR,
Corcione S, Saffioti C, Bartoletti M, Maiuro G, Cardellino CS, Tedeschi
S, Cauda R, Viscoli C, Viale P, Tumbarello M. Risk factors for
carbapenem-resistant Klebsiella pneumoniae bloodstream infection among
rectal carriers: a prospective observational multicentre study. Clin
Microbiol Infect. 2014; 20, 1357-62.
http://dx.doi.org/10.1111/1469-0691.12747
PMid:24980276
 .
. - Daikos GL,
Tsaousi S, Tzouvelekis LS, Anyfantis I, Psichogiou M, Argyropoulou A,
Stefanou I, Sypsa V, Miriagou V, Nepka M, Georgiadou S, Markogiannakis
A, Goukos D, Skoutelis A. Carbapenemase-producing Klebsiella pneumoniae
bloodstream infections: lowering mortality by antibiotic combination
schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;
58, 2322-8. http://dx.doi.org/10.1128/AAC.02166-13 PMid:24514083
PMCid:PMC4023796
 .
. - Qureshi ZA, Paterson DL, Potoski BA,
Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y.
Treatment outcome of bacteremia due to KPC-producing Klebsiella
pneumoniae: superiority of combination antimicrobial regimens.
Antimicrob Agents Chemother. 2012; 56, 2108-13.
http://dx.doi.org/10.1128/AAC.06268-11 PMid:22252816
PMCid:PMC3318350
 .
. - Daikos GL, Markogiannakis A.
Carbapenemase-producing Klebsiella pneumoniae: (when) might we still
consider treating with carbapenems? Clin Microbiol Infect. 2011; 17,
1135-41. http://dx.doi.org/10.1111/j.1469-0691.2011.03553.x
PMid:21635663
 .
. - Daikos GL,
Markogiannakis A, Souli M, Tzouvelekis LS. Bloodstream infections
caused by carbapenemase-producing Klebsiella pneumoniae: a clinical
perspective. Expert Rev Anti Infect Ther. 2012;10(12): 1393-404.
http://dx.doi.org/10.1586/eri.12.138
PMid:23253318
 .
. - Tascini C,
Tagliaferri E, Giani T, Leonildi A, Flammini S, Casini B, Lewis R,
Ferranti S, Rossolini GM, Menichetti F. Synergistic activity of
colistin plus rifampin against colistin-resistant KPC-producing
Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013; 57, 3990-3.
http://dx.doi.org/10.1128/AAC.00179-13 PMid:23752510 PMCid:PMC3719736
 .
. - Jernigan
MG, Press EG, Nguyen MH, Clancy CJ, Shields RK. The combination of
doripenem and colistin is bactericidal and synergistic against
colistin-resistant, carbapenemase-producing Klebsiella pneumoniae.
Antimicrob Agents Chemother. 2012; 56, 3395-8.
http://dx.doi.org/10.1128/AAC.06364-11 PMid:22430958 PMCid:PMC3370798
 .
. - Ceccarelli
G, Falcone M, Giordano A, Mezzatesta ML, Caio C, Stefani S, Venditti M.
Successful ertapenem-doripenem combination treatment of bacteremic
ventilator-associated pneumonia due to colistin-resistant KPC-producing
Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013; 57, 2900-1.
http://dx.doi.org/10.1128/AAC.00188-13 PMid:23571536 PMCid:PMC3716145
 .
. - Oliva
A, D'Abramo A, D'Agostino C, Iannetta M, Mascellino MT, Gallinelli C,
Mastroianni CM, Vullo V. Synergistic activity and effectiveness of a
double-carbapenem regimen in pandrug-resistant Klebsiella pneumoniae
bloodstream infections. J Antimicrob Chemother. 2014; 69, epub feb 11.
 .
. - Bulik
CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing
Klebsiella pneumoniae. Antimicrob Agents Chemother. 2011; 55, 3002-4.
http://dx.doi.org/10.1128/AAC.01420-10 PMid:21422205 PMCid:PMC3101469
 .
. - Trecarichi
EM, Tumbarello M, Caira M, Candoni A, Cattaneo C, Pastore D, Fanci R,
Nosari A, Vianelli N, Busca A, Spadea A, Pagano L. Multidrug resistant
Pseudomonas aeruginosa bloodstream infection in adult patients with
hematologic malignancies. Haematologica. 2011; 96, 1-3.
http://dx.doi.org/10.3324/haematol.2010.036640 PMid:21193424
PMCid:PMC3012771
 .
