Received: June 1, 2016
Accepted: June 15, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016033, DOI 10.4084/MJHID.2016.033
This article is available on PDF format at:


| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Background: Bone is the most
common organ of involvement in patients with Langerhans cell
histiocytosis (LCH), which is often painful and associated with
significant morbidity from pathological fractures. Current first-line
treatments include chemotherapy and steroids that are effective but
often associated with adverse effects, whereas the disease may
reactivate despite an initial response to first-line agents.
Bisphosphonates are osteoclast inhibitors that have shown to be helpful
in treating bone lesions of LCH. To date, there are no large
international studies to describe their role in treating bone lesions
of LCH. Method: We conducted a multicenter retrospective review of 13 patients with histologically proven LCH, who had received bisphosphonates either at diagnosis or at disease reactivation. Results: Ten patients (77%) had a single system bone disease and 3 (23%) had bone lesions as part of multisystem disease. Median follow-up time post-bisphosphonate therapy was 4.6 years (range, 0.8 to 8.2 years). Treatment with bisphosphonates was associated with significant pain relief in almost all patients. Twelve (92%) achieved resolution of active bone lesions, and 10 out of them had no active disease for a median of 3.5 years (range, 0.8 to 5 years). One patient did not respond. No major adverse effects were reported in this series. Conclusion: Bisphosphonates are well-tolerated drugs that can significantly improve bone pain and induce remission in active bone LCH. Future prospective studies evaluating the role of bisphosphonates in LCH are warranted. |
Introduction
Langerhans cell histiocytosis (LCH) is a dendritic cell (DC)
neoplasm defined by the presence of pathologic cells with Langerhans
cell features that are positive for CD1a, Langerin (CD207) and S100
protein.[1] The disease varies widely in clinical
presentation from localized involvement of a single bone to a fatal
disseminated life-threatening disease involving risk organs such as
liver, spleen, or hematopoietic system.[2] Bone is the
commonest area of involvement in about 80% of patients, which can be
painful and associated with significant morbidity from pathologic
fractures. Treatment for bony lesions includes surgical curettage,
intralesional infiltration of corticosteroids,[3] low-dose irradiation, indomethacin or systemic chemotherapy.[4]
Bisphosphonates are chemical analogues of pyrophosphates that act by inhibiting osteoclasts, thus preventing bone resorption.[5]
They were initially found to be effective in multifocal eosinophilic
granuloma of bone (former description of LCH bone lesions) in 1989.[6] Subsequently, this beneficial effect of bisphosphonates in bone lesions of LCH was confirmed in several other case reports.[7-10]
In 2005, da Costa et al showed that multi-nucleated giant cells (MGCs)
in LCH express several osteoclast markers and responsible for producing
osteoclast-inducing cytokines.[11] These osteolytic
cytokines along with various other matrix-degrading enzymes produced by
the MGCs are involved in inducing osteolysis; thus, a rationale for
using bisphosphonates in LCH was established.
Currently,
chemotherapy and steroids remain the standard treatment in most
patients with LCH-related bone disease. Due to immediate and
long-term adverse effects related to chemotherapy and problems of
recurrent reactivations despite standard treatment, less toxic options
like bisphosphonates become attractive. This report summarizes the
international experience describing the role and safety of using
bisphosphonates for patients with bone involvement by LCH.
Materials and Methods
Data collection:
Survey documents were developed at the Hospital for Sick Children
(Toronto), and distributed to five LCH treating centers across North
America and Europe. The participating centers included Toronto and
Quebec City (Canada), New York City (USA), Athens (Greece), and
Amsterdam (The Netherlands). Appropriate ethics approval was obtained
from all participating centers. Data were collected on patients
diagnosed with LCH and treated with bisphosphonates as single agents
between 1995 to 2014, and included age at diagnosis, gender, sites of
disease, disease status (initial diagnosis and number of
reactivations), initial treatment received, type and dose of
bisphosphonates used, number of courses, response to bisphosphonate
therapy including pain grades and radiological assessment pre- and
post-therapy, toxicity, and long-term outcome.
Data analysis:
Statistical analyses were completed using SAS 9.4 (SAS Institute Inc.,
Cary, NC, USA). Categorical variable were summarized using counts
and percentages. Descriptive statistics summarized continuous
variables.
