Received: January 27, 2015
Accepted: July 17, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015048, DOI 10.4084/MJHID.2015.048
This article is available on PDF format at:

Jean El-Cheikh1,2, Roberto Crocchiolo4, Andrea Vai4, Sabine Furst1,2, Stefania Bramanti4, Barbara Sarina4, Angela Granata1,2, Catherine Faucher1,2, Bilal Mohty1,2, Samia Harbi1,2, Reda Bouabdallah2, Norbert Vey2, Armando Santoro4, Christian Chabannon3, Luca Castagna1,4 and Didier Blaise1,2
1 Unité de Transplantation et de Thérapie Cellulaire (U2T), Institut Paoli-Calmettes, Marseille, France.
2 Département d’Onco-Hématologie, Institut Paoli-Calmettes, Marseille, France.
3 Centre de Thérapie Cellulaire, Institut Paoli-Calmettes, Marseille, France.
4 Humanitas cancer Centre, Milan, Rozzano, Italy

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Over the past decade, invasive fungal
infections (IFIs) have remained an important problem in patients
undergoing allogeneic haematopoietic stem cell transplantation (Allo-
HSCT). The optimal approach for prophylactic antifungal therapy has yet
to be determined. We conducted a retrospective analysis, comparing the safety and efficacy of micafungin 50mg/day vs. fluconazole 400mg/day vs. itraconazole 200mg/day as prophylaxis for adult patients with various haematological diseases receiving haploidentical hematopoietic stem cell transplantation (haplo-HSCT) followed by high-dose cyclophosphamide (PT-Cy). Overall, 99 patients were identified: 30 patients received micafungin, 50 and 19 patients received itraconazole and fluconazole, respectively. After a median follow-up of 12 months (range: 1-51), proven or probable IFIs were reported in 3 patients (10%) in the micafungin, 5 patients in the itraconazole (10%) and 2 patients (11%) in the fluconazole group (p=0.998). Fewer patients in the micafungin group had invasive aspergillosis (1 [3%] vs. 3 [6%] in the itraconazole vs. 2 [11%] in the fluconazole group, p=0.589). Four patients (13%) in the micafungin group vs 13 (26%) patients in the itraconazole group and 10 (53%) patients in the fluconazole received empirical antifungal therapy (P = 0.19). No serious adverse events related to treatment were reported by patients, and there was no treatment discontinuation because of drug-related adverse events in both groups. The present analysis shows that micafungin did better than fluconazole in preventing invasive aspergillosis after transplant in these high-risk hematological diseases, as expected. In addition, micafungin was more effective than itraconazole in preventing all IFI episodes when also considering possible fungal infections. Future prospective studies would shed light on this issue, concerning this increasingly used transplant platform. |
Introduction
Invasive fungal infections (IFIs) such as Candida and Aspergillus
species have caused significant morbidity and mortality among
immunocompromised patients in the recent years. Infection is a primary
cause of death in allogeneic hematopoietic stem cell transplant
(allo-HSCT) recipients, with a fatality rate ranging from 50 to 80%
after allo-HSCT.[1-3] Recently, Girmenia et al., in a prospective study, reported a lower attributable mortality rate (AMR),[4]
possibly due to the improvement of diagnostic and
therapeutic tools against infectious complications over the
last years. The risk of developing IFIs is mainly related to the
degree of immunosuppression, duration of neutropenia, the disruption of
protective skin and mucosal surface barriers, graft vs. host disease
(GVHD) and the use of corticosteroids.[5-7] At the
same time, haploidentical HSCT (haplo-HSCT) recipients are at high risk
of IFI involving Candida and Aspergillus species.[2,8]
The
introduction of post-transplant high-dose cyclophosphamide (PT-Cy)
improved the feasibility of haplo-HSCT since an unmanipulated graft is
infused without an elevated risk of GVHD.[9,10] However, IFI prophylaxis, reported in several series, is disparate and reflects the policy of each center.[11-15] As a consequence, the best antifungal prophylaxis in this type of transplant has yet to be determined.
In
a recent study in the setting of haplo-HSCT with PT-Cy, we observed
that the use of micafungin has a good safety and tolerability profile,
with efficacy in preventing IFI in this high-risk population.[16]
We compare here the safety and efficacy of three distinct antifungal
prophylaxis (micafungin, fluconazole or itraconazole) in patients
undergoing haploidentical transplantation using T cell replete and
PT-Cy in current practice.