. - Pe-a C, Gómez-Zorrilla S, Suarez C,
Dominguez MA, Tubau F, Arch O, Oliver A, Pujol M, Ariza J. Extensively
drug-resistant Pseudomonas aeruginosa: risk of bloodstream infection in
hospitalized patients. Eur J Clin Microbiol Infect Dis. 2012; 31,
2791-7. http://dx.doi.org/10.1007/s10096-012-1629-3
PMid:22552893
 .
. - Tascini C,
Gemignani G, Ferranti S, Tagliaferri E, Leonildi A, Lucarini A,
Menichetti F. Microbiological activity and clinical efficacy of a
colistin and rifampin combination in multidrug-resistant Pseudomonas
aeruginosa infections. J Chemother. 2004; 16(3): 282-7.
http://dx.doi.org/10.1179/joc.2004.16.3.282
PMid:15330326
 .
. - Landman D, Bratu
S, Alam M, Quale J. Citywide emergence of Pseudomonas aeruginosa
strains with reduced susceptibility to polymyxin B. J Antimicrob
Chemother. 2005; 55(6): 954-7. http://dx.doi.org/10.1093/jac/dki153
PMid:15883174
 .
. - Sader HS, Farrell
DJ, Flamm RK, Jones RN. Ceftolozane/tazobactam activity tested against
aerobic Gram-negative organisms isolated from intra-abdominal and
urinary tract infections in European and United States hospitals
(2012). J Infect. 2014; 69(3): 266-77.
http://dx.doi.org/10.1016/j.jinf.2014.04.004
PMid:24780763
 .
. - Demiraslan H,
Sevim M, Pala Ç, Durmaz S, Berk V, Kaynar L, Metan G. Risk factors
influencing mortality related to Stenotrophomonas maltophilia infection
in hematology-oncology patients. Int J Hematol. 2013; 97, 414-20.
http://dx.doi.org/10.1007/s12185-013-1296-x
PMid:23430671
 .
. - Micozzi A,
Venditti M, Monaco M, Friedrich A, Taglietti F, Santilli S, Martino P.
Bacteremia due to Stenotrophomonas maltophilia in patients with
hematologic malignancies. Clin Infect Dis. 2000; 31, 705-11.
http://dx.doi.org/10.1086/314043 PMid:11017819
 .
. - Bonfiglio
G, Cascone C, Azzarelli C, Cafiso V, Marchetti F, Stefani S.
Levofloxacin in vitro activity and time-kill evaluation of
Stenotrophomonas maltophilia clinical isolates. J Antimicrob Chemother.
2000; 45, 115-7. http://dx.doi.org/10.1093/jac/45.1.115
PMid:10629022
 .
. - Venditti M,
Monaco M, Micozzi A, Tarasi A, Friedrich A, Martino P. In vitro
activity of moxifloxacin against Stenotrophomonas maltophilia blood
isolates from patients with hematologic malignancies. Clin Microbiol
Infect. . 2001; 7, 37-9.
http://dx.doi.org/10.1046/j.1469-0691.2001.00191.x
PMid:11284945
 .
. - Rizek C, Ferraz
JR, van der Heijden IM, Giudice M, Mostachio AK, Paez J, Carrilho C,
Levin AS, Costa SF. In vitro activity of potential old and new drugs
against multidrug-resistant gram-negatives. J Infect Chemother. 2015;
21(2): 114-7. http://dx.doi.org/10.1016/j.jiac.2014.10.009
PMid:25456893
 .
. - Peleg AY, Seifert
H, Paterson DL. Acinetobacter baumannii: emergence of a successful
pathogen. Clin Microbiol Rev. 2008; 21, 538-82.
http://dx.doi.org/10.1128/CMR.00058-07 PMid:18625687
PMCid:PMC2493088
 .
. - Durante-Mangoni E, Del Franco M,
Andini R, Bernardo M, Giannouli M, Zarrilli R. Emergence of colistin
resistance without loss of fitness and virulence after prolonged
colistin administration in a patient with extensively drug-resistant
Acinetobacter baumannii. Diagn Microbiol Infect Dis. 2015, Vol. Epub
ahead of print.
 .
