Received: June 25, 2016
Accepted: September 12, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016052, DOI 10.4084/MJHID.2016.052
This article is available on PDF format at:

Maria Ilaria Del Principe1, Francesco Buccisano1, Luca Maurillo1, Giuseppe Sconocchia2, Mariagiovanna Cefalo1, Maria Irno Consalvo1, Chiara Sarlo3, Consuelo Conti1, Giovanna De Santis1, Eleonora De Bellis1, Ambra Di Veroli1, Patrizia Palomba1, Cristina Attrotto1, Annagiulia Zizzari1, Giovangiacinto Paterno1, Maria Teresa Voso1, Giovanni Del Poeta1, Francesco Lo-Coco1,4, William Arcese1, Sergio Amadori1 and Adriano Venditti1
1 Ematologia, Dipartimento di Biomedicina e Prevenzione, Università degli studi di Roma “Tor Vergata”, Roma, Italia.
2 Istituto di Farmacologia Translazionale, Dipartimento di Medicina, CNR, Roma, Italia.
3 Ematologia, Policlinico Universitario-Campus Biomedico, Roma, Italia.
4 Laboratorio di Neuro-Oncoematologia, I.R.C.C.S.- Fondazione S. Lucia, Roma, Italia.

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Pretreatment assessment of
cytogenetic/genetic signature of acute myeloid leukemia (AML) has been
consistently shown to play a major prognostic role but also to fail at
predicting outcome on individual basis, even in low-risk AML.
Therefore, we are in need of further accurate methods to refine the
patients’ risk allocation process, distinguishing more adequately those
who are likely to recur from those who are not. In this view, there is
now evidence that the submicroscopic amounts of leukemic cells (called
minimal residual disease, MRD), measured during the course of
treatment, indicate the quality of response to therapy. Therefore, MRD
might serve as an independent, additional biomarker to help to identify
patients at higher risk of relapse. Detection of MRD requires the use
of highly sensitive ancillary techniques, such as polymerase chain
reaction (PCR) and multiparametric flow cytometry (MPFC). In the
present manuscript, we will review the current approaches to
investigate MRD and its clinical applications in AML management. |
Introduction
Acute myeloid leukemia (AML) is a clonal disorder of haemopoietic
stem cells characterized by an abnormal proliferation of myeloid
progenitors and subsequent bone marrow failure. AML response to
chemotherapy is extremely variable with complete remission (CR) rates
ranging from 50% to 80%.[1] Also frequency of relapse is variable,
being reported in 10% to 95% of the cases.[2-4] Currently,
risk-stratification is determined by several factors, patient- and
disease-related, assessed at diagnosis, such as age, performance
status, white blood count (WBC), existence of prior myelodysplastic
syndrome, previous cytotoxic therapy for another disorder and
cytogenetic/molecular abnormalities. Among long-established prognostic
factors, karyotype and genotype of leukemic cells are the strongest for
they impact on response to induction therapy and survival.[5] However,
cytogenetic and molecular findings at diagnosis allow stratification of
~40% of patients in “good risk” or “adverse risk“ groups. The lack of
cytogenetic and molecular markers in approximately 60% of AML, prompts
the need for further accurate methods to select more precisely patients
at high risk of disease recurrence. In this view, there is now evidence
that levels of minimal residual disease (MRD) during the course of
therapy could serve as an independent biomarker to identify such
high-risk patients[6,7] and to plan the therapeutic program
accordingly. We will review the current approaches to investigate MRD
and its clinical applications in AML management.
MRD Detection
The paradigm of a successful treatment of AML is based on the achievement of morphological CR (mCR), defined as less than 5% leukemic cells, counted by light microscopy, within a fully restored bone marrow cellularity. However, it is now clear that classical morphology examination neglects a minority of myeloid blasts that could survive after induction and consolidation cycles. In this view, sophisticated techniques such as polymerase chain reaction (PCR) and multiparametric flow cytometry (MPFC) have been shown to detect leukemic cells at high sensitivity, in conditions of mCR. It is still a matter of debate what is the best method, in terms of clinical usefulness, to measure MRD in AML.
MRD Detection by PCR
In general, PCR is regarded as the most sensitive technique with a detection power of 10-4 to10-6.[7-10] Using PCR, MRD can be monitored by capturing specific leukemia targets such as chimeric fusion genes, mutations and gene overexpression (Table 1).