Patients and Methods
We conducted a retrospective comparative analysis in patients of two Cancer Center, who received itraconazole vs fluconazole or micafungin or for the prophylaxis of IFIs (Paoli- Calmettes Institute of Marseille in France) or Itraconazole vs fluconazole (Humanitas Cancer Center of Rozzano in Italy) while applying common transplant approaches and procedures during the study period. Eligible were adult patients who required a transplant from a haploidentical related donor for the treatment of various high-risk hematological malignancies. They had a history of disease relapse after several chemotherapeutic lines or after a previous autologous or allogeneic HSCT; or they were refractory to conventional salvage chemotherapy and thus, were candidates for an auto-allo strategy consisting of autologous HSCT followed by haplo- HSCT.[17] Main patients' and transplant characteristics are shown in Table 1. Patients with prior IFI were excluded. Study procedures were reviewed and approved by the institutional review board in both comprehensive cancer centers before patient enrollment started. Written informed consent was obtained from each patient before treatment.
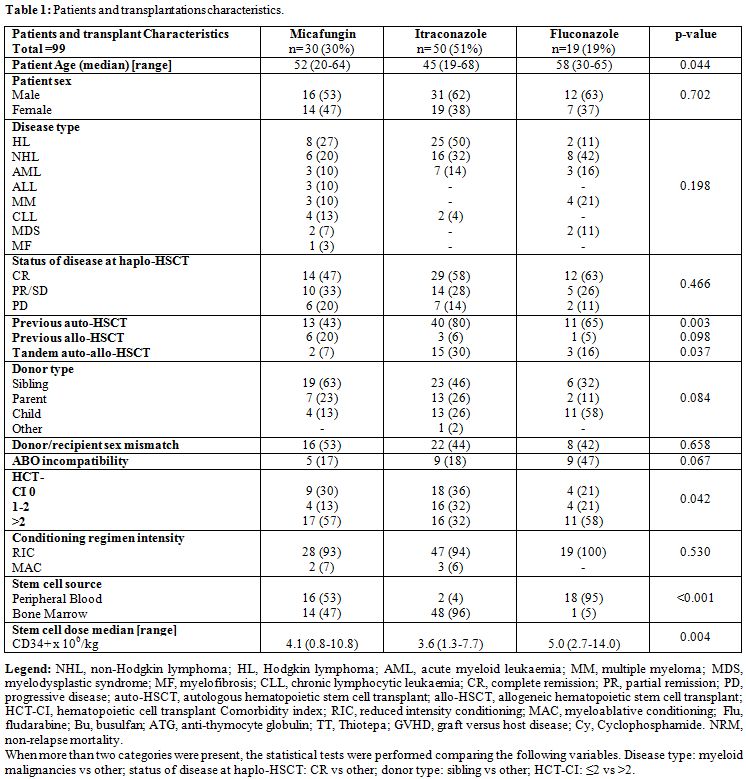 |
Table 1. Patients and transplantations characteristics. |
Conditioning and GVHD prophylaxis.
Conditioning regimens consisted of association of: fludarabine,
cyclophosphamide and total body irradiation (TBI) 2 Gy (n=80);
thiotepa, fludarabine and busulfan (two or three days) (n=15);
thiotepa, fludarabine and cyclophosphamide (n=4). As GVHD prophylaxis
patients received PT-Cy at a dose of 50 mg/ kg i.v. on days +3 and +4;
at day +5 cyclosporin A (3 mg/kg/day) or tacrolimus (1 mg/day) together
with mycophenolate mofetil (15 mg/kg/day) were started. Filgrastim was
prescribed at a dose of 5 mg/kg/day until neutrophil recovery.
Infection prophylaxis and supportive care.
The antifungal prophylaxis regimen was chosen according to
institutional guidelines, regarding patients considered at high risk to
develop IFI. Micafungin was used during a period due to the
availability of this drug. After this period, fluconazole replaced
micafungin. Itraconazole was administered mainly in the first part of
haplo program. Micafungin was administered once daily as a 1-h infusion
(50 mg/day), fluconazole orally at a dose of 400 mg/day, itraconazole
200 mg/day intravenously. Patients received antifungal prophylaxis from
the beginning of the conditioning regimen until hospital discharge, or
the occurrence of an IFI when empirical or targeted treatment was
started. The remaining antimicrobial prophylaxis consisted of
acyclovir, sulfamethoxazole/trimethoprim, levofloxacin (this
latter only at Humanitas Cancer Center). Supportive care, as well as
diagnostic procedures of infections, was similar in the two centers.
Efficacy and Safety Assessments.
