Salvatore Perrone, Gianna Maria D’Elia, Giorgia Annechini and Alessandro Pulsoni
Division of Hematology, Department of Cellular Biotechnologies and Hematology, “Sapienza University” Rome, Italy.
Published: January 1, 2016
Received: Agust 7, 2015
Accepted: November 16, 2015
Mediterr J Hematol Infect Dis 2016, 8(1): e2016006, DOI
10.4084/MJHID.2015.006
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Marginal zone lymphomas have been
associated with several infectious agents covering both viral and
bacterial pathogens and in some cases a clear aetiological role has
been established. Pathogenetic mechanisms are currently not completely
understood. However, the role of chronic stimulation of the host immune
response with persistent lymphocyte activation represents the most
convincing explanation for lymphoproliferation. Gastric MALT lymphoma
is strictly associated with Helicobacter pylori infection
and various eradicating protocols, developed due to increasing
antibiotic resistance, represent the first line therapy for gastric
MALT. The response rate to eradication is good with 80% of response at
1 year; this finding is also noteworthy because it recapitulates cancer
cured only by the antibacterial approach and it satisfies the Koch
postulates of causation, establishing a causative relationship between
Hp and gastric MALT lymphoma. Patients with chronic HCV infection have
5 times higher risk to develop MZL, in particular, an association with
splenic and nodal MZL has been shown in several studies. Moreover,
there is evidence of lymphoma regression after antiviral therapy with
interferon+ribavirin, thus raising hope that newly available drugs,
extremely efficient against HCV replication, could improve outcome also
in HCV-driven lymphomas. Another case-study are represented by those
rare cases of MZL localized to orbital fat and eye conjunctivas that
have been associated with Chlamydophila psittaci
infection carried by birds. Efficacy of antibacterial therapy against
C. psittaci are conflicting and generally poorer than gastric MALT.
Finally, some case reports will cover the relationship between primary
cutaneous B-cell Lymphomas and Borrelia Burgdorferi. |
Introduction
Marginal zone lymphoma (MZL) is a heterogeneous group of low-grade,
B-cell, non-Hodgkin lymphomas comprising three distinct diseases:
extranodal MZL (EMZL) of mucosa-associated lymphoid tissue (MALT),
splenic MZL (SMZL), and nodal MZL (NMZL).[1] In recent European
studies, MZL has been reported in 12% of new lymphoma diagnoses,[2]
while a lower incidence of 3% was reported in the United States from
Surveillance, Epidemiology, and End Results (SEER) registries.[3]
Various contributing factors have been defined in terms of
pathogenesis, including autoimmune diseases (mainly Sjögren syndrome
and chronic autoimmune thyroiditis)[4] while also several infectious
agents are known or simply suspected to cause MZL. Mainly three
mechanisms linking infections to MZL have been hypothesized:
1)
lymphocyte-transforming viruses can infect lymphocytes inducing
dysregulation of normal cell functions and promotion of cell division
(Epstein–Barr virus, Human Herpesvirus 8, Human T-lymphotropic virus
type I);
2) induction of immune suppression through progressive
depletion of CD4+ T-lymphocytes, as in AIDS (Human immunodeficiency
virus) or in patients submitted to therapeutic immunosuppression;
3) stimulation of chronic immune response and persistent lymphocyte activation (Helicobacter pylori, Hepatitis C virus, Chlamydophila psittaci, Borrelia burgdorferi, Campylobacter jejuni).[5]
Specific organisms have been implicated in the aetiology of MZL involving particular anatomic sites:[6] Helicobacter pylori for the stomach, Chlamydophila psittaci for the ocular adnexa, Borrelia burgdorferi for the cutis, Campylobacter jejune for the small intestine, Mycobacterium spp.
For the bronchus, Hepatitis C virus (HCV) for splenic and nodal MZL.
However, a robust association is present only in gastric MALT lymphoma
with Helicobacter pylori (Hp),
where 90% of cases are Hp+.[7] Moreover, Hp eradication therapy is
effective in gastric MALT Hp+ lymphoma with a remission rate around
80%[8,9] and also long-term results are excellent.[10] The studies on Hp
and gastric MALT lymphoma have profoundly influenced our understanding
of the pathogenesis of lymphomas and modified our management, because,
for the first time in the history of medical oncology, cancer has been
cured by antibiotic therapy.
This review focuses on the role of
pathogens and development of MZL, with implication on the therapeutic
option to target the implied infectious agents.
Gastric MALT Lymphoma
The clinical presentation of gastric involvement by MALT lymphoma is
variable and not specific with abdominal pain being the most common
symptom, followed by dyspepsia, vomiting, nausea and anorexia; weight
loss is common; gastric bleeding occurs as presenting symptom in
20%–30% of patients, while gastric occlusion and perforation are less
common.[11] Diagnosis is made after histopathological evaluation of
gastric biopsy, generally after esophagogastroduodenoscopy, and rely on
the morphologic demonstration of the “hallmark” of MALT lymphoma: the
lymphoepithelial lesion that results from invasion by atypical
lymphocytes of epithelial mucosa and invasion of the glandular
epithelium, as well as reactive lymphoid follicles.[12] Paradoxically,
the stomach is the commonest site of MALT lymphoma, despite MALT tissue
is not normally present in gastric mucosa. However, differentiation
from other indolent lymphomas (Follicular lymphoma, Mantle-cell
lymphoma) and aggressive lymphoma (Diffuse large B-cell lymphoma and
Peripheral T-cell lymphoma) is not always straightforward, and a
minimum immunohistochemistry panel including CD20, CD10, CD5 and cyclin
D1 is recommended.[13] Since the diagnosis should be made according to
the WHO criteria,[14] it should be reviewed by an expert
hematopathologist.[15] Since the first discovery in 1982 by the Nobel
prize-winning authors, B. Marshall and R. Warren[16] Helicobacter pylori has
become critical for treating and studying gastric disease like peptic
ulcer, MALT lymphoma and gastric cancer.[17] Hp identification mainly
relies on histology (HE and modified Giemsa staining) and culture or
invasive molecular tests.[18] In the case of negativity, serology
should be performed to identify truly negative gastric MALT
lymphomas,[19] in fact, Hp mucosal colonisation is not uniform, but in
patches;[20] therefore, the infection can go undiagnosed if biopsy
involves a non-colonised area. In addition, it is believed that
extensive mucosal lesion secondary to lymphoma may reduce the density
of the infection to even undetectable levels.[21,22]
Role of H. Pylori in MALT Lymphoma. Helicobacter species are the indigenous biota of mammalian stomachs, and H. pylori is the human-specific inhabitant,[23] there is evidence that H. pylori has
been present in humans at least since ancestors of Amerindians migrated
from Asia more than 11,000 years ago.[24] In this long time span, Hp
has adapted to human gastric environment establishing an interaction
that can be interpreted as both commensalism and long-term
parasitism.[25] Extensive allelic diversity and genetic variability are
hallmarks of this microaerophilic gram-negative bacterium,[26]
resulting from the combination of a high mutation rate[27] and frequent
exchange of genetic material during mixed strains infections.[28] That
extraordinary capacity of adaptation to human host (resembling a
quasispecies) is the key to the success for this microorganism to
infect more than one-half of the human population. H. pylori has been established by International Agency for Research on Cancer
(IARC) to be definitive bacterial carcinogen for humans[29] and is
estimated to be responsible for 5.5% of all human cancer cases, about
592.000 new gastric cancer.[30] Spectrum of H. pylori infection
is wide with most carriers remaining asymptomatic while patients with
duodenal ulcer have antral predominant gastritis with little mucosal
atrophy and hyperacidity; patients with gastric ulcer almost invariably
have corpus predominant gastritis and hypoacidity with various degree
of mucosal atrophy,[31] the latter condition is associated with gastric
cancer.[32] Moreover, the case of gastric MALT lymphoma is a rarer
condition, accounting for <5% of primary gastric neoplasms. The
epidemiological data raise the question why some people develop gastric
cancer (also MALT lymphoma), and others do not? For example, what is
the possible answer to the ‘African enigma’ where ubiquitous H. pylori infections
is not associated with gastric cancer but only gastritis?[33] On the
other hand, in the setting of a developed Country like the Nord-East of
Italy, where there is a higher prevalence of HP infection there is a
concomitant high incidence of gastric lymphoma.[34]
Given that
some simulations indicate that Hp seems to have spread from East Africa
around 58,000 years ago following human migrations,[35] we could
hypothesize that in Africa, where H. pylori have interacted for more time with its human host, it could have reached a better symbiotic state with a decreased virulence.
Pathogenesis of Helicobacter Pylori-Induced Gastric MALT Lymphoma. There are several known mechanism that Helicobacter exploits to interact with the host mucosa. Actually, H. pylori strains
can be broadly categorized into strains that express multiple factors
that interact with host tissue (CagA, s1-VacA, BabA, OipA) and strains
that lack these factors.[36] In 1989, CagA was firstly identified[37]
and is now recognized as a marker for strains that confers increased
risk for peptic ulcer disease[38] and gastric cancer.[39] The Cag Type
IV secretion system (T4SS) is the primary virulence determinant and is
responsible for injecting macromolecules, in particular, CagA, inside
epithelial cells.[40] CagA is then tyrosine-phosphorylated by the host
cell Src kinase; once phosphorylated, CagA interacts with SHP-2, a
tyrosine phosphatase, which affects spreading, migration, and adhesion
of epithelial cells.[41] Moreover, CagA protein interacts with Grb2 and
activates the Ras/MEK/ERK pathway, leading to the phenotypes of cell
scattering and proliferation.[42] In addition, tyrosine-phosphorylated
CagA binds and activates C-terminal Src kinase (Csk) via its SH2
domain, which in turn inactivates the Src family of protein-tyrosine
kinases. Since this signalling may induce apoptosis, the Csk pathway
may attenuate the other CagA interactions.[43] In conclusion,
attenuation of CagA activity by Csk may enable CagA-positive HP
to infect the human stomach persistently for decades while avoiding
excessive CagA toxicity to the host.[43] There is also evidence that
CagA can be directly injected by T4SS inside B-lymphocytes. The
delivered CagA induces the activation and stimulation of B cells
(mediated by intracellular SHP-2 and phosphorylation of ERK and P38
MAPK), and could initiate the first step of transformation, also
upregulating anti-apoptotic proteins BCl-2 and BCl-XL.[44] Moreover,
Kuo and colleagues have demonstrated the presence of the bacterial
protein CagA inside malignant B cells of MALT lymphoma and that those
patients tend to respond faster to HP eradication.[45]
The vacuolating cytotoxin VacA, a high–molecular weight multimeric pore-forming protein is one of virulence factors produced by Hp
and is responsible for epithelial cells by both apoptosis and
programmed cell necrosis.[46] VacA forms a pore that permeabilizes the
epithelial cell plasma membrane to urea,[47] that is an essential
substrate for the H. pylori’s
urease to mitigate acid gastric environment. More importantly, VacA
determines immune suppression by blocking phagosome maturation in
macrophages,[48] inhibiting antigen presentation in T cells,[49]
blocking T cell proliferation with down-regulation of Th1 effects
through interaction with calcineurin to block intracellular
signalling.[50]
Chronic H. pylori infection
can trigger inflammation and immunological responses that stimulate
lymphoid infiltration displaying features of classic MALT
architecture.[51] It is suggested that H. pylori
infection results in T-cell-dependent responses through the classic
germinal centre reaction, with generation of reactive B and T cells;
the H. pylori-specific T