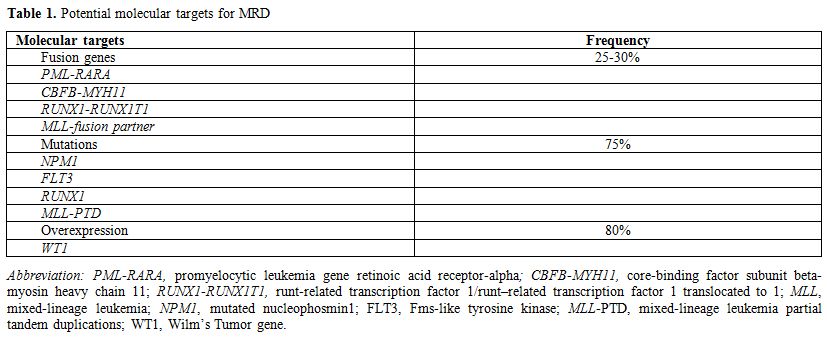 |
Table 1. Potential molecular targets for MRD |
Leukemic Fusion Genes:
This approach relies on cloning of breakpoints of the chromosomal
rearrangements in AML by using reverse transcriptase PCR (RT-PCR) or
quantitative real-time PCR (RQ-PCR). In the situation of mCR, it allows
identification of residual fusion genes in approximately 30% of the
patients. Common targets for PCR-based MRD detection are fusion
transcripts of mixed-lineage leukemia (MLL)-gene and CBF positive AML,
e.g. runt-related transcription factor 1/runt–related transcription
factor 1 translocated to 1 (RUNX1-RUNX1T1, formerly AML1-ETO) and
core-binding factor subunit beta-myosin heavy chain 11 (CBFB-MYH11).
Scholl et al.[11,12] showed that patients achieving a MLL-AF9
PCR-negative state had a very low probability of relapse and a 4-year
overall survival (OS) of 70%, whereas all of those with an RT-PCR
positive finding relapsed and died within 3 years. UK MRC trials group
demonstrated that in CBF positive AML, MRD monitoring by RT-PCR at
different time points identified patients at higher risk of relapse.[9]
However, using RT-PCR, persistent PCR positivity has been observed in
long-term survivors even after allogeneic stem cell transplantation
(ASCT). Therefore, RT-PCR in CBF positive AML may have a limited
clinical applicability since the detection of low transcript levels in
a situation of long-term remission is not likely to anticipate an
impending relapse. Indeed, long-term persistence of CBF RT-PCR signal
would reflect the successful immune surveillance or the presence of MRD
target in the leukemic stem cells (LCS) that requires additional
genetic hits for progression to overt disease.[13] In this view, RQ-PCR
is potentially more advantageous than RT-PCR owing to its capability to
predict impending relapse during long-term follow-up monitoring.[14]
Corbacioglu et al.,[15] using RQ-PCR, established clinically relevant
MRD checkpoints in which persistence of CBFB-MYH11 transcript
positivity singled out patients with significantly increased the risk
of relapse. The authors concluded that monitoring of CBFB-MYH11
transcript levels should be incorporated into future clinical trials to
guide therapeutic decisions. In a prospective multicenter trial,
Jourdan et al.[16] demonstrated, by RQ-PCR, that a less than 3-log MRD
reduction of RUNX1-RUNX1T1 transcript after the first consolidation was
associated with a higher specific hazard of relapse in young CBF-AML
patients. At 36 months, the cumulative incidence of relapse (CIR) and
relapse-free survival (RFS) was lower and longer, respectively, in
patients who achieved 3-log MRD reduction. A decline of RUNX1-RUNX1T1
transcript inferior to 3 logs after 2 courses of consolidation or
within 3-4 months after mCR, were found to predict relapse in other
studies.[17,18] A further multicenter prospective cohort study
confirmed the threshold of >3-log reduction and indicated the second
consolidation as the best timing for MRD examination.[19]
Mutations:
Fusion genes are present in about 30% of AML cases. In fusion gene
negative AML patients, possible targets for PCR-based MRD assessment
are Fms-like tyrosine kinase- internal tandem duplication (FLT3-ITD),
mutated nucleophosmin1 (NPM1),and DNA methyltransferase(DNMT3A). About
25% of AML patients carried FLT3-ITD that predicts poor outcome
especially when it is located in the tyrosine kinase domain.[20]
Several tyrosine kinase inhibitors are currently under investigation
since FLT3 could be a meaningful, actionable therapeutic target
AML.[21] In light of this, detection of MRD by monitoring this marker
would be useful to measure the anti-leukemic activity of FLT3
inhibitors. However, mutational shifts between diagnosis and relapse,
multiclonality at presentation, the outgrowth of clones at relapse
different from those detected at diagnosis, variable insertion sites,
and lengths among patients, make the use of FLT3 mutation still
unreliable for MRD monitoring.[22,23] There is evidence that the lack
of longitudinal stability of gene mutations reflects the insufficient
sensitivity of the currently used methodologies. Next-generation
sequencing (NGS), with its increased sensitivity, might pave the way to
a more accurate MRD monitoring of FLT3-ITD in AML patients.[24-26] In
this regard, Zuffa et al.[27] developed an amplicon based-ultra deep
sequencing (UDS ) approach for FLT3
mutational screening that revealed the presence of small ITD+ clones in
5 of 256 normal karyotypes (CN-) AML patients, who were FLT3 wild-type
at presentation, but tested ITD+ at relapse or disease progression.
