Received: August 9, 2015
Accepted: November 9, 2015
Mediterr J Hematol Infect Dis 2016, 8(1): e2016005, DOI 10.4084/MJHID.2016.005
This article is available on PDF format at:


| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Objective: To identify the pattern of the clinical,
radiological, diagnostic procedures and loss to follow -up of the
diagnosed cases of active tuberculosis (TB) adolescents. Methods: This study was a retrospective analysis of the medical records of 143 adolescents aged 10 to 18 years with tuberculosis who were admitted TB wards of National Research Institute of Tuberculosis and Lung Disease (NRITLD) in Tehran, Iran, between March 2006 and March 2011. Results: Of the 143 patients identified, 62.9% were females. Median age of the patients was 16 years. The contact source was identified in 47.5%. The most common presenting symptom was cough (86%).Isolated pulmonary TB (PTB) was detected in 113 patients (79%), 21 patients (14.7%) had extrapulmonary TB (EPTB), and 9 patients (6.3%) had PTB and EPTB. The most common site of EPTB was pleural (14%). The most common radiographic finding was infiltration (61%). Positive acid fast smears were seen in 67.6%. Positive cultures for Mycobacterium tuberculosis (M. TB) were seen in 44.7%. Positive Polymerase chain reaction (PCR) results were seen in 60%.The adolescents aged 15 to 18 years were more likely to lose weight (p=0.001), smear positive (p=0.001), culture positive (p<0.001) and h ave positive PCR results (p=0.009). The type of TB (p=0.017) was a significant factor influencing loss to follow-up. Conclusions: The study has revealed that the clinical and radiological findings of TB in adolescents are combination as identified in children and adults. The TB control programs should pay more attention to prevention and treatment of TB in adolescents. |
Introduction
Tuberculosis (TB) remains a major health problem globally with 8.6 million people identified in 2012 of whom 1.3 million died.[1] Recent data from Iran estimated the incidence rate of TB as 21 cases per 100,000 population in 2012.[2] According to a recent study in South Africa, among 29,478 newly notified TB cases, the incidence rate started to peak in adolescents.[3] Recent studies have shown that adolescents are a susceptible age group with a higher case incidence compared to young children.[4-6] There are few studies on adolescent TB in the literature and reports indicate that many adolescents with active TB are diagnosed during the late stage of the disease.[7] One study performed in Canada showed that the average interval between the beginning of symptoms and diagnosis of TB was 5 months.[8] The delay in the treatment due to delay in diagnosis of TB leads to increase in the infectivity of the infected person in the community as a result of social interactions in this age group. The aim of this study was to evaluate the demographic data, clinical presentations, radiologic features, microbiological findings, site of TB, treatment, and outcome in this age group.
Material and methods
This study was
conducted on all patients aged 10 to 18 years old with a confirmed
diagnosis of TB, who were admitted to TB wards of National Research
Institute of Tuberculosis and Lung Disease (NRITLD) in Masih Daneshvari
Medical Center between March 2006 and March 2011. NRITLD is a World
Health Organization (WHO) Cooperative health center for TB and lung
disease, located in Tehran, Iran. NRITLD provides particular care for
TB patients who referred from across the country. As in WHO definition,
adolescents group was defined as any person between ages 10 and 18.[9]
The following data was analyzed in this study: demographic data,
presenting symptoms, radiographic features, bacteriological results,
tuberculin skin test, and treatment, outcome, and drug susceptibility
results. Patients with TB disease were defined in two categories: 1)
Definite TB was defined by identification of Mycobacterium tuberculosis
(M.TB) from the sputum, gastric lavage, body secretions, or surgical
specimens, or the histological appearance of biopsy material
representing TB-affected tissues (caseous necrosis or granulomatous
tubercles). 2) Probable TB was referred as the presence of 3 or more of
the following: symptoms and signs consistent with active TB; abnormal
radiography of TB (hilar lymphadenopathy and/or consolidation); history
of TB contact and either response to TB therapy. Then, cases were
divided into two age groups, 10-14 and 15-18 years.