cells then migrate to the marginal zone/tumour area and provide
non-cognate help to auto-reactive neoplastic B cells, which may involve
stimulation of CD40 and other surface receptors by soluble ligands and
cytokines.[52,53]
Host polymorphisms are also a crucial determinant of the interaction with H. pylori and
could elucidate why only rarely MALT lymphoma develops. Determinants of
host immune response variability have been extensively reviewed by
Datta De[36] and mainly consist in variation in an inflammatory gene
like IL-1 (RN 2/2 genotype), glutathione S-transferase T1 null phenotype[54] and a negative association with HLA-B35.[55]
An important proof of concept of the association of Hp
with Gastric MALT were the first report by Wotherspoon of MALT
regression after successful eradication of Hp.[56] Those data have been
corroborated by other studies,[8,10] thus fulfilling Hill’s criteria of
causality.
Gastric MALT Beyond Helicobacter Pylori. After a clonal expansion of B-cell H. pylori-driven
has established in gastric mucosa, other mutational events can explain
the gain of independence from the infection itself. Three chromosomal
translocations are the most frequently detected
t(11;18)(q21;q21)/API2-MALT1, t(1;14)(p22;q32)/BCL10-IGH, and
t(14;18)(q32;q21)/IGH-MALT1, all converging to activation of nuclear
factor kappa-B (NF-κB).[53] Translocation t(11;18)(q21;q21), occurring
in approximately 30% of cases, fuses the N-terminus of the API2 gene to
the C-terminus of the MALT1 gene and generates a functional API2-MALT1
fusion product,[57,58] the other two translocations involve
translocation to immunoglobulin gene loci, and consequently increased
expression, of BCL10 and MALT1 genes.[12] Gastric MALT lymphomas
carrying the t(11;18) are more aggressive with spread to local lymph
nodes[59] and, most importantly, rarely respond to HP
eradication.[60,61] Therefore, its detection can influence the clinical
management and is easily feasible with a commercial MALT1 dual-colour
break-apart probe and an API2-MALT1 dual-colour dual-fusion probe for
FISH, or by rt-PCR of the API2-MALT1 fusion mRNA transcripts.[62] Not
translocated cases frequently carry trisomies of chromosomes 3, 12 and
18.[63]
Gastric MALT lymphoma H. Pylori Negative. Another interesting setting is represented by gastric MALT lymphoma, H. pylori negative that responded to antibiotic treatment in 15.5% of cases, despite H. pylori triple negativity test.[64,65] Those data have been interpreted by authors in light of a limited accuracy of H. pylori
detection (false negative) or the possible presence of other
unidentified bacteria implicated in MALT pathogenesis.[65] Nowadays,
given the limited toxicity, low costs and risk of low-grade lymphoma
progression, antibiotic therapy is also recommended in H. pylori- cases.[66]
Antibiotic Treatment. As previously discussed, targeting H. pylori seems a logical first line approach for gastric MALT lymphoma. Several effective schemes are available for the treatment of H. pylori infection.[67-69] The antibiotic choice should be based mainly on the epidemiology of Hp resistance
to clarithromycin. Therefore, in countries with a prevalence >15%
(That is the case of Europe, with the exception of Northern States)[70]
antimicrobial susceptibility testing could be useful.[18] The most
commonly used regimen is triple therapy: a proton pump inhibitor
(omeprazole)[71] in association with amoxicillin and
clarithromycin.[72] Metronidazole can be substituted to amoxicillin in
penicillin-allergic individuals. An alternative is represented by the
Sequential treatment, which includes a 5-day period with
PPI+amoxicillin, followed by a 5-day period with
PPI-clarithromycin-metronidazole.[73] For failure of the first line
therapy or for clarithromycin-resistant isolated strains, it is
available a bismuth-based quadruple therapy with omeprazole, bismuth
salts, metronidazole and tetracycline (OBMT),[74] an RCT using a
combination of PPI and a single three-in-one capsule tablet showed
improved eradication in comparison with standard triple therapy.[75]
Finally, in case of failure of the second-line treatment
(bismuth-containing quadruple regimen), it is recommended to use the
PPI-levofloxacin-amoxicillin regimen,[76,77] always considering the
rise in epidemiologic H. pylori resistance to levofloxacin.[68]
MALT lymphoma response to H. pylori
eradication is about 80%.[65] However the length of time necessary to
obtain a remission can span from few months to more than 12 months.
Anti-Lymphoma Directed Treatment.
In cases not responding to antibiotic treatment, a control of localised
disease can be achieved with radiation therapy alone with moderate-dose
involved-field radiotherapy (24–30 Gy) to the stomach and perigastric
nodes.[13,78]
Patients with the symptomatic systemic disease
should be considered for systemic treatment that encompass the
association of rituximab + chemotherapy. Rituximab in combination with
chlorambucil has been proven in a randomised study by International
Extranodal Lymphoma Study Group (IELSG)-19 where an excellent
event-free-survival was achieved, superior to chlorambucil alone,
although no overall survival benefit has been shown. Aggressive
anthracycline-containing regimens (CHOP-like) are not usually necessary
and should be reserved for cases with transformation to high grade.[13]
Marginal Zone Lymphoma and HCV
A heterogeneous group of lymphoproliferative disorders have been
long suspected to be associated with HCV infection. HCV-related Mixed
cryoglobulinemia (MC) is considered as a low-grade B-cell
lymphoproliferative disorder, characterized clinically by arthritis,
cutaneous vasculitis (palpable purpura), and, occasionally associated
with glomerulonephritis and by the presence of circulating
cryoprecipitable immune complexes of more than one immunoglobulin
class. MC is defined by laboratory findings: the presence of serum Ig
that precipitate at low temperatures (<37°C) and can solve by
warming serum, that are produced by the lymphoproliferation of B-cells
clones secreting pathogenic IgM with rheumatoid factor activity. MC can
evolve into an overt B-cell NHL in approximately 8–10% of cases after a
long period.[79]
MZLs, in particular splenic (SMZL) and nodal MZL
(NMZL), and other extranodal-MZL are the iNHL subtypes most frequently
described as being HCV-related.[80-82] SMZL is an indolent and rare
entity, separately recognised by WHO,[1,83] usually presenting with
symptomatic splenomegaly, cytopenias, autoimmune phenomena, and serum
monoclonal paraprotein; in some patients a leukemic phase characterized
by circulating lymphocytes with villous projections defines the
so-called Splenic lymphoma with villous lymphocytes (SLVL).[84] SMZL,
albeit rare, in a population-based study, has been reported with an
incidence between 2001 and 2008 of 0.13 per 100,000 person-years,
accounting for 0.6% of all NHL cases[85] and is the most common primary
splenic lymphoma.[86] SMZL incidence is higher among older (median age
is 65 years), white and male (male-to-female ratio of 1.2:1)
population, in the United States.[85]
NMZL is a distinct
clinical-pathological subtype of MZL characterized by exclusive primary
lymph node localization in the absence of extranodal or splenic
disease.[1] NMZLs represent only 1.5% to 1.8% of all lymphoid
neoplasms; the most frequent clinical presentation is a generalized or,
less frequently, localized lymphadenopathy.
Recently, Paulli et
al. have reported a new subset of patients with extranodal HCV-related
MZL characterized by a primary ‘lipoma-like’ subcutaneous presentation
and an indolent clinical course.[87]
Role of HCV in iNHL.
Hepatitis C virus (HCV) is an enveloped, RNA virus of the Flaviviridae
family; it comprises six major genotypes, whose prevalence varies among
different population and countries. HCV is not only hepatotropic,
causing infection of hepatocytes associated with hepatitis, liver
cirrhosis and hepatocellular carcinoma (HCC), but is also responsible
for other extrahepatic manifestations, of whose the most frequent is
lymphoproliferation.[88]
Models of Pathogenesis in HCV-Induced Lymphoproliferation.
The mechanisms underlying B-cell lymphoproliferation possibly induced,
directly or indirectly, by HCV chronic infection are yet not fully
understood. However, few experimental data suggest some clues to
explain this phenomenon and have been reviewed elsewhere.[89,90]
The
first observation derives from the HCV chronic antigenic stimulation
leading to continuous stimulation and selection of reactive B-clones.
HCV-associated NHLs derive from B cells activated during HCV infection,
with some of these B cells being specific for the protein HCV-E2, which
is the primary target of antibody responses against HCV.[91] Moreover,
the monoclonal IgM component of type II MC is often encoded by the same
set of Variable region genes, VH1-69, and Vk3-A27 and similarly the
same genes can be demonstrated to be involved in some cases of
HCV-related NHL.[92,93] The burden of these data suggests that
HCV-associated lymphomas are derived from clonally expanded B cells
stimulated by HCV-E2 protein.
HCV is a positive, single-strand RNA
virus, lacking a DNA intermediate in its replicative cycle. Thus,
insertional mutagenesis in infected B-cells seems not possible.
Moreover, there is conflicting evidence about the demonstration in
B-cells of the negative RNA viral strand, which would be consistent
with active viral replication. In fact, in a Japanese study
negative-strand HCV RNA was detected in B cells only in 4 of the 75
(5%) patients[94] while no replicative intermediates were detected in
another study.[95] In addition, B-cells could be not suitable for virus
entry because claudin-1, a membrane co-receptor required for HCV
infection is not present in peripheral blood cells.[96]
Conversely,
more robust data exist supporting a direct B-cell stimulation by the
engagement of CD81 on their surface by a combination of HCV-E2 and
anti-CD81 antibodies leading to polyclonal activation of naïve, CD27-
B-cells.[97]
Antiviral Therapy.
Since 1990, several Epidemiologic studies suggested an association
between hepatitis C virus (HCV) infection and B-cell NHL, although with
geographical heterogeneity due to the different prevalence of HCV
seropositivity.[98-102] Nevertheless, many of these studies suffered
from methodologic restrictions such as their retrospective nature, the
consideration of prevalent instead of incident cases, missing or
inappropriate controls. In 2003, the GIMEMA group published an Italian
case-control study performed with adequate epidemiologic methods that
demonstrated a clear association between HCV infection and various
types of B-NHL.[103] Recently a larger case-control study of InterLymph
NHL Subtypes Project hepatitis confirmed the increased risk of MZL in C
virus seropositive patients (EMZL OR=5.29, 95% CI=2.48 to 11.28).[104]
In 2002, Hermine et al. reported the first series of nine patients with
SLVL in which a complete regression of lymphoma was obtained with
antiviral treatment (AT) consisting of interferon (IFN) +/-
ribavirin.[105] As a control, the authors reported 6 pts. with the same
disease, but without HCV infection, not responding to the same
treatment. This argument suggested for the first time that viral
eradication and not the direct anti-proliferative effect of IFN was
responsible for HCV-related NHL regression. Many single cases
subsequently confirmed this observation, although, due to the rarity of
HCV-related NHL, very few prospective studies have been published. In
20 patients, Vallisa et al. demonstrated a complete response to
antiviral treatment of different subtypes in 70% of indolent
i-NHL.[106] Recently a multicenter study of Fondazione Italiana Linfomi
(FIL) recorded more than 700 patients with i-NHL and HCV
seropositivity, demonstrating that AT used in the first line in 100 pts
produced 44 CR and 33 PR.[107] A French prospective study of 116
HCV-positive patients with B-NHL revealed that within the MZL subgroup
(n=45 pts.), 84% (n=38) received AT and 61% (n=23) achieved a Sustained
Virological Response.[108] Moreover, outcome analysis showed a
favourable association between OS and AT in MZL patients (P =
0.04).[108] From these experiences, AT is, to date, considered the
first-line approach for the cases of HCV-related indolent lymphoma, not