Evaluation criteria and definitions: The National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE)[12]
was used to define the pain response pre- and post-bisphosphonate
therapy; 0 for no pain, 1 for mild pain, 2 for moderate pain, and 3 for
severe pain. The “best response” in bone lesions following
bisphosphonates was evaluated using the disease state categories
proposed by LCH-III protocol.[13] The “no active
disease” (NAD) status was defined as the disappearance of all signs and
symptoms of disease with the exception of diabetes insipidus (DI) and
central nervous system degeneration (CNS-ND), or residual radiological
findings of bone lesions showing regression or stabilization with bone
remodeling. The “no response” (NR) status was defined as unequivocal
enlargement of the size of the existing bone lesion and/or appearance
of new lesions. In this study, we acknowledged the variability in the
response of bone lesions in children and adults, and exercised caution
while interpreting the post-therapy response individually. An
experienced radiologist at the respective institution assessed and
evaluated the radiological findings.
Results
The characteristics and treatment outcome of all the patients are summarized in Table 1. Thirteen patients (male/female ratio, 8:5 and age range, 2.8 to 55 years) with histologically proven LCH were included in this series. Ten patients (77%) had a single system bone disease, and 3 (23%) had bone lesions as part of multisystem disease. None of the 3 patients with the multisystem disease had risk organ (liver, spleen and/or hematopoeitic system) involvement. The median age at initiation of bisphosphonate therapy was 21.4 years (range, 2.7 to 55.3 years) and the median post- bisphosphonate therapy follow-up period was 4.6 years (range, 0.8 to 8.2 years).
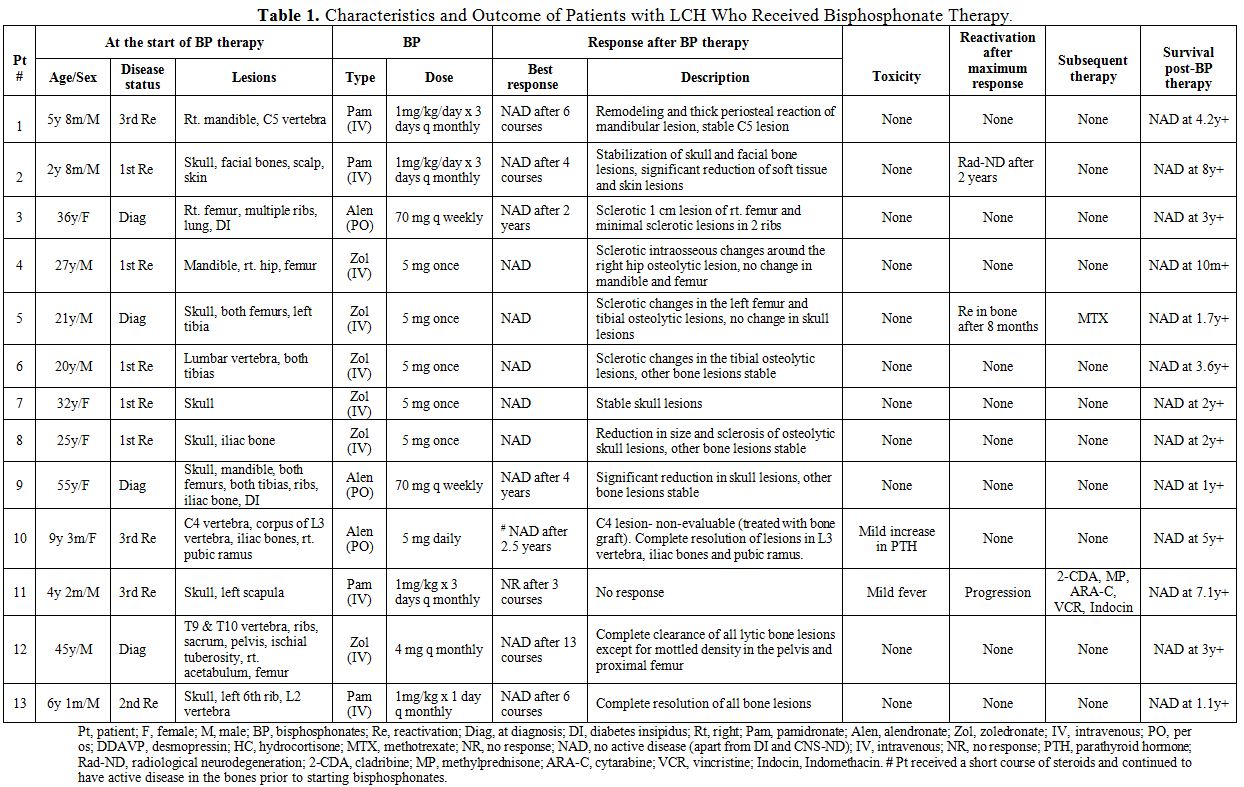 |
Table 1. Characteristics and Outcome of Patients with LCH Who Received Bisphosphonate Therapy. |
Management of LCH with bisphosphonates: Four patients (31%) received pamidronate, 3 (23%) received alendronate, and 6 (46%) received zoledronate. All children in the series received either pamidronate or alendronate therapy while all adults received zoledronate. Pamidronate was administered intravenously (IV) at a median dose of 1 mg/kg/course between 3 to 6 “monthly” courses and alendronate was administered orally as a single “daily” dose of 5 mg or “weekly” course of 70 mg. Most of the patients who were treated with zoledronate received a single IV dose of 5mg, while one patient received a “monthly” course of 4mg. Most of the patients had received some form of chemotherapy and/or radiotherapy and had progressed before starting bisphosphonate therapy, while a few received bisphosphonates as upfront therapy. No other LCH-directed treatment was administered during the course of bisphosphonate therapy except for desmopressin (DDAVP) in patients with DI (nos. 3, 9) and hydrocortisone replacement therapy for pituitary insufficiency in one patient (no. 4). Patient 10 received a short course of steroids and yet continued to have active bone disease prior to starting therapy with bisphosphonates.