The incidence of proven or probable IFI in the three groups was
calculated. Criteria for diagnosis were those for IFI as described by
the Invasive Fungal Infections Cooperative Group of the European
Organization for Research and Treatment of Cancer, National Institute
of Allergy and Infectious Diseases Mycoses Study Group.[2]
Proven infection was defined as biopsy-proven invasive or disseminated
infection. Sinus or pulmonary infection with Aspergillus, Fusarium, or
Zygomycetes organisms also was considered to be proven if results of
cultures of specimens obtained from the respiratory tract were positive
in conjunction with compatible diagnostic imaging findings. Patients
were deemed to have probable pulmonary aspergillosis if lower
respiratory tract diagnostic studies revealed fungal elements in
conjunction with compatible clinical and radiographic findings. Fungal
infection was defined as suspected if fevers (temperature, > 38°C),
persisted for > 96 h during the neutropenic phase, despite broad-
spectrum antibacterial therapy, and led to the initiation of empirical
antifungal therapy.[2,7]
Severe,
non-hematological adverse event related to antifungal drugs, death from
any cause, time to death, non-relapse mortality (NRM) and GVHD were
evaluated as well. NRM and progression-free survival (PFS) were also
estimated after 1 year of follow-up.
Statistical analysis.
Categorical variables were expressed as proportions and continuous
variables as the median with the respective range. Comparisons were
performed with the Chi-square and the Mann- Whitney test, or the Mood's
median test when appropriate, for categorical and continuous variables,
respectively. Probabilities of overall survival (OS) were estimated by
the Kaplan-Meier method, using the log-rank test for univariate
comparisons; the cumulative incidence of IFI and NRM was calculated by
a competing risk analysis, with the Gray test for univariate
comparisons. Death was the competing event when calculating IFI,
relapse or progression was competing event when calculating NRM. A
p-value <0.05 was considered significant. Statistical analysis was
performed with SPSS software (version 15.0) and R (version 2.12.2),
package "cmprsk".
Results
From January 2009 to May 2013, a total of 99 patients were
identified; 30 patients were treated with micafungin, and 69 patients
were treated with itraconazole (n=50) or fluconazole (n=19). Main
patients' and transplant characteristics are detailed in Table 1.
The most representative diagnoses are: Hodgkin's
lymphoma (HL), non-Hodgkin's lymphoma (NHL) and acute myeloid leukemia
(AML), accounting for 35%, 30% and 13% of patients' population,
respectively. The median follow-up from haplo-HSCT was 13 months
(range: 5-23) in the micafungin group, 18 months (1-51) in the
itraconazole and 3 months (1-6) in the fluconazole group
(p<0.001).
Engraftment and GVHD. Rate of engraftment was 89%. The median number of days to reach an ANC more than 500/mm3
was 20 (14-42) days in the micafungin, 20 (6-32) in the itraconazole
and 21 (15-38) in the fluconazole group (p=0.835). The median number of
days to reach a platelet count more than 20 x 109/l
was 29 (14-47) days in the micafungin, 27 (8-187) in the
itraconazole and 30 (10-53) in the fluconazole group (p=0.497). The
cumulative incidence of acute GVHD grade 2-4 was 22% (95% confidence
interval [CI]: 9-40), 24% (95% CI: 12-38) and 26% (95% CI: 3-58) in the
micafungin, itraconazole and fluconazole groups, respectively
(p=0.951). The cumulative incidence of acute GVHD grade 3-4 was 12%
(95% CI: 3-27), 2% (0-11) and 0% in the micafungin, itraconazole and
fluconazole groups, respectively (p=0.163). The cumulative incidence of
extensive chronic GVHD was 0%, 8% (95% CI: 1-22) and 0% in the
micafungin, itraconazole and fluconazole groups, respectively (p=0.481).
Invasive fungal infections.
Proven or probable IFI were reported in 3 patients (10%) in the
micafungin group, 5 patients (10%) in the itraconazole and 2 patients
(11%) in the fluconazole group (p= 0.998). The cumulative incidence of
proven/probable IFI was 11% (95% CI: 5-18). As expected, the rate of
aspergillosis was higher after fluconazole prophylaxis compared with
the micafungin and itraconazole groups (11% vs. 3% and 6%,
respectively, p=0.589; see Table 2).
Taking into account also possible IFI, aspergillosis occurred in 2
patients after fluconazole (11%) vs. two patients after micafungin (7%)
vs. 13 patients after itraconazole (26%, p=0.148).