requiring immediate chemo-immunotherapy. The recent introduction of
new, highly active, antiviral treatments could clarify whether a pure
antiviral approach,[109-111] free from the confounding role of
interferon,[112] is equally or even more efficacious against lymphoma.
Ocular Adnexa MALT Lymphoma
Ocular adnexa MALT lymphoma (OAML), although uncommon, is the third
most frequently involved site of MZL with an incidence rate of
1.4/1,000,000 person-years, with a median age of 65 years,6 thus
accounting for 50-78% of all ocular lymphomas in Western
Countries.[113] Moreover, Danish and American SEER data from
surveillance registries have reported an increase in the incidence of
OAML.[114,115] Clinical presentation of orbital fat lymphomas
involvement (75% of OAML) includes exophthalmos (27% of cases),
palpable mass (19%), eyelid ptosis (6%), diplopia (2%), eyelid nodule,
orbital oedema, epiphora and a variable degree of impaired ocular
motility, while the most common sign for conjunctival lymphomas (25% of
OAML) is the characteristic ‘salmon red patch’.[116] OAML is an
indolent lymphoma with a favourable clinical outcome emphasized by a
10-year overall survival of 81%, with no deaths from lymphoma for the
patients treated with radiotherapy[117] and 94% for the patients
managed with ‘watch and wait’.[118] Microscopically neoplastic cells
are monocytoid, centrocytic-like or lymphoplasmocitoid and their
immunophenotype is similar to that of other MALT lymphomas: CD20+,
CD79a+, usually IgM+ with light-chain restriction, PAX5+, bcl-2+,
TCL1+, CD11c+/-, CD43+/-,CD21+/-, CD35+/-, and IgD2, CD32, CD52, CD102,
CD23-, cyclin D1-, bcl-6-, MUM1-.[119] Frequent chromosomal alteration
encompasses trisomy 3 in 62% of cases and trisomy 18 in 47% of
cases.[120] In addition, the most common translocations in ocular
adnexal MALT lymphomas are the t(11;18)(q21;q21)/API2-MALT1 and the
t(14;18) (q32;q21)/IGH-MALT1.[121] The immunoglobulin heavy-chain gene
rearrangement is clonal in 55% of cases[122] and shows somatic
hypermutation in two-thirds of these,[123] with a majority of selected
genes commonly implicated in the assembly of autoantibodies, hence
supporting the view that OAML represents a clonal expansion of
post-germinal-centre memory B-cells, where selection may have occurred
driven by antigen stimulation.[123] Moreover, in OAML a possible
infectious association has been long proposed, Chlamidophila spp.
and, to a lesser extent, H. pylori and HCV[124] have been proposed as
causative agents. Although the association with Hp is
controversial,[125] a more robust evidence is available for the role of
C. psittaci.[119,126]
Role of C. Psittaci in Ocular Adnexa MALT Lymphoma. Chlamydophila psittaci (CP)
is an obligate intracellular bacterium responsible for
psittacosis/ornithosis in birds and in humans after zoonotic infection
through inhalation of aerosolized bacteria when exposed to infected
birds or handling contaminated feathers, faecal material or
carcasses.[127] CP infection is commonly asymptomatic with repeated
infection cycles in humans but mainly involves the respiratory tract.
CP has been reported as a potential trigger for OAML, and Ferreri et
colleagues firstly showed the efficacy of antibiotic treatment.[126] In
this work CP DNA was found in lymphoma samples from 32 of the 40 (80%)
case-patients analysed, thus revealing a strong association between
OAML and CP infection, also in light of the low seroprevalence in
general population studies varying between 0 and 49% (median:
5–10%).[128] Moreover, Ponzoni et al. reported the presence ofCP in
74% OAML specimens by different techniques such as
immunohistochemistry, immunofluorescence and laser-capture
microdissection-assisted PCR inside infiltrating
monocytes/macrophages.[129] Nevertheless, significant variability in CP
association with OAML has been reported in different geographical
areas, ranging from 47% in Germany to 35% in the East Coast of the USA,
29% in the Netherlands, 13% in Italy, 12% in UK and 11% in Southern
China,[130] while no evidence of CP infection was found in cases from
the South Florida[131] and Japan.[132] To sum up, the overall
prevalence of CP infection in
423 cases of OAML reported in 14 different papers is 19%.[128] In
conclusion, the possible role of methodological pitfalls and other
interpretation bias or confounding factors should be carefully
considered when interpreting the bacteria–lymphoma association, also
focussing on the potential role for C. psittaci infection in lymphomagenesis.[128]
Treatment.
OAML is a rare indolent lymphoma, for its treatment no consensus is
available because no prospective clinical trials have been conducted to
define the optimal treatment approach for these patients.[133] However,
patients managed by a watch & wait for approach have a 10-years OS
of 94%.[118] Moreover, limited toxicity and costs associated with
antibiotic treatment should suggest the opportunity to target the
possibly correlated infection by C. psittaci,
in analogy with gastric MALT lymphoma and Hp eradication. A single
antibiotic course of oral doxycycline at a dose of 100 mg, given twice
a day, for 3 weeks is the most popular regimen. In the first
prospective trial of doxycycline, 20 of the 27 patients were
progression free at 2 years; interestingly also 6 CP DNA–negative
patients of the 16 treated experienced lymphoma regression.[134] A
subsequent international prospective phase II trial was performed by
IELSG: the prevalence of Cp positivity in OAML was 89%; in these naïve
patients, after CP eradication, lymphoma regressed with an ORR of 65%,
with 6 complete and 16 partial responses.[135] A larger Korean
prospective trial, enrolling 90 patients, showed an ORR of 27%.[136]
Actually, a new prospective study (IELSG 39) is enrolling patients (http://www.ielsg.org/trialsonfr.html).
On the contrary, another retrospective study did not show any
response,[137] even if the short median follow-up of 9 months could
have hampered the proper assessment of response. Altogether, in 9
studies identified in the literature by Kiesewetter and Raderer, 131
patients were treated with doxycycline resulting in an ORR of 45%, with
CR achieved in 23 patients (18%) and PR achieved by 36 pts. (27%).[138]
An interesting Italian work by Govi et al. showed the efficacy of
administration of 500 mg clarithromycin, twice a day, for 6-months, in
relapsed/refractory EMZL after treatment with doxycycline.[139] Over
the anti-bacterial effect on unidentified pathogens, clarithromycin
could exert a direct anti-proliferating effect on OAML. Patients who
fail to respond to doxycycline therapy can be successfully salvaged
with chemotherapy and/or radiotherapy.
Anti-Lymphoma Directed Treatment.
Standard treatment is based on surgical resection of the single lesion.
Radiotherapy is known as a treatment modality with a high local control
rate for primary OAML.[140,141] However, orbital irradiation can induce
complications such as cataracts, keratitis, dry eye syndrome, and
retinopathy.[142] Even if no universally accepted radiation schedule is
available, National Cancer Center Network guidelines recommend
radiotherapy of 20 to 30 Gy for initial treatment of early-stage
non-gastric MZL of all sites and reirradiation for locally recurrent
disease.
Only limited data on chemotherapy for patients with OAML
suggest different association and schedule. The oral agent chlorambucil
is the most frequently used chemotherapy agent and has an extremely
favourable toxicity. Complete responses are observed in 67% to 100% of
patients; however, long-term outcome data suggest that local recurrence
occurs in up to 29% of patients.[143-146] Only a few cases of OAML have
been treated with rituximab as single-agent, demonstrating high
activity in both newly diagnosed and relapsed disease, although early
recurrence is common.[147-150] Also, the association of rituximab and
chlorambucil has been tested with encouraging results.[151] CNS
prophylaxis is not recommended since OAML rarely recurs to CNS.[152]
Role of Borrelia Burgdorferi in Primary Cutaneous B-cell Lymphomas
MZL of the skin has an incidence rate of 1.1/1,000,000 person-years[6] and is predominant among males across all ages. B. burgdorferi (Bb)
infection has been associated with skin MZL in some cases in Europe,
but not in the U.S., Asia and some parts of Europe, thereby challenging
the aetiological role of this agent.[153,154] In particular, in Bb
endemic areas such as the Scottish Highlands[155] or Austria,[156]
cutaneous MZL patients have demonstrated Borrelia infection in up to
40% cases, while no association was detected in two Italian case
series.[157,158] However, in a nonendemic region like France, Bb DNA
was found in 19% of 16 cases with primary cutaneous MALT lymphoma.[159]
Bb
infection might be associated with chronic antigen-driven
lymphomagenesis in the skin, which is the port of entry of this
gram-negative spirochete, through a bite from Ixodid tick and is also
the infectious agent of Lyme borreliosis.[5,160] Moreover, in late Lyme
borreliosis, lymphocytes may infiltrate the dermis and produce the
characteristic borrelia “lymphocytoma”, a cutaneous B-cell
pseudolymphoma characterized by ‘top-heavy’, mixed-cell lymphoid
infiltrate, usually accompanied by the formation of lymphoid follicles
with germinal centres.[161] Lyme disease and primary cutaneous lymphoma
may represent a continuous spectrum of pathological states viewed as a
multistep progression from lymphocytoma to “pseudolymphoma” eventually
leading to primary cutaneous B-cell Lymphoma, where evidence of B-cell
monoconality may help distinguish between the different stages of the
disease.[5]
Discordant data exist about cutaneous MZL recession
after antibiotic treatment of Bb infection (generally consisting of
cephalosporins +/- tetracyclines) and are based on case reports.[138]
In conclusion, Bb and its association with cutaneous MZL are currently
the object of investigation, even if an antibiotic treatment may be
attempted given the indolent nature of the disease.[162]
Concluding Remarks
Marginal zone lymphoma is a
fascinating clinical setting in which it has been clearly shown in
several trials (see Table 1) that several therapies targeting the
putative oncogenic infectious agent can induce steady lymphoma
regression. It represents the proof of concept that a chronic stimulus
on the immune system induced by an infectious agent, under particular
host predisposition, may lead to the selection of abnormal B-clones and
a more selection may result in overt lymphoma development. For gastric
and the ocular adnexa MALT lymphomas the compelling evidence, to date,
provides a rationale to implement actively antibiotics regimens that
can be an effective first-line treatment due to the peculiar indolent
course of the disease and the high therapeutic index of these drugs.
Indeed, international guidelines state that “Helicobacter pylori
eradication therapy must be given to all gastric MALT lymphomas,
independently of stage”. Conversely, in OAML and MZL of the skin
antibiotic treatment remains investigational, given the questionable
results of the former and the paucity of data for the latter clinical
setting.
With the recent availability of new direct-acting
antiviral agents for HCV infection, that promise a rapid and sustained
virological response, there is increasing interest in their employment
to treat HCV-related SMZL and NMZL; however, further studies are needed
to assess this strategy. In conclusion, we hope new evidence can
improve our understanding of the pathogenesis of lymphoma mediated by
antigen-dependent infectious agents, thus enabling the availability of
other alternative and efficacious anti-lymphoma treatments.
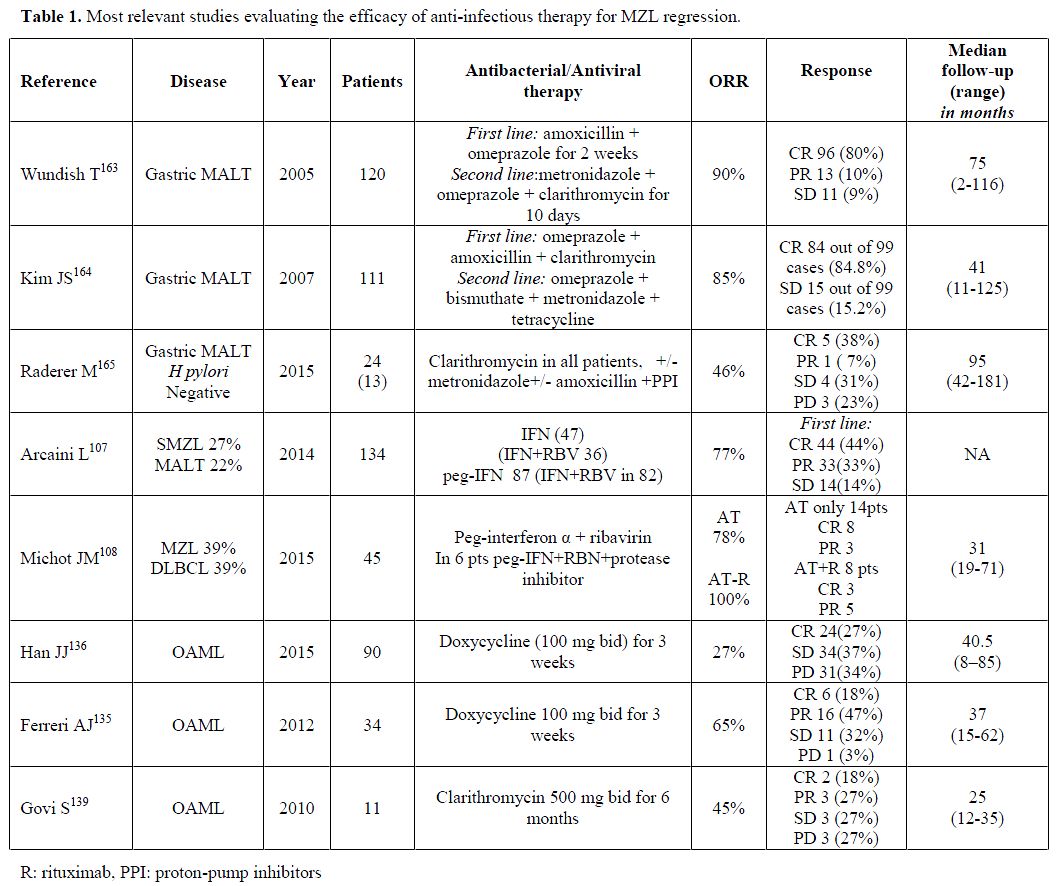 |
Table 1. Most relevant studies evaluating the efficacy of anti-infectious therapy for MZL regression. |
References
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri
SA, Stein H, et al. WHO classification of tumours of haematopoietic and
lymphoid tissues. 4th ed. Lyon, France: International Agency for
Research on Cancer; 2008.
- Smith A, Crouch
S, Lax S, Li J, Painter D, Howell D, et al. Lymphoma incidence,
survival and prevalence 2004-2014: sub-type analyses from the UK's
Haematological Malignancy Research Network. Br J Cancer.
2015;112(9):1575-84. http://dx.doi.org/10.1038/bjc.2015.94 PMid:25867256 PMCid:PMC4453686

