Received: May 22, 2016
Accepted: June 14, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016034, DOI 10.4084/MJHID.2016.034
This article is available on PDF format at:


| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Background: In March 2015, the
International Network of Clinicians for Endocrinopathies in Thalassemia
and Adolescent Medicine (ICET-A) implemented a two-step survey on
central adrenal insufficiency (CAI) assessment in TM patients and after
analysis of the collected data, recommendations for the assessment of
hypothalamic-pituitary- adrenal (HPA) axis in clinical practice were
defined. Methods: To ascertain the current practice for assessment of CAI in thalassemia, the Coordinator of ICET-A sent two questionnaires by email: i) The first to evaluate the current interpretation of basal serum cortisol level (first step) and ii) The second to assess the current usage of ACTH test and the variability in practice" (second step). Based on the surveys the core ICET-A group prepared the recommendations for the assessment of suspected CAI in thalassemia (third step). Results: A total of 19 thalassemologists/endocrinologists have participated in the first survey and 35 specialists participated in the second step questionnaire. The study demonstrated a considerable variability in almost all aspects of relevant current criteria used for the diagnosis of CAI. An ROC analysis using peak value > 20 μg/dl (> 550 nmol/L), after ACTH stimulation test, was performed with the aim of identifying the optimal basal serum cortisol cut-off. The optimal threshold that maximizes sensitivity plus specificity for morning basal cortisol against peak post- ACTH value >20 μg/dl (>550 nmol/L) was 10 μg/dl (275 nmol/L). Furthermore, the values associated with the highest negative predictive value (NPV) and highest, positive predictive value (PPV) were 4.20 (115 nmol/L) and 18.45 μg/dl (510 nmol/L), respectively. Surprisingly, 20 specialists in thalassemia working in blood bank, thalassemia centres (day hospital), internal medicine, hematology and onco-hematology had poor knowledge and experience in testing for CAI and stopped filling the questionnaire after the second question. In contrast, 9 endocrinologists (8 pediatricians) and 6 hematologists working in collaboration with endocrinologists completed the questionnaire. Conclusions: While waiting for more extensive adequately powered and targeted studies, physicians should adopt an acceptable policy for accurate assessment of HPA in TM patients. Regular surveillance, early diagnosis, treatment and follow-up in a multi-disciplinary specialized setting are also recommended. The ICET-A recommendations are reported in order to facilitate for interested physicians the approach to a successful assessment of adrenal function in thalassemia. |
Introduction
Accurate assessment of the hypothalamic-pituitary- adrenal (HPA)
axis is essential for the management of patients with potential or
suspected pituitary or hypothalamic disease that is frequent in
patients with thalassemia major (TM). The diagnosis of adrenal
insufficiency (AI) is relatively simple when glucocorticoid secretion
is profoundly depressed. However, AI can present a difficult diagnostic
challenge, especially when adrenal insufficiency is partial. This is a
particularly important issue as acute crises may occur during stress
periods in undiagnosed patients.
Recently, several studies
reported a significant prevalence of “biochemical” central adrenal
insufficiency (CAI), ranging from 15% to 53.6
%,[1-5] in children, adolescent and adults with TM. In
one study the youngest patient reported with “biochemical” CAI was 9
years old.[4] The age of patients varied from 12 and 20 years,[1] 8 to 26 years,[2] 10.2 ± 3.7 years (ranges are not available),[3] 3 to 18 years[4] and 18 to 50 years.[5]
The prevalence of CAI was higher in adult TM patients (32.1%; age range 18–50 years, median 30).[1–4]
Extreme variability in the prevalence of CAI has been attributed to the
variable duration of regular transfusion (p=0.016, 95% CI: -28.5/-
3.24), iron overload status and the use of different tests for
assessing adrenal function as well as different cut-off
values for diagnosing AI among the various centres.[1-5]
The
pathophysiological basis of “biochemical” AI in TM has not yet been
well-defined. Chronic transfusions induce iron overload in several
organs, including adrenal and pituitary glands.[1-5] Therefore, it is possible that pituitary iron deposition might reduce ACTH secretion leading to CAI.[2]
Furthermore, the adrenal glands might also be directly affected by iron
toxicity. In two studies, patients with TM had higher baseline
adrenocorticotrophic hormone (ACTH) levels than do controls, suggesting
primary impairment of adrenocortical function.[2,5]
There
are two methods to differentiate between primary and secondary AI.