Tuberculin
skin test (TST) and radiological findings could not be determined for
all patients through the information in the medical files since the
study was retrospective. TB treatment has been started at the time of
diagnosis, as recommended by the WHO guidelines, consisting of
isoniazid, rifampin, ethambutol and pyrazinamide for an initial 2-
month phase followed by isoniazid and rifampin for a maintenance
4-month phase.[10]
SPSS version 21 was used for data analysis.
The Chi-square or Fisher’s exact test was used to evaluate the level of
significance. The Mann-Whitney and Kruskal-Wallis were used to compare
the relationship between an increase in age and positive acid-fast
smears, and increase in age and cavitary lesions, respectively. A
p-value less than 0.05 was considered significant.
Results
We identified 143 patients with TB, 115 (80.4%) definite TB and 28 (19.6%) probable TB (Table 1).
Ninety (62.9%) were female, and 53 (37.1%) were male. The median age
was 16 years. Of these, 79 (55.2%) were Iranian and 64 (44.8%) Afghan.
Scar of Bacille Calmette-Guerin (BCG) vaccine injection was seen in 27
(19%), in 10 (7%) not seen the scar and in 106 (74%) the scar was
unknown. The contact source was identified in 68 cases (47.5%); 24(60%)
in 10-14 years and 44 (42.7%) in 15-18 years old. The difference
between two age groups is described in Table 2.
Common contact sources were parents 26 (18.2%). HIV was only tested for
56 out of 143 patients, among them two patients (3.5%) were HIV
positive and presented with pulmonary localization.
A TST result
was available in 61 patients. Of these, Thirty-two (22.3%) patients had
induration that exceeded 10mm. There was no significant difference in
TST positivity between two groups. (p-value: 0.167) (Table 2).
However, there may have been some selection bias regarding whose
results were available for TST, making a difference in TST positivity
between the groups.
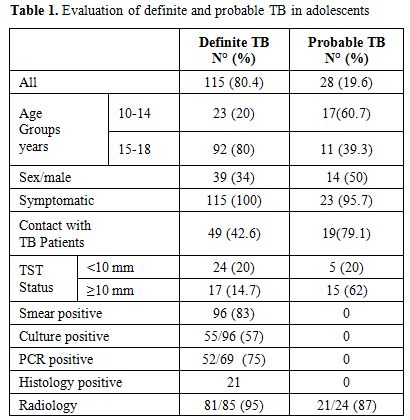 |
Table 1. Evaluation of definite and probable TB in adolescents |
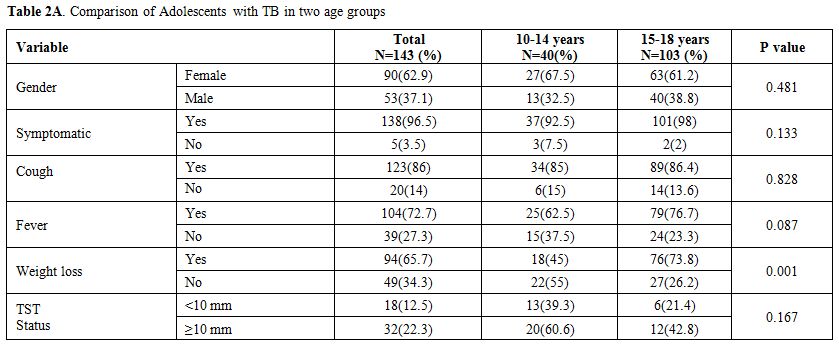 |
Table 2a. Comparison of Adolescents with TB in two age groups |
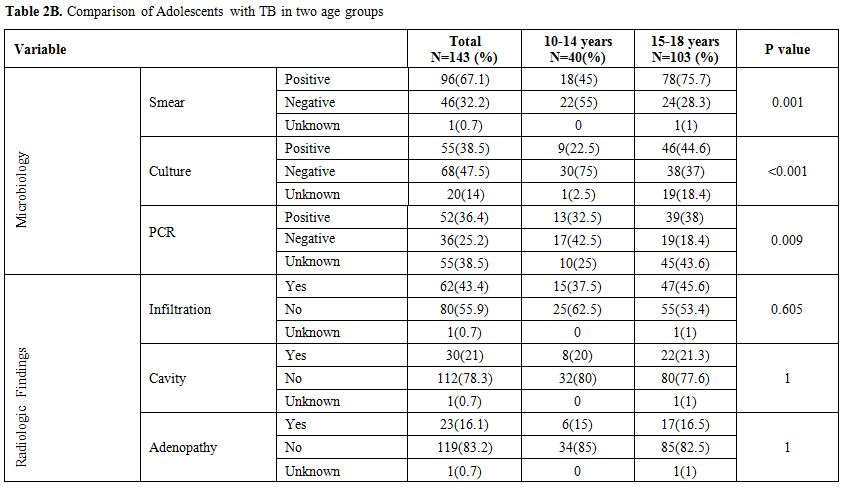 |
Table 2b. Comparison of Adolescents with TB in two age groups |
Most patients 138 (96.5%) were recognized by presenting
symptomatically. The most common symptoms were a cough 123 (86%), fever