- Morton
LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma
incidence patterns by WHO subtype in the United States, 1992-2001.
Blood. 2006;107(1):265-76. http://dx.doi.org/10.1182/blood-2005-06-2508 PMid:16150940 PMCid:PMC1895348

- Wohrer
S, Troch M, Streubel B, Zwerina J, Skrabs C, Formanek M, et al. MALT
lymphoma in patients with autoimmune diseases: a comparative analysis
of characteristics and clinical course. Leukemia. 2007;21(8):1812-8. http://dx.doi.org/10.1038/sj.leu.2404782 PMid:17554381

- Suarez
F, Lortholary O, Hermine O, Lecuit M. Infection-associated lymphomas
derived from marginal zone B cells: a model of antigen-driven
lymphoproliferation. Blood. 2006;107(8):3034-44. http://dx.doi.org/10.1182/blood-2005-09-3679 PMid:16397126

- Khalil
MO, Morton LM, Devesa SS, Check DP, Curtis RE, Weisenburger DD, et al.
Incidence of marginal zone lymphoma in the United States, 2001-2009
with a focus on primary anatomic site. Br J Haematol.
2014;165(1):67-77. http://dx.doi.org/10.1111/bjh.12730 PMid:24417667 PMCid:PMC3967856

- Weber
DM, Dimopoulos MA, Anandu DP, Pugh WC, Steinbach G. Regression of
gastric lymphoma of mucosa-associated lymphoid tissue with antibiotic
therapy for Helicobacter pylori. Gastroenterology. 1994;107(6):1835-8.
PMid:7958698

- Zullo
A, Hassan C, Cristofari F, Andriani A, De Francesco V, Ierardi E, et
al. Effects of Helicobacter pylori eradication on early stage gastric
mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol.
2010;8(2):105-10. http://dx.doi.org/10.1016/j.cgh.2009.07.017 PMid:19631287

- Gisbert
JP, Calvet X. Review article: common misconceptions in the management
of Helicobacter pylori-associated gastric MALT-lymphoma. Aliment
Pharmacol Ther. 2011;34(9):1047-62. http://dx.doi.org/10.1111/j.1365-2036.2011.04839.x PMid:21919927

- Nakamura
S, Sugiyama T, Matsumoto T, Iijima K, Ono S, Tajika M, et al. Long-term
clinical outcome of gastric MALT lymphoma after eradication of
Helicobacter pylori: a multicentre cohort follow-up study of 420
patients in Japan. Gut. 2012;61(4):507-13. http://dx.doi.org/10.1136/gutjnl-2011-300495 PMid:21890816

- Psyrri
A, Papageorgiou S, Economopoulos T. Primary extranodal lymphomas of
stomach: clinical presentation, diagnostic pitfalls and management. Ann
Oncol. 2008;19(12):1992-9. http://dx.doi.org/10.1093/annonc/mdn525 PMid:18647965 PMCid:PMC2733120

- Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4(8):644-53. http://dx.doi.org/10.1038/nrc1409 PMid:15286744

- Zucca
E, Copie-Bergman C, Ricardi U, Thieblemont C, Raderer M, Ladetto M.
Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24
Suppl 6:vi144-8. http://dx.doi.org/10.1093/annonc/mdt343 PMid:24078657

- Isaacson
P, Chott A, Nakamura S, Muller-Hermelink H, Harris N, Swerdlow S.
Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid
tissue (MALT lymphoma). In: Press I, editor. WHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. 4 th ed. Lyon, France
2008. p. 214-7.
- Dreyling M, Thieblemont
C, Gallamini A, Arcaini L, Campo E, Hermine O, et al. ESMO Consensus
conferences: guidelines on malignant lymphoma. part 2: marginal zone
lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann Oncol.
2013;24(4):857-77. http://dx.doi.org/10.1093/annonc/mds643 PMid:23425945

- Marshall
BJ, Warren JR. Unidentified curved bacilli in the stomach of patients
with gastritis and peptic ulceration. Lancet. 1984;1(8390):1311-5. http://dx.doi.org/10.1016/S0140-6736(84)91816-6

- Parsonnet
J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et
al. Helicobacter pylori infection and the risk of gastric carcinoma. N
Engl J Med. 1991;325(16):1127-31. http://dx.doi.org/10.1056/NEJM199110173251603 PMid:1891020

- Mégraud
F, Lehours P. Helicobacter pylori detection and antimicrobial
susceptibility testing. Clin Microbiol Rev. 2007;20(2):280-322. http://dx.doi.org/10.1128/CMR.00033-06 PMid:17428887 PMCid:PMC1865594

- Morgner
A, Schmelz R, Thiede C, Stolte M, Miehlke S. Therapy of gastric mucosa
associated lymphoid tissue lymphoma. World J Gastroenterol.
2007;13(26):3554-66. http://dx.doi.org/10.3748/wjg.v13.i26.3554 PMid:17659705 PMCid:PMC4146794

- Xu
W, Zhou C, Zhang G, Wang H, Wang L, Guo J. Repeating gastric biopsy for
accuracy of gastric lymphoma diagnosis. Gastroenterol Nurs.
2010;33(4):313-7. http://dx.doi.org/10.1097/SGA.0b013e3181ea9035 PMid:20679784

- Eck
M, Greiner A, Schmausser B, Eck H, Kolve M, Fischbach W, et al.
Evaluation of Helicobacter pylori in gastric MALT-type lymphoma:
differences between histologic and serologic diagnosis. Mod Pathol.
1999;12(12):1148-51. PMid:10619268

- Eck
M, Schmausser B, Greiner A, Muller-Hermelink HK. Helicobacter pylori in
gastric mucosa-associated lymphoid tissue type lymphoma. Recent Results
Cancer Res. 2000;156:9-18. http://dx.doi.org/10.1007/978-3-642-57054-4_2 PMid:10802858

- Blaser
MJ. Helicobacters are indigenous to the human stomach: duodenal
ulceration is due to changes in gastric microecology in the modern era.
Gut. 1998;43(5):721-7. http://dx.doi.org/10.1136/gut.43.5.721 PMid:9824358 PMCid:PMC1727310

- Ghose
C, Perez-Perez GI, Dominguez-Bello MG, Pride DT, Bravi CM, Blaser MJ.
East Asian genotypes of Helicobacter pylori strains in Amerindians
provide evidence for its ancient human carriage. Proc Natl Acad Sci U S
A. 2002;99(23):15107-11. http://dx.doi.org/10.1073/pnas.242574599 PMid:12417749 PMCid:PMC137551

- Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113(3):321. http://dx.doi.org/10.1172/JCI20925 PMid:14755326 PMCid:PMC324548

- Kennemann
L, Didelot X, Aebischer T, Kuhn S, Drescher B, Droege M, et al.
Helicobacter pylori genome evolution during human infection.
Proceedings of the National Academy of Sciences. 2011;108(12):5033-8. http://dx.doi.org/10.1073/pnas.1018444108 PMid:21383187 PMCid:PMC3064335

- Björkholm
B, Sjölund M, Falk PG, Berg OG, Engstrand L, Andersson DI. Mutation
frequency and biological cost of antibiotic resistance in Helicobacter
pylori. Proceedings of the National Academy of Sciences.
2001;98(25):14607-12. http://dx.doi.org/10.1073/pnas.241517298 PMid:11717398 PMCid:PMC64729