First is done by measuring plasma ACTH concentration in the basal
fasting AM blood sample. If it is higher than normal, the patient has
primary AI, whereas if it is low, the diagnosis of secondary or
tertiary AI should be considered. The second method assesses the serum
cortisol values in response to exogenous corticotropin (ACTH)
stimulation or insulin tolerance test (ITT). The agent most commonly
used is synthetic ACTH [1-24] (cosyntropin), which has the full
biologic potency of native ACTH [1-39]. The text is useful for the
diagnosis
of AI but not for the differential
diagnosis between peripheral and central forms.[6]
Therefore, a prolonged corticotropin administration may become helpful
in the differential diagnosis. Unfortunately, this diagnostic approach
has not been validated in patients with TM.
In March 2015, the
International Network of Clinicians for Endocrinopathies in Thalassemia
and Adolescent Medicine (ICET-A) promoted a two-steps survey on the
assessment CAI in TM patients among Endocrinologists and Hematologists
working with thalassemia patients in different countries. After
collecting and analysing the data, the ICET-A group prepared relevant
clinical and practical recommendations for the assessment of HPA axis
in these patients. The results of the ICET-A project are presented in
this paper.
Methods
To ascertain the current practice for assessment of CAI in
thalassemia, the Coordinator (VDS) of ICET-A sent by email two
questionnaires: i) “to evaluate the current interpretation of basal
serum cortisol level” to 19 thalassemologists/ endocrinologists members
of ICET-A (first step) and ii) a copy of modified questionnaire survey used by Elder et al[7]
”to evaluate the current usage of ACTH test and the variability in
practice” among other 20 additional specialists taking care of TM
patients (second step). A total of 35 specialists participated in the second step questionnaire.
Based on the surveys the core ICET-A group prepared the recommendations for the assessment of suspected CAI (third step).
The recommendations were based on published, peer- reviewed scientific
evidence, expert opinion, and accumulated professional knowledge and
experience. Recommendations from published guidelines
were used when available and appropriate. The ICET-A Network also
issued expert consensus opinions on topics for which limited or low
level evidence is available in the literature. Since not all published
references were based on randomised controlled trials, the
recommendations have been scored according to the following criteria:
A.
High confidence indicates that further research is unlikely to
change the confidence in the estimate of effect (●●●)
B.
Moderate confidence indicates that further research may change
the confidence in the estimate of effect (●●○)
C.
Low confidence indicates that further research would likely have
a significant impact on the confidence in the estimate of effect (●○○)
D. Insufficient indicates that the evidence is unavailable or does not permit a conclusion (○○○)
Statistical Analysis
A ROC analysis using peak value >20 μg/dl (>550 nmol/L) after ACTH stimulation test as the classification variable and basal value as the continuous predictor variable was performed using the data from the literature.[2-4] and the personal experience, in 80 TM patients (aged 3-50 years) with the aim of identifying the optimal basal cut-off. The optimal cutoff was determined by the Youden Index, which is defined as Sensitivity plus Specificity-1. All analyses and calculations were done using R version 3.3.0, with the open-source package “pROC”. [8]
Results
First step: Answered questionnaire was received from 15 out of 19 ICET-A members (78.9% response rate). Responders, who are collectively following 1895 TM patients, were asked to report their position on the lowest basal cortisol threshold used to diagnose CAI and the highest basal threshold excluding CAI. The results are summarized in table 1.
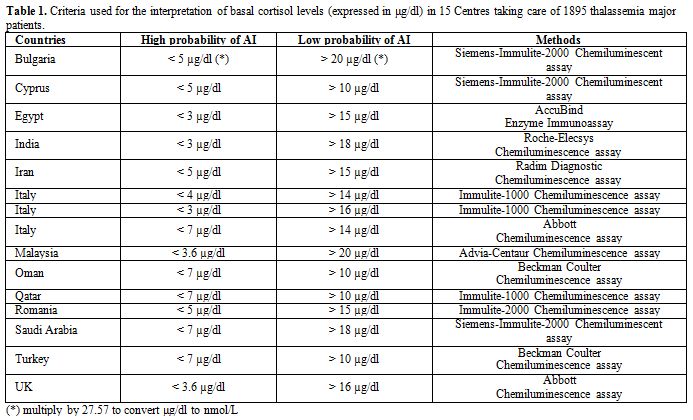 |
Table 1. Criteria used for the interpretation of basal cortisol levels (expressed in μg/dl) in 15 Centres taking care of 1895 thalassemia major patients. |
In the survey, the lowest basal cortisol threshold reported
was ≤3 μg/dl (88 nmol/l) to exclude CAI and the highest threshold was
≤7 μg/dl (<190 nmol/l) to diagnose CAI. Values greater than 20 μg/dl
(550 nmol/l) were reported to predict best normal HPA axis.