Thus, UDS appears as a valuable tool not only for FLT3 mutational
screening but also MRD monitoring. NPM1 mutations are very stable at
relapse[28] thus that they might have a role in MRD assessment. NPM1
gene mutations are present in 30% of all AML patients and in 50% of
those with CN.[29] Several studies have shown a favorable impact of
NPM1-mutated (NPM1mut) on clinical
outcome in the CN-AML setting.[20,29] Nevertheless, a substantial
proportion of patients with NPM1 mutations will eventually experience a
disease recurrence. In a retrospective analysis performed on 155
patients, increasing MRD levels of NPM1 were predictive of relapse
after chemotherapy or allogeneic hematopoietic stem cell
transplantation (ASCT).[30] These data are in concordance with previous
reports investigating comparable data sets. Schnittger et al.[31]
developed a highly sensitive RQ-PCR assay able to prime 17 different
mutations of NPM1. In 252 NPM1mutAML, high levels of NPM1mut
were significantly correlated with outcome, at each of four time-points
of monitoring. In multivariate analysis, including age, FLT3-ITD status
and the level of residual NPM1, it was demonstrated that the latter was
the most relevant prognostic factor affecting event free survival (EFS)
during first-line treatment, also in the subgroup of patients
undergoing ASCT. In a further refinement of such an approach, Kronke et
al.[32] demonstrated that NPM1mut transcripts
levels measured at two distinct checkpoints, after double induction and
consolidation therapy, impacted on OS and CIR (p<0.001 for all
comparisons). Recently, Ivey et al.[33] confirmed the prognostic role
of residual NPM1mut transcripts. After the second cycle of chemotherapy, the persistence of NPM1mut
transcripts was observed in the peripheral blood of 15% previously
untreated patients. Such a persistence was associated with a 3-year
greater risk of relapse (82% vs. 30%) and a lower rate of survival (24%
vs. 75%) than in a situation of transcript undetectability. In
multivariate analysis, the presence in the peripheral blood of MRD was
the only independent prognostic factor associated with death. Another
possible target for MRD monitoring is DNMT3A, found in 15-25% of AML
patients, particularly in CN AML patients.[34,35] The presence of
DNMT3A mutations is an independent determinant of dismal prognosis both
in the overall population and high-risk category (FLT3-ITD, age older
than 60 years).[34] To explore the utility of DNMT3A mutations as
biomarkers for MRD in AML, Pløen et al.[36] developed assays for
sensitive detection of recurrent mutations affecting residue R882.
Analysis of DNA from 298 diagnostic AML samples revealed DNMT3A
mutations in 45 cases (15%), which coincided with mutations in NPM1,
FLT3 and isocitrate dehydrogenase 1. DNMT3A mutations were stable in 12
of 13 patients presenting with relapse or secondary myelodysplastic
syndrome, but were also present in remission samples of 14 patients
until 8 years after initial AML diagnosis, despite the loss of all
other molecular AML markers. Based on these data, the suitability of
DNMT3A as MRD marker is still questioned.
Gene overexpression:
MRD can also be monitored through detection of gene overexpression.