- Suerbaum
S, Josenhans C. Helicobacter pylori evolution and phenotypic
diversification in a changing host. Nature reviews microbiology.
2007;5(6):441-52. http://dx.doi.org/10.1038/nrmicro1658 PMid:17505524

- Schistosomes,
liver flukes and Helicobacter pylori. IARC Working Group on the
Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC
Monogr Eval Carcinog Risks Hum. 1994;61:1-241. PMid:7715068

- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030-44.http://dx.doi.org/10.1002/ijc.21731 PMid:16404738

- Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347(15):1175-86. http://dx.doi.org/10.1056/NEJMra020542 PMid:12374879

- Uemura
N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al.
Helicobacter pylori infection and the development of gastric cancer. N
Engl J Med. 2001;345(11):784-9. http://dx.doi.org/10.1056/NEJMoa001999 PMid:11556297

- Holcombe C. Helicobacter pylori: the African enigma. Gut. 1992;33(4):429-31. http://dx.doi.org/10.1136/gut.33.4.429 PMid:1582581 PMCid:PMC1374052

- Doglioni
C, Wotherspoon AC, Moschini A, de Boni M, Isaacson PG. High incidence
of primary gastric lymphoma in northeastern Italy. Lancet.
1992;339(8797):834-5. http://dx.doi.org/10.1016/0140-6736(92)90280-G

- Linz
B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, et al. An
African origin for the intimate association between humans and
Helicobacter pylori. Nature. 2007;445(7130):915-8. http://dx.doi.org/10.1038/nature05562 PMid:17287725 PMCid:PMC1847463

- Datta
De D, Roychoudhury S. To be or not to be: The host genetic factor and
beyond in Helicobacter pylori mediated gastro-duodenal diseases. World
J Gastroenterol. 2015;21(10):2883-95. http://dx.doi.org/10.3748/wjg.v21.i10.2883 PMid:25780285 PMCid:PMC4356907

- Cover
TL, Dooley CP, Blaser MJ. Characterization of and human serologic
response to proteins in Helicobacter pylori broth culture supernatants
with vacuolizing cytotoxin activity. Infect Immun. 1990;58(3):603-10.
PMid:2307514 PMCid:PMC258508

- Crabtree
JE, Taylor JD, Wyatt JI, Heatley RV, Shallcross TM, Tompkins DS, et al.
Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic
ulceration, and gastric pathology. Lancet. 1991;338(8763):332-5. http://dx.doi.org/10.1016/0140-6736(91)90477-7

- Blaser
MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, et al.
Infection with Helicobacter pylori strains possessing cagA is
associated with an increased risk of developing adenocarcinoma of the
stomach. Cancer Res. 1995;55(10):2111-5. PMid:7743510

- Odenbreit
S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of
Helicobacter pylori CagA into gastric epithelial cells by type IV
secretion. Science. 2000;287(5457):1497-500. http://dx.doi.org/10.1126/science.287.5457.1497 PMid:10688800

- Yamazaki
S, Yamakawa A, Ito Y, Ohtani M, Higashi H, Hatakeyama M, et al. The
CagA protein of Helicobacter pylori is translocated into epithelial
cells and binds to SHP-2 in human gastric mucosa. J Infect Dis.
2003;187(2):334-7. http://dx.doi.org/10.1086/367807 PMid:12552462

- Mimuro
H, Suzuki T, Tanaka J, Asahi M, Haas R, Sasakawa C. Grb2 is a key
mediator of helicobacter pylori CagA protein activities. Mol Cell.
2002;10(4):745-55. http://dx.doi.org/10.1016/S1097-2765(02)00681-0

- Tsutsumi
R, Higashi H, Higuchi M, Okada M, Hatakeyama M. Attenuation of
Helicobacter pylori CagA x SHP-2 signaling by interaction between CagA
and C-terminal Src kinase. J Biol Chem. 2003;278(6):3664-70. http://dx.doi.org/10.1074/jbc.M208155200 PMid:12446738

- Lin
WC, Tsai HF, Kuo SH, Wu MS, Lin CW, Hsu PI, et al. Translocation of
Helicobacter pylori CagA into Human B lymphocytes, the origin of
mucosa-associated lymphoid tissue lymphoma. Cancer Res.
2010;70(14):5740-8. http://dx.doi.org/10.1158/0008-5472.CAN-09-4690 PMid:20587516

- Kuo
SH, Chen LT, Lin CW, Wu MS, Hsu PN, Tsai HJ, et al. Detection of the
Helicobacter pylori CagA protein in gastric mucosa-associated lymphoid
tissue lymphoma cells: clinical and biological significance. Blood
Cancer Journal. 2013;3(7):e125. http://dx.doi.org/10.1038/bcj.2013.22 PMid:23852160 PMCid:PMC3730200

- de Bernard M, Josenhans C. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2014;19 Suppl 1:11-8. http://dx.doi.org/10.1111/hel.12160 PMid:25167940

- Tombola
F, Morbiato L, Del Giudice G, Rappuoli R, Zoratti M, Papini E. The
Helicobacter pylori VacA toxin is a urea permease that promotes urea
diffusion across epithelia. J Clin Invest. 2001;108(6):929-37. http://dx.doi.org/10.1172/JCI13045 PMid:11560962 PMCid:PMC200932

- Zheng
PY, Jones NL. Helicobacter pylori strains expressing the vacuolating
cytotoxin interrupt phagosome maturation in macrophages by recruiting
and retaining TACO (coronin 1) protein. Cell Microbiol.
2003;5(1):25-40. http://dx.doi.org/10.1046/j.1462-5822.2003.00250.x

- Molinari
M, Salio M, Galli C, Norais N, Rappuoli R, Lanzavecchia A, et al.
Selective inhibition of Ii-dependent antigen presentation by
Helicobacter pylori toxin VacA. J Exp Med. 1998;187(1):135-40. http://dx.doi.org/10.1084/jem.187.1.135 PMid:9419220 PMCid:PMC2199184

- Gebert
B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori
vacuolating cytotoxin inhibits T lymphocyte activation. Science.
2003;301(5636):1099-102. http://dx.doi.org/10.1126/science.1086871 PMid:12934009

- Bamford
KB, Fan X, Crowe SE, Leary JF, Gourley WK, Luthra GK, et al.
Lymphocytes in the human gastric mucosa during Helicobacter pylori have
a T helper cell 1 phenotype. Gastroenterology. 1998;114(3):482-92. http://dx.doi.org/10.1016/S0016-5085(98)70531-1

- Craig
VJ, Cogliatti SB, Arnold I, Gerke C, Balandat JE, Wundisch T, et al.
B-cell receptor signaling and CD40 ligand-independent T cell help
cooperate in Helicobacter-induced MALT lymphomagenesis. Leukemia.
2010;24(6):1186-96. http://dx.doi.org/10.1038/leu.2010.76 PMid:20428202

- Du MQ. MALT lymphoma: many roads lead to nuclear factor-kappab activation. Histopathology. 2011;58(1):26-38. http://dx.doi.org/10.1111/j.1365-2559.2010.03699.x PMid:21261681

- Rollinson
S, Levene AP, Mensah FK, Roddam PL, Allan JM, Diss TC, et al. Gastric
marginal zone lymphoma is associated with polymorphisms in genes
involved in inflammatory response and antioxidative capacity. Blood.
2003;102(3):1007-11. http://dx.doi.org/10.1182/blood-2002-12-3803 PMid:12676777

- Reimer
P, Fischbach W, Goebeler ME, Kraus MR, Goldmann S, Muller C, et al.
Decreased frequency of HLA-B35 in patients with gastric MALT lymphoma.
Ann Hematol. 2004;83(4):232-6. http://dx.doi.org/10.1007/s00277-003-0809-8 PMid:14634793

- Wotherspoon
AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, et al.
Regression of primary low-grade B-cell gastric lymphoma of
mucosa-associated lymphoid tissue type after eradication of
Helicobacter pylori. Lancet. 1993;342(8871):575-7. http://dx.doi.org/10.1016/0140-6736(93)91409-F

- Akagi
T, Motegi M, Tamura A, Suzuki R, Hosokawa Y, Suzuki H, et al. A novel
gene, MALT1 at 18q21, is involved in t(11;18) (q21;q21) found in
low-grade B-cell lymphoma of mucosa-associated lymphoid tissue.
Oncogene. 1999;18(42):5785-94. http://dx.doi.org/10.1038/sj.onc.1203018 PMid:10523859

- Dierlamm
J, Baens M, Wlodarska I, Stefanova-Ouzounova M, Hernandez JM, Hossfeld
DK, et al. The apoptosis inhibitor gene API2 and a novel 18q gene, MLT,
are recurrently rearranged in the t(11;18)(q21;q21) associated with
mucosa-associated lymphoid tissue lymphomas. Blood. 1999;93(11):3601-9.
PMid:10339464

- Liu
H, Ye H, Dogan A, Ranaldi R, Hamoudi RA, Bearzi I, et al.
T(11;18)(q21;q21) is associated with advanced mucosa-associated
lymphoid tissue lymphoma that expresses nuclear BCL10. Blood.
2001;98(4):1182-7. http://dx.doi.org/10.1182/blood.V98.4.1182 PMid:11493468

- Nakamura
S, Ye H, Bacon CM, Goatly A, Liu H, Banham AH, et al. Clinical impact
of genetic aberrations in gastric MALT lymphoma: a comprehensive
analysis using interphase fluorescence in situ hybridisation. Gut.
2007;56(10):1358-63. http://dx.doi.org/10.1136/gut.2007.123729 PMid:17525089 PMCid:PMC2000261

- Liu
H, Ye H, Ruskone-Fourmestraux A, De Jong D, Pileri S, Thiede C, et al.
T(11;18) is a marker for all stage gastric MALT lymphomas that will not
respond to H. pylori eradication. Gastroenterology.
2002;122(5):1286-94. http://dx.doi.org/10.1053/gast.2002.33047 PMid:11984515

- Du
MQ, Atherton JC. Molecular subtyping of gastric MALT lymphomas:
implications for prognosis and management. Gut. 2006;55(6):886-93. http://dx.doi.org/10.1136/gut.2004.061663 PMid:16698756 PMCid:PMC1856234

- Streubel
B, Simonitsch-Klupp I, Mullauer L, Lamprecht A, Huber D, Siebert R, et
al. Variable frequencies of MALT lymphoma-associated genetic
aberrations in MALT lymphomas of different sites. Leukemia.
2004;18(10):1722-6. http://dx.doi.org/10.1038/sj.leu.2403501 PMid:15356642

- Park
HS, Kim YJ, Yang WI, Suh CO, Lee YC. Treatment outcome of localized
Helicobacter pylori-negative low-grade gastric MALT lymphoma. World J
Gastroenterol. 2010;16(17):2158-62. http://dx.doi.org/10.3748/wjg.v16.i17.2158 PMid:20440857 PMCid:PMC2864842

- Zullo
A, Hassan C, Ridola L, De Francesco V, Rossi L, Tomao S, et al.
Eradication therapy in Helicobacter pylori-negative, gastric low-grade
mucosa-associated lymphoid tissue lymphoma patients: a systematic
review. J Clin Gastroenterol. 2013;47(10):824-7. http://dx.doi.org/10.1097/MCG.0b013e318286ff72 PMid:23442842