. - Tascini C, Menichetti F, Bozza S, Del
Favero A, Bistoni F. Evaluation of the activities of two-drug
combinations of rifampicin, polymyxin B and ampicillin/sulbactam
against Acinetobacter baumannii. J Antimicrob Chemother. 1998; 42,
270-1. http://dx.doi.org/10.1093/jac/42.2.270
PMid:9738852
 .
. - Durante-Mangoni E,
Signoriello G, Andini R, Mattei A, De Cristoforo M, Murino P, Bassetti
M, Malacarne P, Petrosillo N, Galdieri N, Mocavero P, Corcione A,
Viscoli C, Zarrilli R, Gallo C, Utili R. Colistin and rifampicin
compared with colistin alone for the treatment of serious infections
due to extensively drug-resistant Acinetobacter baumannii: a
multicenter, randomized clinical trial. Clin Infect Dis. 2013, Vol. 57,
349-58. http://dx.doi.org/10.1093/cid/cit253
PMid:23616495
 .
. - Metan G, Alp E,
Yildiz O, Percin D, Aygen B, Sumerkan B. Clinical experience with
tigecycline in the treatment of carbapenem-resistant Acinetobacter
infections. J Chemother. 2010; 22(2): 110-4.
http://dx.doi.org/10.1179/joc.2010.22.2.110
PMid:20435570
 .
. - Metan G, Pala Ç,
Kaynar L, Cevahir F, Alp E. A nightmare for haematology clinics:
extensively drug-resistant (XDR) Acinetobacter baumannnii. Infez Med.
2014; 22, 277-82. PMid:25551842
 .
. - Nesher
L, Rolston KV. The current spectrum of infection in cancer patients
with chemotherapy related neutropenia. Infection. 2014; 42(1): 5-13.
http://dx.doi.org/10.1007/s15010-013-0525-9
PMid:23975584
 .
. - Orasch C,
Averbuch D, Mikulska M, Cordonnier C, Livermore DM, Gyssens IC,
Klyasova G, Engelhard D, Kern W, Viscoli C, Akova M, Marchetti O; 4th
European Conference on Infections in Leukemia (ECIL-4); joint venture
of Infectious Diseases Working Party of the European Group for Blood
and Marrow Transplantation (IDWP-EBMT); Infectious Diseases Group of
the European Organization for Research and Treatment of Cancer
(IDG-EORTC); International Immunocompromised Host Society (ICHS);
European Leukemia Net (ELN) and European Study Group on Infections in
Immunocompromised Hosts of the European Society for Clinical
Microbiology and Infectious Diseases (ESGICH-ESCMID). Discontinuation
of empirical antibiotic therapy in neutropenic leukaemia patients with
fever of unknown origin is ethical. Clin Microbiol Infect. 2015; 21(3):
e25-7.
 .
. - Korucu B, Inkaya AC, Erbil AA, Okay M, Ascioglu
S, Akova M. Early cessation of empirical antibacterial therapy in
high-risk febrile neutropenic patients with FUO. 25th European Congress
of Clinical Microbiology and Infectious Diseases (ECCMID), 25-28 April
2015, Copenhagen, Denmark. P1206.
- Retamar P,
López-Cerero L, Muniain MA, Pascual Á, Rodríguez-Ba-o J e Group.,
ESBL-REIPI/GEIH. Impact of the MIC of piperacillin-tazobactam on the
outcome of patients with bacteremia due to
extended-spectrum-ß-lactamase-producing Escherichia coli. Antimicrob
Agents Chemother. 2013; 57, 3402-4.
http://dx.doi.org/10.1128/AAC.00135-13 PMid:23612190 PMCid:PMC3697383
 .
. - Pagano
L, Caira M, Trecarichi EM, Spanu T, Di Blasi R, Sica S, Sanguinetti M,
Tumbarello M. Carbapenemase-producing Klebsiella pneumoniae and
hematologic malignancies. Emerg Infect Dis. 2014; 20, 1235-6.
http://dx.doi.org/10.3201/eid2007.130094
PMid:24960464 PMCid:PMC4073839
 .
. - Freifeld AG, Bow EJ,
Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young
JA, Wingard JR, Infectious Diseases Society of America. Clinical
practice guideline for the use of antimicrobial agents in neutropenic
patients with cancer: 2010 update by the Infectious Diseases Society of
America. Clin Infect Dis. 2011; 15, e56-93.
http://dx.doi.org/10.1093/cid/cir073 PMid:21258094
 .