Clinical effects of bisphosphonates: Twelve of 13 patients (92%) achieved NAD with radiological
re-ossification and normalization of active bone lesions either during
or after cessation of bisphosphonates. Of the 12 patients who had
obtained NAD, 10 continued to have a complete radiographic resolution
for a median of 3.5 years (range, 0.8 to 5 years) since the
commencement of bisphosphonates. One patient (no. 5) had bone
reactivation 8 months following cessation of bisphosphonates and
subsequently achieved NAD with methotrexate therapy. One child (no. 11)
who received pamidronate for his third bone reactivation did not
respond and continued to progress. The same patient achieved NAD after
treatment with multiple chemotherapeutic agents. Patient no. 3
developed radiographic central nervous system neurodegeneration
(CNS-ND) 2 years after stopping pamidronate therapy. However the
radiographic findings remained stable during the last follow up without
further therapeutic intervention.
Eleven
of 13 patients reported either moderate or severe pain prior to
starting therapy with bisphosphonates and required analgesic
medications. Seventy five percent (75%) of them reported no pain during
or after cessation of bisphosphonate therapy along with restoration of
functional status, while the rest reported improvement to only mild
pain.
In Figure 1, a complete resolution of mandibular lytic lesions following pamidronate
therapy is demonstrated in a 3-dimensional CT scan. In Figure 2, CT scan showed bone remodeling and reduction in orbital soft tissue mass following pamidronate therapy.
| Figure 2. Skull CT before (a) and after (b) 2 courses of pamidronate therapy. a) Permeative bone lesions in the lateral wall of orbit and soft tissue mass involving the right lacrimal gland and lateral rectus muscle. b) Bone remodeling and reduction in orbital soft tissue mass |
Adverse effects: Bisphosphonate therapy was well tolerated by all patients without major adverse effects. One patient (no. 11) had a fever during initial courses of IV pamidronate administration and another (no. 10) had mild elevation in parathyroid hormone (PTH) levels following alendronate.
Discussion
In the present study, bisphosphonates appear to be an effective
option in treating bone lesions of LCH. The majority of patients in
this series demonstrated significant improvement in pain symptoms
related to the disease with return of functional status and 92% were
able to achieve complete resolution of active bone lesions. Several
case reports have been published suggesting the beneficial effects of
bisphosphonates in LCH, but there is currently no international
consensus on the role of bisphosphonates in LCH. A nationwide
survey from Japan comprising of 16 children with LCH investigated the
role of pamidronate in reactivated LCH and concluded that pamidronate
was effective in the resolution of bone lesions in 75% of children with
acceptable toxicity profile.[14] Our report is the
first international study that included both children and adults, and
described the role and safety of various bisphosphonates in the
treatment of bone lesions of LCH suggesting that this is a class
effect.