104 (72.7%) and weight loss 94 (65.7%). The median of symptoms duration
was two months. Five patients were asymptomatic at presentation; all of
them had the history of contact, reactive TST and pulmonary involvement
in chest radiographic.
Isolated pulmonary disease occurred in 113
(79%), 21 (14.7%) had extrapulmonary disease, and 9 (6.3%) had
pulmonary TB(PTB) and extrapulmonary TB(EPTB); EPTB distributed
as pleural 20 (14%), central nervous system (CNS) 4 (2.8%), lymph node
1 (0.7%), pleural with peritoneal 2 (1.4%), pleural with skeletal
1 (0.7%), and CNS with lymph node 1 (0.7%).
Positive acid-fast
smears were seen in 96 of 142 patients (67.6%). The most common site of
smear positive was sputum 76 (53%). The smear-positive rate increased
with age (33% at ten years to 76% at 18 years) (Mann-Whitney: p=0.003)
peak age was 16 years (80%) (Figure 1).
Cultures were achieved in 123 patients. Positive cultures for M.TB were
seen in 55 (44.7%); fifty patients with pulmonary disease, one patient
with EP TB and four patients with both disease. Culture-positive rate
increased with age (17% at 10 years to 48% at 18 years) (Mann-Whitney:
p=0.039) (Figure 1). Polymerase
chain reaction (PCR) test of M.TB were performed for 88 patients;
positive PCR was seen in 52 patients (60%). The common site of positive
PCR test was sputum in 38 patients (73%). PCR-positive rate increased
with age (40% at 10 years to 80% at 18 years) (Mann-Whitney: p=0.018) (Figure 1).
| Figure 1. Age distribution of adolescents with smear positivity, culture positivity , PCR positivity and with the presence of cavity lesion. |
Twenty-one patients were confirmed as TB cases by a
histological diagnosis (pleural n=14, bronchial n=6, lymph nodes
n=2, bone n=1). Drug susceptibility testing (DST) for M.TB was
performed in 15 patients. Seven patients had susceptible M.TB strains
to all anti-TB drugs, eight patients had isolates of M.TB
drug-resistant: 5 had multidrug-resistant TB (three with resistance to
rifampin and isoniazid, another with additional resistance to
ethambutol, amikacin, and kanamycin), three patients had drug
resistance to isoniazid and one to pyrazinamide.
Radiography
reports were available in 107 patients; 23 cases had chest X-ray, and
84 cases had CT scan reports. Abnormalities were found in 102 (95.3%).