- Ruskone-Fourmestraux
A, Fischbach W, Aleman BM, Boot H, Du MQ, Megraud F, et al. EGILS
consensus report. Gastric extranodal marginal zone B-cell lymphoma of
MALT. Gut. 2011;60(6):747-58. http://dx.doi.org/10.1136/gut.2010.224949 PMid:21317175

- Fuccio
L, Laterza L, Zagari RM, Cennamo V, Grilli D, Bazzoli F. Treatment of
Helicobacter pylori infection. BMJ. 2008;337:a1454. http://dx.doi.org/10.1136/bmj.a1454 PMid:18794181

- Malfertheiner
P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, et al.
Management of Helicobacter pylori infection—the Maastricht IV/Florence
consensus report. Gut. 2012;61(5):646-64. http://dx.doi.org/10.1136/gutjnl-2012-302084 PMid:22491499

- Chey
WD, Wong BC. American College of Gastroenterology guideline on the
management of Helicobacter pylori infection. Am J Gastroenterol.
2007;102(8):1808-25. http://dx.doi.org/10.1111/j.1572-0241.2007.01393.x PMid:17608775

- Megraud
F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, et al.
Helicobacter pylori resistance to antibiotics in Europe and its
relationship to antibiotic consumption. Gut. 2013;62(1):34-42. http://dx.doi.org/10.1136/gutjnl-2012-302254 PMid:22580412

- Borody
TJ, Andrews P, Fracchia G, Brandl S, Shortis NP, Bae H. Omeprazole
enhances efficacy of triple therapy in eradicating Helicobacter pylori.
Gut. 1995;37(4):477-81. http://dx.doi.org/10.1136/gut.37.4.477 PMid:7489931 PMCid:PMC1382896

- Bazzoli
F, Zagari RM, Fossi S, Pozzato P, Alampi G, Simoni P, et al. Short-term
low-dose triple therapy for the eradication of Helicobacter pylori. Eur
J Gastroenterol Hepatol. 1994;6(9):773-8. http://dx.doi.org/10.1097/00042737-199409000-00004

- Gisbert
JP, Calvet X, O'Connor A, Megraud F, O'Morain CA. Sequential therapy
for Helicobacter pylori eradication: a critical review. J Clin
Gastroenterol. 2010;44(5):313-25. http://dx.doi.org/10.1097/mcg.0b013e3181c8a1a3

- de
Boer WA, Driessen WM, Potters VP, Tytgat GN. Randomized study comparing
1 with 2 weeks of quadruple therapy for eradicating Helicobacter
pylori. Am J Gastroenterol. 1994;89(11):1993-7. PMid:7942724

- Malfertheiner
P, Bazzoli F, Delchier JC, Celinski K, Giguere M, Riviere M, et al.
Helicobacter pylori eradication with a capsule containing bismuth
subcitrate potassium, metronidazole, and tetracycline given with
omeprazole versus clarithromycin-based triple therapy: a randomised,
open-label, non-inferiority, phase 3 trial. Lancet.
2011;377(9769):905-13. http://dx.doi.org/10.1016/S0140-6736(11)60020-2

- Gisbert
JP, Morena F. Systematic review and meta-analysis: levofloxacin-based
rescue regimens after Helicobacter pylori treatment failure. Aliment
Pharmacol Ther. 2006;23(1):35-44. http://dx.doi.org/10.1111/j.1365-2036.2006.02737.x PMid:16393278

- Saad
RJ, Schoenfeld P, Kim HM, Chey WD. Levofloxacin-based triple therapy
versus bismuth-based quadruple therapy for persistent Helicobacter
pylori infection: a meta-analysis. Am J Gastroenterol.
2006;101(3):488-96. http://dx.doi.org/10.1111/j.1572-0241.2006.00637.x PMid:16542284

- Wirth
A, Gospodarowicz M, Aleman BM, Bressel M, Ng A, Chao M, et al.
Long-term outcome for gastric marginal zone lymphoma treated with
radiotherapy: a retrospective, multi-centre, International Extranodal
Lymphoma Study Group study. Ann Oncol. 2013;24(5):1344-51. http://dx.doi.org/10.1093/annonc/mds623 PMid:23293112

- Ferri
C, Sebastiani M, Giuggioli D, Cazzato M, Longombardo G, Antonelli A, et
al. Mixed cryoglobulinemia: demographic, clinical, and serologic
features and survival in 231 patients. Semin Arthritis Rheum.
2004;33(6):355-74. http://dx.doi.org/10.1016/j.semarthrit.2003.10.001 PMid:15190522

- Libra
M, Polesel J, Russo AE, De Re V, Cina D, Serraino D, et al.
Extrahepatic disorders of HCV infection: a distinct entity of B-cell
neoplasia? Int J Oncol. 2010;36(6):1331-40. http://dx.doi.org/10.3892/ijo_00000618 PMid:20428756

- Saadoun
D, Suarez F, Lefrere F, Valensi F, Mariette X, Aouba A, et al. Splenic
lymphoma with villous lymphocytes, associated with type II
cryoglobulinemia and HCV infection: a new entity? Blood.
2005;105(1):74-6. http://dx.doi.org/10.1182/blood-2004-05-1711 PMid:15353484

- Arcaini
L, Burcheri S, Rossi A, Paulli M, Bruno R, Passamonti F, et al.
Prevalence of HCV infection in nongastric marginal zone B-cell lymphoma
of MALT. Ann Oncol. 2007;18(2):346-50. http://dx.doi.org/10.1093/annonc/mdl388 PMid:17071937

- Schmid C, Kirkham N, Diss T, Isaacson PG. Splenic marginal zone cell lymphoma. Am J Surg Pathol. 1992;16(5):455-66. http://dx.doi.org/10.1097/00000478-199205000-00004 PMid:1599024

- Matutes
E, Morilla R, Owusu-Ankomah K, Houlihan A, Catovsky D. The
immunophenotype of splenic lymphoma with villous lymphocytes and its
relevance to the differential diagnosis with other B-cell disorders.
Blood. 1994;83(6):1558-62. PMid:8123845

- Liu
L, Wang H, Chen Y, Rustveld L, Liu G, Du XL. Splenic marginal zone
lymphoma: a population-based study on the 2001-2008 incidence and
survival in the United States. Leuk Lymphoma. 2013;54(7):1380-6. http://dx.doi.org/10.3109/10428194.2012.743655 PMid:23101590

- Kraus MD, Fleming MD, Vonderheide RH. The spleen as a diagnostic specimen. Cancer. 2001;91(11):2001-9. http://dx.doi.org/10.1002/1097-0142(20010601)91:11<2001::AID-CNCR1225>3.0.CO;2-3

- Paulli
M, Arcaini L, Lucioni M, Boveri E, Capello D, Passamonti F, et al.
Subcutaneous 'lipoma-like' B-cell lymphoma associated with HCV
infection: a new presentation of primary extranodal marginal zone
B-cell lymphoma of MALT. Ann Oncol. 2010;21(6):1189-95. http://dx.doi.org/10.1093/annonc/mdp454 PMid:19858084

- Zignego
AL, Ferri C, Pileri SA, Caini P, Bianchi FB. Extrahepatic
manifestations of Hepatitis C Virus infection: a general overview and
guidelines for a clinical approach. Dig Liver Dis. 2007;39(1):2-17. http://dx.doi.org/10.1016/j.dld.2006.06.008 PMid:16884964

- Marcucci
F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology,
mechanisms of tumorigenesis, and therapeutic opportunities. Blood.
2011;117(6):1792-8. http://dx.doi.org/10.1182/blood-2010-06-275818 PMid:20959600

- Landau
DA, Saadoun D, Calabrese LH, Cacoub P. The pathophysiology of HCV
induced B-cell clonal disorders. Autoimmun Rev. 2007;6(8):581-7. http://dx.doi.org/10.1016/j.autrev.2007.03.010 PMid:17854753

- Lesniewski
R, Okasinski G, Carrick R, Van Sant C, Desai S, Johnson R, et al.
Antibody to hepatitis C virus second envelope (HCV-E2) glycoprotein: a
new marker of HCV infection closely associated with viremia. J Med
Virol. 1995;45(4):415-22. http://dx.doi.org/10.1002/jmv.1890450411 PMid:7545212

- Quinn
ER, Chan CH, Hadlock KG, Foung SK, Flint M, Levy S. The B-cell receptor
of a hepatitis C virus (HCV)-associated non-Hodgkin lymphoma binds the
viral E2 envelope protein, implicating HCV in lymphomagenesis. Blood.
2001;98(13):3745-9. http://dx.doi.org/10.1182/blood.V98.13.3745 PMid:11739181

- Chan
CH, Hadlock KG, Foung SK, Levy S. V(H)1-69 gene is preferentially used
by hepatitis C virus-associated B cell lymphomas and by normal B cells
responding to the E2 viral antigen. Blood. 2001;97(4):1023-6. http://dx.doi.org/10.1182/blood.V97.4.1023 PMid:11159532

- Inokuchi
M, Ito T, Uchikoshi M, Shimozuma Y, Morikawa K, Nozawa H, et al.
Infection of B cells with hepatitis C virus for the development of
lymphoproliferative disorders in patients with chronic hepatitis C. J
Med Virol. 2009;81(4):619-27. http://dx.doi.org/10.1002/jmv.21388 PMid:19235854

- Lanford
RE, Chavez D, Chisari FV, Sureau C. Lack of detection of
negative-strand hepatitis C virus RNA in peripheral blood mononuclear
cells and other extrahepatic tissues by the highly strand-specific rTth
reverse transcriptase PCR. J Virol. 1995;69(12):8079-83. PMid:7494326
PMCid:PMC189758

- Evans
MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, et al.
Claudin-1 is a hepatitis C virus co-receptor required for a late step
in entry. Nature. 2007;446(7137):801-5. http://dx.doi.org/10.1038/nature05654 PMid:17325668

- Rosa
D, Saletti G, De Gregorio E, Zorat F, Comar C, D'Oro U, et al.
Activation of naive B lymphocytes via CD81, a pathogenetic mechanism
for hepatitis C virus-associated B lymphocyte disorders. Proc Natl Acad
Sci U S A. 2005;102(51):18544-9. http://dx.doi.org/10.1073/pnas.0509402102 PMid:16339892 PMCid:PMC1310512

- Engels
EA, Chatterjee N, Cerhan JR, Davis S, Cozen W, Severson RK, et al.
Hepatitis C virus infection and non‐Hodgkin lymphoma: results of the
NCI‐SEER multi‐center case‐control study. Int J Cancer.
2004;111(1):76-80. http://dx.doi.org/10.1002/ijc.20021 PMid:15185346

- Ferri
C, Caracciolo F, Zignego AL, Civita LL, Monti M, Longombardo G, et al.
Hepatitis C virus infection in patients with non‐Hodgkin's lymphoma. Br
J Haematol. 1994;88(2):392-4. http://dx.doi.org/10.1111/j.1365-2141.1994.tb05036.x PMid:7803287