The results of the ROC analysis are shown in table 2 and figure 1.
Using the Youden index, the optimal threshold that maximizes
sensitivity plus specificity for morning basal cortisol against peak
post-ACTH value >20 μg/dl (>550nmol/L) was 10 μg/dl (275 nmol/L).
Three chemilumininescent assays (one Beckman Coulter and two Immulite
1000 kits) and one competitive enzyme-linked immunoassay were used for
cortisol measurements (AccuBind kit). Furthermore, the values
associated with the highest negative predictive value (NPV) and highest
positive predictive value (PPV) were 4.20 (115 nmol/L) and 18.45 μg/dl
(510 nmol/L), respectively.
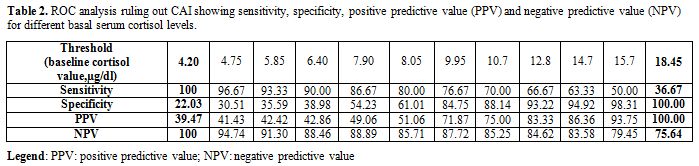 |
Table 2. ROC analysis ruling out CAI showing sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for different basal serum cortisol levels. |
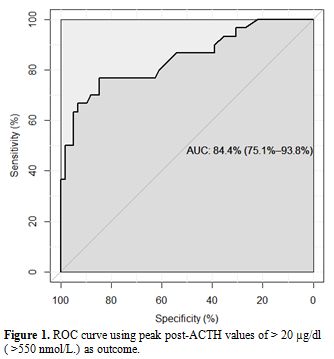 |
Figure 1. ROC curve using peak post-ACTH values of > 20 µg/dl ( >550 nmol/L) as outcome |
Second step: Thirty five centres following a total of 3433 TM patients shared the second questionnaire. Taking into consideration that several protocols have been used to assess the response to ACTH test, the aim of the survey was to collect 5 pieces of information regarding: a) How many patients are you regularly following in your hospital? b) Does your centre carry out? Synacthen testing? Are you familiar with the indication and interpretation of Synacthen test, c) What dose of Synacthen does your Unit use? d) At what age you screen your patients by measuring serum cortisol? e) What cut off point you accept for diagnosing normal and abnormal adrenal function? f) For a normal result what do you require? (Table 3).
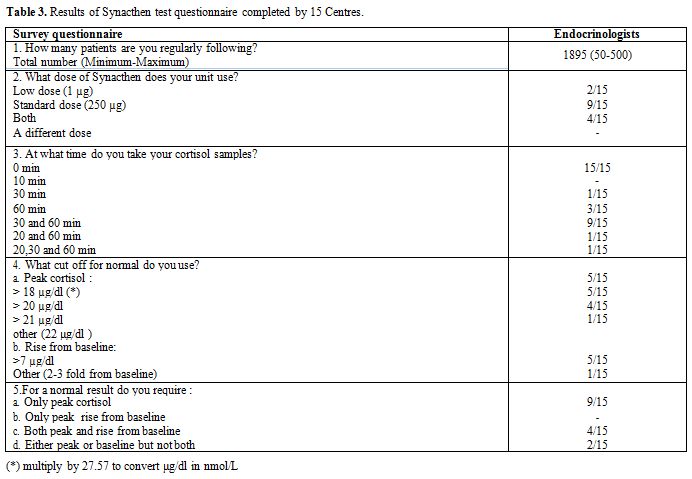 |
Table 3. Results of Synacthen test questionnaire completed by 15 Centres. |
Surprisingly, 20 specialists working in blood banks,
thalassemia centres (day hospital), internal medicine, hematology and
onco-hematology had poor knowledge about the test and stopped filling
the questionnaire after the second question. On the contrary 9
endocrinologists (8 were pediatricians) and 6 hematologists working in
collaboration with endocrinologists completed the survey (Table 3).
9/15 (60%) of responding centres employed a standard-dose (SDT)
corticotropin stimulation test (Synacthen test: 250 µg intravenously)
and 2/15 (26.6%) a low dose (LDT) stimulation test (Synacthen test: 1
µg intravenously). There was a variation in the timing of cortisol
sampling. 60% collected blood samples at 0', and at 30' and 60' minutes
after ACTH injection. The maximum number of samples per test was 3. The
diagnostic cut-off values used by different centres are reported in table 3.