Of these patients, 62 of 102 (61%) had infiltration, 52 of 102 (51%)
had consolidation, 30 (29%) cavity, 25 (24.5%) pleural effusion and 23
(22.5%) adenopathy. Cavitary lesion was increased with age (0 at ten
years to 23% at 18 years) but the difference is not statistically
significant (by Kruskalwallis: p=0.373) (Figure 1)
Eighty-nine
patients (61%) had complete follow-up; 71 (49.7%) improvement, 14
(9.8%) relapse, 3 (2.1%) expire and 1 (0.7%) sequelae. Fifty-four (39%)
adolescents identified as a loss to follow-up TB treatment. The
comparison between adolescents loss to follow–up and not were performed
to found if any factors based on our research that influenced loss to
follow–up. A significant statistical difference was found for type TB
(p=0.017) between adolescents identified as a loss to follow–up and not
(Table 3).
Elevation of
AST and ALT enzymes occurred in 9 of 143 patients (6.2%); seven
patients were between 15 and 18 years. However, restored to the normal
range without any disruption of treatment.
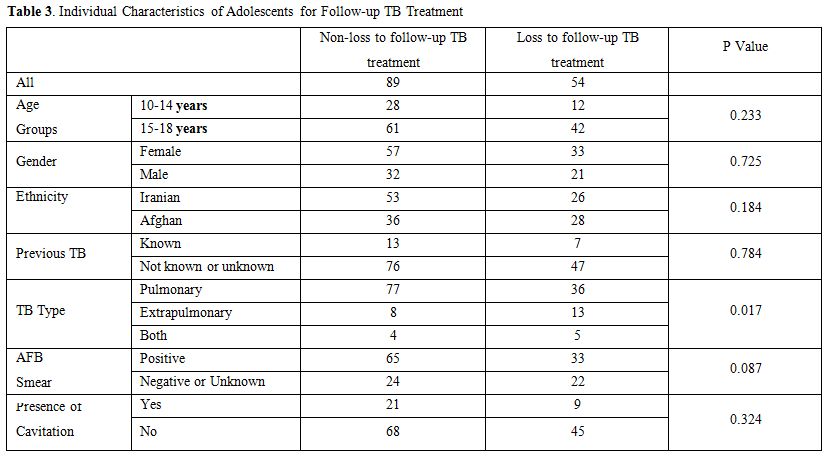 |
Table 3. Individual Characteristics of Adolescents for Follow-up TB Treatment |
Discussion
This study reports the epidemiology and characteristics of TB in
adolescents from a developing country. To our knowledge, no similar
study has been reported from other Middle East countries. Clinical and
radiological features of adolescents TB are different from children and
adults. Older adolescents had a severity of TB disease than younger
adolescents.
Adolescents are more frequently
symptomatic.[8,11,12] We also found most patients (96.5%) were
recognized after presenting with symptoms. Isolated PTB and EPTB were
detected in 79.7% and 14.7% of the patients in this study,
respectively. Previous studies detected PTB and EPTB in 22% to 78.6%
and 17% to 35% of the adolescents with TB, respectively.[8,11,12] The
proportion of patients with EPTB compared with those of PTB varies
among countries and depends on host-related factors such as ethnicity,
and associated diseases.[13] In this study, the frequency of EPTB was
closer to the rate in adults (16%) as compared to children (27.3%).[14]
The most common form of EPTB in our adolescents was pleural, similar to
studies conducted in France and Canada.[8,12] This is in contrast to
studies conducted in the USA and South Africa that found the most
common site of EPTB was peripheral lymphadenopathy.[11,15] These
differences propose that the type of EPTB may be specific to
adolescents in different population; more population-based studies in
different geographic regions are required.