- Mazzaro
C, Zagonel V, Monfardini S, Tulissi P, Pussini E, Fanni M, et al.
Hepatitis C virus and non‐Hodgkin's lymphomas. Br J Haematol.
1996;94(3):544-50. http://dx.doi.org/10.1046/j.1365-2141.1996.6912313.x PMid:8790157

- Silvestri
F, Barillari G, Fanin R, Salmaso F, Pipan C, Falasca E, et al. Impact
of hepatitis C virus infection on clinical features, quality of life
and survival of patients with lymphoplasmacytoid lymphoma/immunocytoma.
Ann Oncol. 1998;9(5):499-504. http://dx.doi.org/10.1023/A:1008265804550 PMid:9653490

- Sansonno
D, De Vita S, Cornacchiulo V, Carbone A, Boiocchi M, Dammacco F.
Detection and distribution of hepatitis C virus-related proteins in
lymph nodes of patients with type II mixed cryoglobulinemia and
neoplastic or non-neoplastic lymphoproliferation. Blood.
1996;88(12):4638-45. PMid:8977256

- Mele
A, Pulsoni A, Bianco E, Musto P, Szklo A, Sanpaolo MG, et al. Hepatitis
C virus and B-cell non-Hodgkin lymphomas: an Italian multicenter
case-control study. Blood. 2003;102(3):996-9. http://dx.doi.org/10.1182/blood-2002-10-3230 PMid:12714514

- Bracci
PM, Benavente Y, Turner JJ, Paltiel O, Slager SL, Vajdic CM, et al.
Medical history, lifestyle, family history, and occupational risk
factors for marginal zone lymphoma: the InterLymph Non-Hodgkin Lymphoma
Subtypes Project. J Natl Cancer Inst Monogr. 2014;2014(48):52-65. http://dx.doi.org/10.1093/jncimonographs/lgu011 PMid:25174026 PMCid:PMC4207869

- Hermine
O, Lefrere F, Bronowicki JP, Mariette X, Jondeau K, Eclache-Saudreau V,
et al. Regression of splenic lymphoma with villous lymphocytes after
treatment of hepatitis C virus infection. N Engl J Med.
2002;347(2):89-94. http://dx.doi.org/10.1056/NEJMoa013376 PMid:12110736

- Vallisa
D, Bernuzzi P, Arcaini L, Sacchi S, Callea V, Marasca R, et al. Role of
anti-hepatitis C virus (HCV) treatment in HCV-related, low-grade,
B-cell, non-Hodgkin's lymphoma: a multicenter Italian experience. J
Clin Oncol. 2005;23(3):468-73. http://dx.doi.org/10.1200/JCO.2005.06.008 PMid:15659492

- Arcaini
L, Vallisa D, Rattotti S, Ferretti VV, Ferreri AJ, Bernuzzi P, et al.
Antiviral treatment in patients with indolent B-cell lymphomas
associated with HCV infection: a study of the Fondazione Italiana
Linfomi. Ann Oncol. 2014;25(7):1404-10. http://dx.doi.org/10.1093/annonc/mdu166 PMid:24799461

- Michot
JM, Canioni D, Driss H, Alric L, Cacoub P, Suarez F, et al. Antiviral
therapy is associated with a better survival in patients with hepatitis
C virus and B-cell non-Hodgkin lymphomas, ANRS HC-13 lympho-C study. Am
J Hematol. 2015;90(3):197-203. http://dx.doi.org/10.1002/ajh.23889 PMid:25417909

- Sultanik
P, Klotz C, Brault P, Pol S, Mallet V. Regression of an HCV-associated
disseminated marginal zone lymphoma under IFN-free antiviral treatment.
Blood. 2015;125(15):2446-7. http://dx.doi.org/10.1182/blood-2014-12-618652 PMid:25858892

- Cornberg M, Manns MP. New kids on the block--step by step to an ideal HCV therapy. Lancet. 2015;385(9973):1050-2. http://dx.doi.org/10.1016/S0140-6736(14)62008-0

- Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368(20):1907-17. http://dx.doi.org/10.1056/NEJMra1213651 PMid:23675659 PMCid:PMC3893124

- Florian
J, Mishra P, Arya V, Harrington P, Connelly S, Reynold KS, et al.
Direct-acting antiviral drugs for the treatment of chronic hepatitis c
virus infection: Interferon free is now. Clin Pharmacol Ther.
2015:n/a-n/a.

- Mannami
T, Yoshino T, Oshima K, Takase S, Kondo E, Ohara N, et al. Clinical,
Histopathological, and Immunogenetic Analysis of Ocular Adnexal
Lymphoproliferative Disorders: Characterization of MALT Lymphoma and
Reactive Lymphoid Hyperplasia. Mod Pathol. 0000;14(7):641-9. http://dx.doi.org/10.1038/modpathol.3880366 PMid:11454995

- Moslehi
R, Devesa SS, Schairer C, Fraumeni JF, Jr. Rapidly increasing incidence
of ocular non-hodgkin lymphoma. J Natl Cancer Inst. 2006;98(13):936-9. http://dx.doi.org/10.1093/jnci/djj248 PMid:16818858

- Sjo
LD, Ralfkiaer E, Prause JU, Petersen JH, Madsen J, Pedersen NT, et al.
Increasing incidence of ophthalmic lymphoma in Denmark from 1980 to
2005. Invest Ophthalmol Vis Sci. 2008;49(8):3283-8. http://dx.doi.org/10.1167/iovs.08-1768 PMid:18390644

- Collina
F, De Chiara A, De Renzo A, De Rosa G, Botti G, Franco R. Chlamydia
psittaci in ocular adnexa MALT lymphoma: a possible role in
lymphomagenesis and a different geographical distribution. Infect Agent
Cancer. 2012;7:8. http://dx.doi.org/10.1186/1750-9378-7-8 PMid:22472082 PMCid:PMC3355003

- Fung
CY, Tarbell NJ, Lucarelli MJ, Goldberg SI, Linggood RM, Harris NL, et
al. Ocular adnexal lymphoma: clinical behavior of distinct World Health
Organization classification subtypes. Int J Radiat Oncol Biol Phys.
2003;57(5):1382-91. http://dx.doi.org/10.1016/S0360-3016(03)00767-3

- Tanimoto
K, Kaneko A, Suzuki S, Sekiguchi N, Maruyama D, Kim SW, et al.
Long-term follow-up results of no initial therapy for ocular adnexal
MALT lymphoma. Ann Oncol. 2006;17(1):135-40. http://dx.doi.org/10.1093/annonc/mdj025 PMid:16236754

- Ferreri
AJ, Dolcetti R, Du MQ, Doglioni C, Resti AG, Politi LS, et al. Ocular
adnexal MALT lymphoma: an intriguing model for antigen-driven
lymphomagenesis and microbial-targeted therapy. Ann Oncol.
2008;19(5):835-46. http://dx.doi.org/10.1093/annonc/mdm513 PMid:17986622

- Tanimoto
K, Sekiguchi N, Yokota Y, Kaneko A, Watanabe T, Maeshima AM, et al.
Fluorescence in situ hybridization (FISH) analysis of primary ocular
adnexal MALT lymphoma. BMC Cancer. 2006;6:249. http://dx.doi.org/10.1186/1471-2407-6-249 PMid:17052360 PMCid:PMC1630703

- Ye
H, Gong L, Liu H, Hamoudi RA, Shirali S, Ho L, et al. MALT lymphoma
with t (14; 18)(q32; q21)/IGH‐MALT1 is characterized by strong
cytoplasmic MALT1 and BCL10 expression. The Journal of pathology.
2005;205(3):293-301. http://dx.doi.org/10.1002/path.1715 PMid:15682443

- Mannami
T, Yoshino T, Oshima K, Takase S, Kondo E, Ohara N, et al. Clinical,
histopathological, and immunogenetic analysis of ocular adnexal
lymphoproliferative disorders: characterization of malt lymphoma and
reactive lymphoid hyperplasia. Mod Pathol. 2001;14(7):641-9. http://dx.doi.org/10.1038/modpathol.3880366 PMid:11454995

- Coupland
SE, Foss HD, Anagnostopoulos I, Hummel M, Stein H. Immunoglobulin VH
gene expression among extranodal marginal zone B-cell lymphomas of the
ocular adnexa. Invest Ophthalmol Vis Sci. 1999;40(3):555-62.
PMid:10067957

- Ferreri
AJ, Viale E, Guidoboni M, Resti AG, De Conciliis C, Politi L, et al.
Clinical implications of hepatitis C virus infection in MALT-type
lymphoma of the ocular adnexa. Ann Oncol. 2006;17(5):769-72. http://dx.doi.org/10.1093/annonc/mdl027 PMid:16524978

- Goebel
N, Serr A, Mittelviefhaus H, Reinhard T, Bogdan C, Auw-Haedrich C.
Chlamydia psittaci, Helicobacter pylori and ocular adnexal lymphoma-is
there an association? The German experience. Leuk Res.
2007;31(10):1450-2. http://dx.doi.org/10.1016/j.leukres.2006.12.005 PMid:17257672

- Ferreri
AJ, Guidoboni M, Ponzoni M, De Conciliis C, Dell'Oro S, Fleischhauer K,
et al. Evidence for an association between Chlamydia psittaci and
ocular adnexal lymphomas. J Natl Cancer Inst. 2004;96(8):586-94. http://dx.doi.org/10.1093/jnci/djh102 PMid:15100336

- Knittler
MR, Sachse K. Chlamydia psittaci: update on an underestimated zoonotic
agent. Pathog Dis. 2015;73(1):1-15. PMid:25853998

- Decaudin
D, Dolcetti R, de Cremoux P, Ponzoni M, Vincent-Salomon A, Doglioni C,
et al. Variable association between Chlamydophila psittaci infection
and ocular adnexal lymphomas: methodological biases or true
geographical variations? Anticancer Drugs. 2008;19(8):761-5. http://dx.doi.org/10.1097/CAD.0b013e32830b58c4 PMid:18690086

- Ponzoni
M, Ferreri AJ, Guidoboni M, Lettini AA, Cangi MG, Pasini E, et al.
Chlamydia infection and lymphomas: association beyond ocular adnexal
lymphomas highlighted by multiple detection methods. Clin Cancer Res.
2008;14(18):5794-800. http://dx.doi.org/10.1158/1078-0432.CCR-08-0676 PMid:18794089

- Chanudet
E, Zhou Y, Bacon CM, Wotherspoon AC, Muller-Hermelink HK, Adam P, et
al. Chlamydia psittaci is variably associated with ocular adnexal MALT
lymphoma in different geographical regions. J Pathol.
2006;209(3):344-51. http://dx.doi.org/10.1002/path.1984 PMid:16583361

- Rosado
MF, Byrne GE, Ding F, Fields KA, Ruiz P, Dubovy SR, et al. Ocular
adnexal lymphoma: a clinicopathologic study of a large cohort of
patients with no evidence for an association with Chlamydia psittaci.
Blood. 2006;107(2):467-72. http://dx.doi.org/10.1182/blood-2005-06-2332 PMid:16166588 PMCid:PMC1895606

- Daibata
M, Nemoto Y, Togitani K, Fukushima A, Ueno H, Ouchi K, et al. Absence
of Chlamydia psittaci in ocular adnexal lymphoma from Japanese
patients. Br J Haematol. 2006;132(5):651-2. http://dx.doi.org/10.1111/j.1365-2141.2005.05943.x PMid:16445841