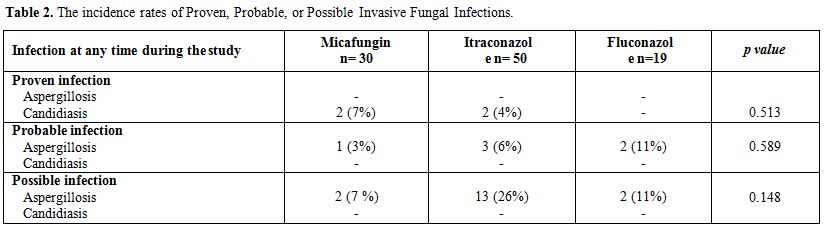 |
Table 2. The incidence rates of Proven, Probable, or Possible Invasive Fungal Infections. |
The etiology of the proven/probable IFI was Aspergillus
(n=6), Candida albicans (n=2) and Candida non Albicans (n=2). The
clinical syndrome was pneumonia in 7 cases, colitis in 1 case, sepsis
in 1 case and sinusitis in 1 case. The median day of occurrence of
proven/probable IFI was 28 days after haplo-HSCT (range: 0-69). In 7
out of 10 cases, the patient was experiencing neutropenia at the time
of infection. No patient was diagnosed with acute GVHD before the onset
of proven/probable IFI.
As reported in Figure 1A,
the cumulative incidence of proven/probable IFI was 10% (95% CI:
2-24), 11% (95% CI: 4-22) and 12% (95% CI: 2-33)
in the micafungin, itraconazole and fluconazole groups,
respectively (p=0.998).
The cumulative incidence of
proven/probable/possible IFI was 13% (95% CI: 4-28), 24% (95% CI:
13-38) and 12% (95% CI: 1-33) in the micafungin, itraconazole and
fluconazole groups, respectively (p=0.402, see Figure 1B).
| Figure 1A. The cumulative incidence of proven/probable IFI in the micafungin, itraconazole and fluconazole groups. |
| Figure 1 B. The cumulative incidence of proven/probable/possible IFI in the micafungin, itraconazole and fluconazole groups. |
A total of 4 (13%) patients in the micafungin group vs 13
(26%) patients in the itraconazole group and 10 (53%) patients in the
fluconazole received empirical antifungal therapy (P = 0.19). After
hospital discharge, 18 patients (60%) of the micafungin group received
fluconazole 400 mg/day until day 90 after haplo-HSCT because of the
lack of GVHD and 17 patients (57%) received posaconazole because they
presented acute GVHD. In the itraconazole group 14 patients (28%)
presented acute GVHD received posaconazole after hospital discharge.
Patients in the fluconazole group continue under fluconazole until day
90 after haplo- HSCT.
Invasive aspergillosis occurred in 1 (3%)
patient while receiving micafungin, 3 (6%) while receiving Itraconazole
and 2 (11%) patients while receiving fluconazole. Invasive candidiasis
occurred in 2 (7%) patients while receiving micafungin, 2 (4%) patients
while receiving itraconazole, and none while receiving fluconazole. (Table 2)
Adverse events.
No serious adverse events related to antifungal treatment were reported
and there was no treatment discontinuation because of drug-related
adverse event in any group. In the micafungin group, no
infusion-related adverse event nor hepatotoxicity were observed.
Elevated bilirubin levels of NCI-CTC grade 3 (or 4) reported in two
patients were related to their haematological disease.
Overall survival, progression-free survival, and non-relapse mortality.
OS was 65% (95% CI: 46-83), 62% (95% CI: 47-76)
and 95% (95% CI: 85-100) in the
micafungin, itraconazole and fluconazole group, respectively (p=0.387).
NRM at 100 days and 1 year was 7% (95% CI: 1-21), 17% (95% CI: 8-29),
0% and 12% (95% CI: 3-28), 24% (95% CI: 13-38), 0% in the micafungin,
itraconazole and fluconazole groups, respectively (p=0.097). Causes of
NRM were: pneumonia (n=1), JC virus encephalopathy (n=1), GVHD (n=1),
graft failure (n=1) in the micafungin group and pneumonia (n=5), JC
virus encephalopathy (n=1), septic shock (n=1), liver failure (n=1),
heart failure (n=1), multiorgan failure (n=1), secondary cancer (n=1)
in the itraconazole group. One death attributable to IFI was recorded
in the itraconazole group and the etiologic agent was probable
Aspergillosis.
A total of 13 (13%) patients died of disease
relapse or progression after haplo-HSCT; diagnoses were: HL (n=3), NHL
(n=3), AML (n=2), multiple myeloma (n=2), other (n=2). There was no
significant difference in mortality due to relapse among the three
groups (p=0.658).
Discussion
Funding
We would like to thank the Association pour la Recherche sur le Cancer
(ARC) (Pole ARECA) for their generous support of our research. Our
group is supported by several grants from the French Ministry of
Health as part of the Programme Hospitalier de Recherche Clinique (PHRC).
Transparency declaration
The authors have no conflicts of interest to declare.Acknowledgments
We thank the nursing staff for providing excellent care for our patients and the physicians of the Haematology Department at the Institut Paoli-Calmettes and Humanitas Cancer Center (Rozzano) for their important study contributions and dedicated patient care.References




