Approximately fifty
percent of our adolescents were exposed to a known adult with TB. This
finding shows that contact investigation remains an essential tool for
the control of TB disease. Previous contact investigation may have
prevented these cases of adolescents TB. Positive source cases were
identified in 25–66% of the children with TB in previous
studies.[16-19] In our study, the rate of exposure to known adults with
TB was close to the rate of children with TB. According to some
studies, the history of contact with TB can be determined in 12%-19% of
adult patients.[20-22]
Similar to the study conducted in the US,
we found that adolescents aged 15 to 18 years were more likely to have
smear-positive TB and severity of the disease.[23] An
epidemiological study on age and pathogenesis of TB has revealed that
older adolescents are at higher risk of developing the disease than
younger adolescents after infection with M.TB.[24] Since most of these
age groups have considerable social connections and interactions in
colleges or schools; these patients are more likely to transmit TB to
the community. Therefore, it is important to screen older adolescents
for TB. Studies in children have revealed that the yields of culture
and PCR are higher than that of the smear.[25] Nevertheless, we found
that the rate of smear positivity was higher than that of culture and
PCR. Smear positive /culture negative results may be due to the
presence of nonviable mycobacteria in the sample receiving anti-TB
treatment. The low yields of culture and PCR might be due to low sample
volume. More effort is needed to improve both quality and quantity of
samples to have a better diagnosis of TB in adolescents.[26]
Lymphadenopathy
was the most common radiographic feature in young children.[27] After
puberty, the radiological findings of TB are similar to adults include
parenchymal lesions and cavities.[28] In the present study, the most
common radiologic finding was infiltration, which is similar to studies
conducted in the United States and Brazil.[11,29] In contrast to the
study conducted in France, most patients had mediastinal
lymphadenopathy. However, despite this finding, a notable number of
patients continue to present with a parenchymal lesion in France
study.[12] Although not statistically significant, cavitary lesion rate
increased with age in our study. The studies on adolescent TB in
France,[12] Brazil[21] and South Africa[15] found that the cavity
lesion rate increased with age. Seventy-one percent (n=101) of our
patients were probably preventable through screening immigrants from
Afghanistan (64) and contact investigation (37). Our study was
consistent with the study performed in the US.[11] This finding
demonstrates the importance of developing new strategies for the
prevention and treatment of TB to improve children’s and adolescents’
survival to achieve Millennium Development Goals.[30]
TB patients
who are lost to follow-up are at increased risk of developing
drug-resistant TB and treatment failure that further increases the risk
of TB transmission.[31] In this study, 54 patients (37.7%) were lost to
follow-up after starting treatment. It is necessary to identify the
components of potential behavioral intervention strategies for future
program implementation among adolescents entering and receiving TB
care. Contracting the contingency and peer counseling are two such
intervention methods that have been used in adolescents.[32] Peer
counseling is an adolescent with a successful TB treatment encouraging
a newly diagnosed adolescent patient. Contingency contracting is
improving the desired behavior of adherence to the medical regimen by
providing incentives.[33] It has been suggested that prospective
studies are required to evaluate such strategies to increase adherence
to TB treatment.
Patients with EPTB were more likely to become
lost to follow-up than those with PTB in this study. This result is in
agreement with findings in previous studies.[34,35] However, other
studies have found no relationship between the type of TB and the
successful TB treatment outcome.[36-38] Despite the increasing
proportion of patients with EPTB in many countries, these patients
often receive less priority on the international health system.[39]
Therefore, more attention must be paid to the patients with EPTB, who
are usually neglected in the TB control program.
Directly observed
treatments (DOTs) should be attended to improve treatment compliance.
There is a need to conduct prospective studies on the risk factors of
loss to follow-up in adolescents TB treatment such as inadequate
knowledge about TB, living far from health facilities, and drug side
effects. Only one recent study revealed that the lowest rates of
completing TB treatment were associated with older adolescent age,
ethnicity, parental responsibility, and access to the clinic.[40]
Conclusions
The study has shown that the clinical and radiological findings of TB in adolescents are a combination as identified in children and adults. The data presented in this study have implications for the development of strategies for early screening of TB in high-risk categories, prompt diagnosis of TB, and improving the rate of completion of care among adolescents treated for TB.
Acknowledgment
References

































[TOP]