Peak cortisol level was used on its own as the diagnostic criteria in
60% of centres and in association with cortisol rise from baseline
in 26.6% of centres. Peak cortisol value >18-20 µg/dl (200- 550
nmol/l) was diagnostic for normal adrenal function in 10/15 centres
(66.6%) while 5/15 centres (33.3%) required values > 20 µg/dl (550
nmol/l). Rise from baseline, defined as >7 µg/dl (> 200
nmol/l) or 2-3 fold from baseline was required for diagnosing normal
adrenocotical function by 40% of the centres.
LDT were performed
in 6/15 centres diluting one vial of 250 microgram ACTH into 250 mL
sterile normal saline. 1 mL (=1 microgram ACTH) of the solution was
then injected as an intravenous bolus.
Discussion
Our
survey demonstrated a considerable variability in the utilization of
the current criteria used for the diagnosis of AI. These included serum
basal cortisol level, ACTH dose, the timing of cortisol sampling and
cut-offs for AI. In our study, the optimal threshold that maximizes
sensitivity plus specificity for morning basal cortisol against peak
post-ACTH value >20 μg/dl (>550 nmol/L) was 10 μg/dl (275 nmol/L).
Furthermore, the values associated with highest negative predictive value (NPV) and
highest positive predictive value (PPV) were 4.20 (115 nmol/L) and
18.45 μg/dl (510 nmol/L), respectively.
The
lower cut-off is in line with the published data while the upper
cut-off is markedly lower than the one reported in the meta-analysis.[9]
In fact, in a meta-analysis of 12 studies on adults (635 subjects
without thalassemia), a basal cortisol less than 5 μg/dl (< 138
nmol/l) strongly predicted CAI, while values greater than 13 μg/dl (365
nmol/l) a normal HPA axis.[9]
The
lack of uniformity in cut-off levels could in part be attributed to
differences in study populations, the variability of dynamic tests,
different serum cortisol assays used, the cut-off of peak serum
cortisol that was deemed indicative of a normal HPA axis response, and
the clinical context in which the studies were done. Therefore,
additional studies are required to further elucidate these differences.
Dynamic
testing is performed to establish the diagnosis in patients with
equivocal cortisol levels in whom hypoadrenalism is suspected. Several
protocols have been used to assess the response to exogenous
corticotrophin (ACTH). The agent used is synthetic ACTH [1-24]
(cosyntropin), which has the full biologic potency of native ACTH
[1-39]. There is controversy whether the low-dose test (LDT) is
superior to the high-dose ACTH stimulation test (HDT).
The
existing controversies in the literature about the use of different
Synachten stimulation tests in the assessments of the HPA axis are
thought to be related to the use of inappropriate cut-off values.[1-5,10-14]
Conventionally, adrenal insufficiency is likely if serum cortisol level
is less than 18-20 μg/dL (500-550 nmol/L) at 30-60 minutes after
administration of ACTH and or an increments of less than 7 μg/dl (200 nmol/L) above basal cortisol, a criterion described by Crowley et al for LDT.[10] Olkers et al[11]
suggested the importance of using different cut-off points for HDT and
LDT. A raised cut-off of 22 μg/dl (600 nmo/l) could result in higher
sensitivity for the diagnosis.
Mayenknecht et al.[12] established normal ranges for cortisol responses in the LDT (0.5 mg/m2
tetracosactin injection) and HDT in 35 endocrinologically normal
healthy subjects. Mean responses minus 2 standard deviations
were used as
the cut-off point. The result for the LDT at 30 min after injection was
20 μg/dL (550 nmol/L); for the HDT at 30 min after injection: 22 μg/dL
(600 nmol/L) and at 60 min: 26 μg/dL (715 nmol/L).
The authors concluded that it was crucial to use different cut-off points in the HDT and LDT tests.
It
is possible that TM patients with mild/ partial or recent-onset
pituitary ACTH or hypothalamic corticotropin-releasing hormone (CRH)
deficiency may have a normal response to 250 μg of Synacthen because
the adrenal glands have not undergone significant atrophy and still
responds to very high concentrations of ACTH.[13,14] This is an especially important issue because acute crisis may occur during stress periods in undiagnosed patients.