- Stefanovic A, Lossos IS. Extranodal marginal zone lymphoma of the ocular adnexa. Blood. 2009;114(3):501-10. http://dx.doi.org/10.1182/blood-2008-12-195453 PMid:19372259 PMCid:PMC2713468

- Ferreri
AJ, Ponzoni M, Guidoboni M, Resti AG, Politi LS, Cortelazzo S, et al.
Bacteria-eradicating therapy with doxycycline in ocular adnexal MALT
lymphoma: a multicenter prospective trial. J Natl Cancer Inst.
2006;98(19):1375-82. http://dx.doi.org/10.1093/jnci/djj373 PMid:17018784

- Ferreri
AJ, Govi S, Pasini E, Mappa S, Bertoni F, Zaja F, et al. Chlamydophila
psittaci eradication with doxycycline as first-line targeted therapy
for ocular adnexae lymphoma: final results of an international phase II
trial. J Clin Oncol. 2012;30(24):2988-94. http://dx.doi.org/10.1200/JCO.2011.41.4466 PMid:22802315

- Han
JJ, Kim TM, Jeon YK, Kim MK, Khwarg SI, Kim CW, et al. Long-term
outcomes of first-line treatment with doxycycline in patients with
previously untreated ocular adnexal marginal zone B cell lymphoma. Ann
Hematol. 2015;94(4):575-81. http://dx.doi.org/10.1007/s00277-014-2240-8 PMid:25338969

- Grunberger
B, Hauff W, Lukas J, Wohrer S, Zielinski CC, Streubel B, et al. 'Blind'
antibiotic treatment targeting Chlamydia is not effective in patients
with MALT lymphoma of the ocular adnexa. Ann Oncol. 2006;17(3):484-7. http://dx.doi.org/10.1093/annonc/mdj143 PMid:16500916

- Kiesewetter
B, Raderer M. Antibiotic therapy in nongastrointestinal MALT lymphoma:
a review of the literature. Blood. 2013;122(8):1350-7. http://dx.doi.org/10.1182/blood-2013-02-486522 PMid:23770778

- Govi
S, Dognini GP, Licata G, Crocchiolo R, Resti AG, Ponzoni M, et al.
Six-month oral clarithromycin regimen is safe and active in extranodal
marginal zone B-cell lymphomas: final results of a single-centre phase
II trial. Br J Haematol. 2010;150(2):226-9. http://dx.doi.org/10.1111/j.1365-2141.2010.08179.x

- De
Cicco L, Cella L, Liuzzi R, Solla R, Farella A, Punzo G, et al.
Radiation therapy in primary orbital lymphoma: a single institution
retrospective analysis. Radiation Oncology. 2009;4(1):60. http://dx.doi.org/10.1186/1748-717X-4-60 PMid:19968864 PMCid:PMC2794866

- Nam
H, Ahn Y, Kim Y, Ko Y, Kim W. Prognostic significance of anatomic
subsites: results of radiation therapy for 66 patients with localized
orbital marginal zone B cell lymphoma. Radiother Oncol. 2009;90:236 -
41. http://dx.doi.org/10.1016/j.radonc.2008.09.011 PMid:18950885

- Cho
WK, Lee SE, Paik JS, Cho SG, Yang SW. Risk potentiality of frontline
radiotherapy associated cataract in primary ocular adnexal
mucosa-associated lymphoid tissue lymphoma. Korean J Ophthalmol.
2013;27(4):243-8. http://dx.doi.org/10.3341/kjo.2013.27.4.243 PMid:23908569 PMCid:PMC3730065

- Charlotte
F, Doghmi K, Cassoux N, Ye H, Du M-Q, Kujas M, et al. Ocular adnexal
marginal zone B cell lymphoma: a clinical and pathologic study of 23
cases. Virchows Arch. 2006;448(4):506-16. http://dx.doi.org/10.1007/s00428-005-0122-0 PMid:16323006

- Ben
Simon GJ, Cheung N, McKelvie P, Fox R, McNab AA. Oral chlorambucil for
extranodal, marginal zone, B-cell lymphoma of mucosa-associated
lymphoid tissue of the orbit. Ophthalmology. 2006;113(7):1209-13. http://dx.doi.org/10.1016/j.ophtha.2006.01.057 PMid:16647129

- Song
E-K, Kim S-Y, Kim TM, Lee K-W, Yun T, Na I-I, et al. Efficacy of
chemotherapy as a first-line treatment in ocular adnexal extranodal
marginal zone B-cell lymphoma. Ann Oncol. 2008;19(2):242-6. http://dx.doi.org/10.1093/annonc/mdm457 PMid:17947227

- Tanimoto
K, Kaneko A, Suzuki S, Sekiguchi N, Watanabe T, Kobayashi Y, et al.
Primary Ocular Adnexal MALT Lymphoma: A Long-term Follow-up Study of
114 Patients. Jpn J Clin Oncol. 2007;37(5):337-44. http://dx.doi.org/10.1093/jjco/hym031 PMid:17562719

- Ferreri
A, Ponzoni M, Martinelli G, Muti G, Guidoboni M, Dolcetti R, et al.
Rituximab in patients with mucosal-associated lymphoid tissue-type
lymphoma of the ocular adnexa. Haematologica. 2005;90(11):1578-9.
PMid:16266908

- Benetatos
L, Alymara V, Asproudis I, Bourantas KL. Rituximab as first line
treatment for MALT lymphoma of extraocular muscles. Ann Hematol.
2006;85(9):625-6. http://dx.doi.org/10.1007/s00277- 006-0134-0 PMid:16691396

- Heinz
C, Merz H, Nieschalk M, Mueller-Miny H, Koch P, Heiligenhaus A.
Rituximab for the treatment of extranodal marginal zone B-cell lymphoma
of the lacrimal gland. Br J Ophthalmol. 2007;91(11):1563-4. http://dx.doi.org/10.1136/bjo.2007.115626 PMid:17947275 PMCid:PMC2095418

- Zinzani
PL, Alinari L, Stefoni V, Loffredo A, Pichierri P, Polito E. Rituximab
in primary conjunctiva lymphoma. Leuk Res. 2005;29(1):107-8. http://dx.doi.org/10.1016/j.leukres.2004.05.011 PMid:15541482

- Rigacci
L, Nassi L, Puccioni M, Mappa S, Polito E, Dal Pozzo S, et al.
Rituximab and chlorambucil as first-line treatment for low-grade ocular
adnexal lymphomas. Ann Hematol. 2007;86(8):565-8. http://dx.doi.org/10.1007/s00277-007-0301-y PMid:17483948

- Restrepo
A, Raez LE, Byrne GE, Jr., Johnson T, Ossi P, Benedetto P, et al. Is
central nervous system prophylaxis necessary in ocular adnexal
lymphoma? Crit Rev Oncog. 1998;9(3-4):269-73. PMid:10201631

- Willemze
R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC
classification for cutaneous lymphomas. Blood. 2005;105(10):3768-85. http://dx.doi.org/10.1182/blood-2004-09-3502 PMid:15692063

- Takino
H, Li C, Hu S, Kuo TT, Geissinger E, Muller-Hermelink HK, et al.
Primary cutaneous marginal zone B-cell lymphoma: a molecular and
clinicopathological study of cases from Asia, Germany, and the United
States. Mod Pathol. 2008;21(12):1517-26. http://dx.doi.org/10.1038/modpathol.2008.159 PMid:18820662

- Goodlad
JR, Davidson MM, Hollowood K, Ling C, MacKenzie C, Christie I, et al.
Primary cutaneous B-cell lymphoma and Borrelia burgdorferi infection in
patients from the Highlands of Scotland. Am J Surg Pathol.
2000;24(9):1279-85. http://dx.doi.org/10.1097/00000478-200009000-00012 PMid:10976703

- Cerroni
L, Zochling N, Putz B, Kerl H. Infection by Borrelia burgdorferi and
cutaneous B-cell lymphoma. J Cutan Pathol. 1997;24(8):457-61. http://dx.doi.org/10.1111/j.1600-0560.1997.tb01318.x PMid:9331890

- Ponzoni
M, Ferreri AJ, Mappa S, Pasini E, Govi S, Facchetti F, et al.
Prevalence of Borrelia burgdorferi infection in a series of 98 primary
cutaneous lymphomas. Oncologist. 2011;16(11):1582-8. http://dx.doi.org/10.1634/theoncologist.2011-0108 PMid:22071292 PMCid:PMC3233293

- Goteri
G, Ranaldi R, Simonetti O, Capretti R, Menzo S, Stramazzotti D, et al.
Clinicopathological features of primary cutaneous B-cell lymphomas from
an academic regional hospital in central Italy: No evidence of Borrelia
burgdorferi association. Leuk Lymphoma. 2007;48(11):2184-8. http://dx.doi.org/10.1080/10428190701618250 PMid:17926178

- de
la Fouchardiere A, Vandenesch F, Berger F. Borrelia-associated primary
cutaneous MALT lymphoma in a nonendemic region. Am J Surg Pathol.
2003;27(5):702-3. http://dx.doi.org/10.1097/00000478-200305000-00017 PMid:12717258

- Ferreri AJ, Govi S, Ponzoni M. Marginal zone lymphomas and infectious agents. Semin Cancer Biol. 2013;23(6):431-40. http://dx.doi.org/10.1016/j.semcancer.2013.09.004 PMid:24090976

- Colli
C, Leinweber B, Müllegger R, Chott A, Kerl H, Cerroni L. Borrelia
burgdorferi-associated lymphocytoma cutis: clinicopathologic,
immunophenotypic, and molecular study of 106 cases. J Cutan Pathol.
2004;31(3):232-40. http://dx.doi.org/10.1111/j.0303-6987.2003.00167.x PMid:14984575

- Zenahlik
P, Fink-Puches R, Kapp KS, Kerl H, Cerroni L. [Therapy of primary
cutaneous B-cell lymphomas]. Hautarzt. 2000;51(1):19-24. http://dx.doi.org/10.1007/s001050050005 PMid:10663035

- Wundisch
T, Thiede C, Morgner A, Dempfle A, Gunther A, Liu H, et al. Long-term
follow-up of gastric MALT lymphoma after Helicobacter pylori
eradication. J Clin Oncol. 2005;23(31):8018-24. http://dx.doi.org/10.1200/JCO.2005.02.3903 PMid:16204012

- Kim
JS, Chung SJ, Choi YS, Cheon JH, Kim CW, Kim SG, et al. Helicobacter
pylori eradication for low-grade gastric mucosa-associated lymphoid
tissue lymphoma is more successful in inducing remission in distal
compared to proximal disease. Br J Cancer. 2007;96(9):1324-8. http://dx.doi.org/10.1038/sj.bjc.6603708

- Raderer
M, Wohrer S, Kiesewetter B, Dolak W, Lagler H, Wotherspoon A, et al.
Antibiotic treatment as sole management of Helicobacter pylori-negative
gastric MALT lymphoma: a single center experience with prolonged
follow-up. Ann Hematol. 2015;94(6):969-73. http://dx.doi.org/10.1007/s00277-014-2298-3 PMid:25579756





































































































































