Bisphosphonates have been used as standard of care for bone pain caused by various metastatic tumors,[15,16]
and interestingly, the bone pain of LCH shares similar properties with
cancer-induced pain. LCH cells, like tumor cells, release certain
cytokines including Tumor Necrosis Factor (TNF)-α and Interleukin-1[17,18]
that stimulate osteoclast activity leading to fragility of bone and
increased fracture risk. Moreover, both receptor activator of nuclear
factor B ligand (RANKL) and osteoprotegerin (OPG), which are crucial
key factors for the maturation and activation of osteoclasts, have
recently been implicated in the disease process both at the lesional
and systemic level.[19-21] Amelioration of pain
symptoms combined with the resolution of bone lesions is probably
explained by the anti-osteoclastic property of bisphosphonates that
helps reduce noxious inflammatory substances and other matrix degrading
cytokines in the active lesions, thereby conferring its analgesic and
bone-remodeling properties.
Pamidronate is the only bisphosphonate
that is most widely used in children for various bone conditions with a
well-established safety profile.[22,23] Similarly,
the safety and efficacy of zoledronate has been extensively reviewed in
adults, and especially found to be effective in decreasing the risk of
skeletal-related events secondary to breast cancer such as pathological
fractures, spinal cord compressions and hypercalcemia.[24]
Although there are few reports demonstrating the role and safety of
alendronate in certain skeletal conditions such as avascular necrosis
and osteoporosis, large-scale prospective studies evaluating its safety
profile and efficacy is still lacking.[25] Despite
the small sample size of this series, we were able to observe clinical
activity with a wide range of bisphosphonates at different doses.
However no positive correlation could be established between the type
of agent used, the dose administered and outcomes.
Bisphosphonates especially pamidronate has shown some efficacy for nonostotic LCH such as skin and soft tissue lesions.[14]
This concurs with the laboratory findings of da Costa et al who
demonstrated the presence of CD68+ osteoclast-like MGCs in nonostotic
lesions that also co-express CD1a.[11] The assessment
of response in nonostotic lesions was not the intent of this study, but
interestingly one child (no. 2) in our series demonstrated significant
response in skin and soft tissue lesions following pamidronate therapy (Figure 2).
Given the varying natural course of the disease, no favorable
conclusion can be derived from this favorable response; however the
effect of bisphosphonates in nonostotic lesions cannot be completely
ruled out and need to be further explored in a large prospective
study.
The most commonly reported toxicities with bisphosphonates are the acute phase reaction and hypocalcemia,[16]
the latter especially among vitamin D deficient patients. One patient
in our series developed mild fever with initial courses of
bisphosphonate that responded to antipyretics. PTH abnormality was
reported in one patient manifesting as secondary hyperparathyroidism
due to mild hypocalcemia, which was appropriately monitored and
managed. It is possible that the PTH abnormality following
bisphosphonates could be underreported in this series, as not all the
patients would have been systematically tested for it during the
therapy. Another rare yet significant side effect associated with IV
bisphosphonates is osteonecrosis of the jaw (ONJ);[26]
however none of the patients in our cohort was reported with ONJ. In
addition, ONJ is almost never seen in children who only receive a much
lower dose of bisphosphonates to treat bone lesions.[27]
Thus, both oral and intravenous bisphosphonates appear to be a safe
option to treat bone lesions of LCH in adults as well as in children.
Ensuring adequate vitamin D repletion prior to bisphosphonate therapy
and monitoring serum calcium and PTH pre- and during bisphosphonate
therapy is highly recommended.
Our study has a few limitations
that warrant consideration. The retrospective nature of the study can
potentially introduce reporting biases and confounding factors, which
makes interpretation of these data challenging. The longer study
period, approximately 19 years, precluded us from performing a central
radiographic review. Nevertheless, our data suggests that
bisphosphonates might be an effective treatment option for symptomatic
pain relief and resolution of active bone lesions in patients with LCH,
and may obviate the need for toxic chemotherapy in less advanced cases.
Future prospective studies are required to optimize the strategy of
bisphosphonate treatment in LCH, including the ideal agent in different
age groups, the timing of treatment initiation, optimal dose and
duration of therapy, and long-term efficacy and safety.
Acknowledgements
The authors would like to thank Dr. Marina Tsoli (National and Kapodistrian University of Athens) and Christopher Famulare (Memorial Sloan-Kettering Cancer Center, New York) for assistance with clinical data collection. We would like to thank Shiyi Chen (Clinical Research Services, The Hospital for Sick Children, Toronto) for her support with the data analysis. We thank the physicians, nurses, and staff who provided care for these patients, as well as the patients and families themselves.
References



























[TOP]