. - Viale P, Tumietto
F, Giannella M, Bartoletti M, Tedeschi S, Ambretti S, Cristini F,
Gibertoni C, Venturi S, Cavalli M, De Palma A, Puggioli MC, Mosci D,
Callea E, Masina R, Moro ML, Lewis RE. Impact of a hospital-wide
multifaceted programme for reducing carbapenem-resistant
Enterobacteriaceae infections in a large teaching hospital in northern
Italy. Clin Microbiol Infect. 2015, Vol. 21, 242-7.
http://dx.doi.org/10.1016/j.cmi.2014.10.020
PMid:25658534
 .
. - Cattaneo C,
Antoniazzi F, Casari S, Ravizzola G, Gelmi M, Pagani C, D'Adda M,
Morello E, Re A, Borlenghi E, Manca N, Rossi G. P. aeruginosa
bloodstream infections among hematological patients: an old or new
question? Ann Hematol. 2012; 91, 1299-304.
http://dx.doi.org/10.1007/s00277-012-1424-3
PMid:22349723
 .
. - Lodise TP Jr,
Patel N, Kwa A, Graves J, Furuno JP, Graffunder E, Lomaestro B,
McGregor JC. Predictors of 30-day mortality among patients with
Pseudomonas aeruginosa bloodstream infections: impact of delayed
appropriate antibiotic selection. Antimicrob Agents Chemother. 2007;
51, 3510-5. http://dx.doi.org/10.1128/AAC.00338-07 PMid:17646415
PMCid:PMC2043259
 .
. - Deris ZZ, Yu HH, Davis K, Soon RL,
Jacob J, Ku CK, Poudyal A, Bergen PJ, Tsuji BT, Bulitta JB, Forrest A,
Paterson DL, Velkov T, Li J, Nation RL. The combination of colistin and
doripenem is synergistic against Klebsiella pneumoniae at multiple
inocula and suppresses colistin resistance in an in vitro
pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother.
2012; 56, 5103-12.
 . http://dx.doi.org/10.1128/AAC.01064-12 PMid:22802247
PMCid:PMC3457376
. http://dx.doi.org/10.1128/AAC.01064-12 PMid:22802247
PMCid:PMC3457376  .
. - Sbrana F, Malacarne P, Viaggi B,
Costanzo S, Leonetti P, Leonildi A, Casini B, Tascini C, Menichetti F.
Carbapenem-sparing antibiotic regimens for infections caused by
Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in
intensive care unit. Clin Infect Dis. 2013; 56, 697-700.
http://dx.doi.org/10.1093/cid/cis969
PMid:23155147
 .
. - De Pascale G,
Montini L, Pennisi M, Bernini V, Maviglia R, Bello G, Spanu T,
Tumbarello M, Antonelli M. High dose tigecycline in critically ill
patients with severe infections due to multidrug-resistant bacteria.
Crit Care. 2014, 5, 10.1186/cc13858.
http://dx.doi.org/10.1186/cc13858
 .
. - Fehér C, Rovira
M, Soriano A, Esteve J, Martínez JA, Marco F, Carreras E, Martínez C,
Fernández-Avilés F, Suárez-Lledó M, Mensa J. Effect of meropenem
administration in extended infusion on the clinical outcome of febrile
neutropenia: a retrospective observational study. J Antimicrob
Chemother. 2014; 69, 2556-62. http://dx.doi.org/10.1093/jac/dku150
PMid:24855125
 . . .
. . . - Garonzik SM, Li
J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest
A, Nation RL. Population pharmacokinetics of colistin methanesulfonate
and formed colistin in critically ill patients from a multicenter study
provide dosing suggestions for various categories of patients.
Antimicrob Agents Chemother. 2011; 55, 3284-94.
http://dx.doi.org/10.1128/AAC.01733-10 PMid:21555763 PMCid:PMC3122440
 .
.
[TOP]








 .
.  .
.
 .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
. 
 .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  . http://dx.doi.org/10.1128/AAC.01064-12 PMid:22802247
PMCid:PMC3457376
. http://dx.doi.org/10.1128/AAC.01064-12 PMid:22802247
PMCid:PMC3457376  .
.  .
.  .
.  . . .
. . .  .
.