A
meta-analysis including 30 studies (1209 adults and 228 children)
compared the results of high- and low-dose ACTH stimulation tests using
different peak serum cortisol cut-offs. The analysis showed that both
tests had similar diagnostic accuracy in adults and children. In
general, both tests had low sensitivity and high specificity
resulting in
reasonable likelihood ratios for a positive test, but a
relatively suboptimal likelihood ratio for a negative test.[15] Another survey, published in 2016, supports that there is no clear evidence to indicate that one test is superior to another.[16]
This report conflicts with earlier studies with a small number of
patients, suggesting that the low-dose test was more sensitive.[17-21]
In
patients with TM, an AI was demonstrated in 30 of 56 patients (53.6%)
after an LDT. To assess more precisely the adrenal function, the
insulin tolerance test (ITT) was performed in 26 of 30 TM patients
(86.7%) who had peak total cortisol less than 16 µg/dl (440 nmol/l),
after ACTH test. The remaining four patients declined the testing. The
time interval between the 1 µg ACTH test and ITT was
approximately 4–5 wk. Five of 26 patients (19.2%) had peak total
cortisol after an ITT of 20 µg/dl or greater. Therefore, about one
fifth of patients who failed the 1 µg ACTH test had normal peak total
cortisol levels after an ITT. Thus, by using an ITT, the estimated
frequency of adrenal insufficiency in the entire patient group was
reduced by approximately 20%.[3]
Soliman et al.[2]
using the apparently more “physiologic” LDT and a normal peak total
cortisol cut-off level of 20 μg/dl (550 nmol/L) and increment >7
µd/dl (>200 nmol/l), diagnosed a prevalence of CAI in 8 out of 23
(34.7%) in TM patients (6 adolescents and 2 children). Using the HDT
and the same cut-off levels diagnosed AI in 8.7% (2/23) of these
adolescents. Therefore, about 75% of patients who failed the LDT had
normal peak total cortisol levels after the HDT. Similar results were
reported by Pang et al. in 6/8 TM patients.[4]
In
conclusion, further studies and more normative data are urgently needed
because neither over diagnosis nor under diagnosis of HPA insufficiency
should be acceptable in patients who potentially may be treated with
steroids unnecessarily or who may have impaired cortisol in times of
stress and are in need of steroids.[2,22]
Twenty
specialists working in blood bank, thalassemia centres (day hospital),
internal medicine, hematology, and onco-hematology had poor knowledge
of the test and stopped to fill the questionnaire after the second
question. On the contrary, 9 endocrinologists and 6 hematologists
working in collaboration with endocrinologists completed the survey
questionnaire.
Therefore,
a collaborative working arrangement between professionals is needed to
meet all the required comprehensive care to patients. We believe that
one of the near future ICET-A tasks is to set up a collaborative
enterprise to identify and address the underlying factors that lead to
barrier inter-professional team work and thereby to facilitate
inter-professional collaboration.
The
lack of treatment guidelines and published research often leave
hematologists and internists with hesitant to approach TM patients
presenting uncommon endocrine complications. Therefore, as a third
step, we thought worth to prepare clinical practice recommendations for
all those taking care of TM patients on current criteria for the
assessment of CAI (Table 4). The recommendations provide helpful
information on laboratory parameters and their interpretation, as well
as adrenal hormone replacement dosages and management strategies. The
guidelines emphasize that clinicians need to suspect AI earlier in TM
patients with risk factors, such as advanced age, severe iron overload
and/or poor compliance to therapy, and with multiple endocrine
complications.
 |
Table 4. Practical recommendations for the assessment of suspected adrenal insufficiency (AI) in thalassemia. |
If corticotropin testing is not feasible, a combination of a morning plasma ACTH and cortisol levels (less than 4.2 µg/dL=115 nmo/L) can be used as an initial screening; based on the results, a confirmatory testing with corticotropin stimulation is strongly recommended. Because tests are not perfect, there is still an important role for clinical judgment, especially regarding the use of glucocorticoid supplementation during extreme stress, such as surgery.[2,22]
In summary, our survey provides a better understanding of current physician clinical practices and beliefs in the assessment of the hypothalamic-pituitary-adrenal axis in TM patients. While waiting for more extensive, adequately powered and targeted studies, physicians should adopt an applicable, common sense policy for accurate assessment of HPA in TM patients. Regular surveillance, early diagnosis, treatment and follow-up in a multi-disciplinary specialized setting are also recommended.
Aknowledgements
We wish to thank dr CE Elder, University of Sheffield, Academic Unit of Child Health, Stephenson Wing, Sheffield Children's Hospital, Western Bank, Sheffield for giving us the opportunity to use a copy of the ACTH test questionnaire used in her publication.
References






























[TOP]