Several genes have been proposed as candidates, with Wilm’s Tumor gene
(WT1) being the most reliable. WT1 is a tumor suppressor gene that
encodes for a zinc-finger transcription factor that is aberrantly
overexpressed in 85-90% of AML cases.[10] The value of WT1 monitoring in
AML has been a matter of debate, mainly due to differences among the
assays in use. This led to the development of a standardized WT1 assay,
validation of which involved a network of 11 laboratories and provided
independent prognostic information in AML. Among a cohort of 129 AML
patients, a WT1 reduction below 200 copies after the first induction
chemotherapy was associated with a longer duration of CR, independently
from age, WBC count or cytogenetic risk group.[10] Based on the post
induction WT1 level, Nomdedeu et al.[37] identified three prognostic
AML groups: group 0 (no. ofWT1 copies 0-17.5, in 134 patients), group 1
(no. ofWT1 copies 17.6-170.5, in 160 patients), and group 3 (no. of WT1
copies >170.5, in 71 patients). Outcomes of these groups differed
significantly in terms of OS (59±4%, 59±4%, 72±5%), leukemia free
survival (24±7%, 46±4%, 65±5%) and relapse probability (CIR 72±4%,
45±4%,25±5%). In line with these data, the RQ-PCR positivity of WT1-MRD
(defined as >0.5% in peripheral blood) after induction, was
associated with a higher risk of relapse and a shorter OS in a further
series of 183 AML patients with WT1 overexpression.[38] The post
induction time-point was confirmed in 45 AML patients, in whom a
post-induction WT1 log clearance <1.96 predicted disease
recurrence.[39] Levels of WT1 higher than 150 copies/104ABL
after induction course are associated with a shorter RFS, also in
childhood AML patients.[40] Furthermore, Pozzi et al. found that WT1
expression>100 copies predicted relapse even after ASCT. Actually,
patients who received donor lymphocyte infusion after ASCT, because of
high WT1 levels, had an OS significantly longer than those who
expressed the same high levels but were not given donor
lymphocytes.[41] Finally, there is evidence that the presence of high
levels of WT1 gene in circulating RNA after ASCT predicts AML
recurrence.[42] Moreover, WT1 was listed as the theoretically best
single universal molecular marker for MRD detection in AML.[10,42] In
practice its monitoring cannot be applied in all AML cases, which can
exhibit significantly different patterns of expression.[43,44]
Furthermore, since the expression of WT1 is not leukemia-specific,
discriminating genuine residual disease from background expression can
be problematic.[6,38] In order to mitigate the limitations of this
promising but sub-optimally used marker for MRD detection, Goswami et
al.[45] developed a technique based on the identification of a panel of
genes, including WT1, which are overexpressed in AML. They concluded
that multiple gene based MRD assay was superior to the use of WT1 alone
for MRD purposes. In fact, this approach allowed WT1 MRD negative
patients to be reclassified as positive on the basis of the measure of
other genes.
MRD Detection by MPFC
MPFC provides a quick and relatively inexpensive method for MRD detection, which is applicable to the vast majority of patients with AML. In fact, ≥ 85% of AML cases exhibits an aberrant phenotype called “leukemia-associated immunophenotype” (LAIP). LAIP is defined as the combination of antigens and/or flow-cytometry physical abnormalities that are absent or very infrequent in healthy bone marrow.[46] Phenotypic abnormalities in AML include expression of markers not expressed on myeloid cells (lymphoid-affiliated antigens such as CD7, CD19, and CD56), co-expression of markers commonly expressed at different stages of maturation as well as over-expression and under-expression of myeloid markers (e.g. CD33)(Table 2).[47,48] Initial studies of normal and leukemic phenotypes were performed in 2-3 color-assays.[47,49] With the time it became manifest that implementing LAIP identification required a more comprehensive diagnostic antibody panel. In this regard, international efforts are being made to generate standardized MPFC protocols, which cover the phenotypic heterogeneity of AML and the large number of potential LAIPs.[50,51] Actually, the diffusion of devices equipped with multiple lasers has implemented multiple color assays (>6–10 monoclonal antibody combinations) thus favoring increment of sensitivity from 10-3 to 10-5.[52-54] Accordingly, MPFC appears a highly sensitive and specific method to monitor MRD in AML patients. Transposition of MPFC approach to the clinical reality, requires that key-issues, such as MRD thresholds and appropriate time-points to determine MRD, are adequately addressed. Ideally, threshold and time-point should be the ones, assessment of which provides the most informative prognostic indication, thus that the choice of post-remission therapy is driven by the actual risk of relapse. The German AML Cooperative group demonstrated that MRD persistence on day 16 and the log-difference between MRD positive cells on day 1 and day 16, was an independent prognostic factor affecting CR, EFS, OS and RFS.[53-56] In the same line of research, two different studies[57,58] have established a correlation between the degree of peripheral blood and BM blast clearance as measured on day 14 after induction. In turn, these parameters correlated with achievement of morphological CR at the end of the induction cycle. Levels of MRD, as determined after induction therapy, also seem to correlate with the quality of peripheral recovery at the time of morphologic remission. In a retrospective study including 245 adults with AML, those who achieved CR had detectable MRD less frequently and at lower levels (median, 0.5%; range 0.004% to 3.9%) than patients achieving CR with incomplete platelet or WBC recovery. This finding suggests that failure in the resumption of normal peripheral blood values may result not only from the commonly assumed toxicity to normal progenitors but also from the persistence of residual leukemia. Furthermore, although peripheral blood recovery and MRD level are linked, each of them was an independent prognostic factor impacting on relapse rate, OS and RFS.[3] MRD status may also serve as a surrogate for optimal biological dosing of chemotherapeutic agents. To explore this hypothesis, we carried out a retrospective analysis of 130 patients who achieved an mCR after one cycle of either standard dose (SDAC) or high doses of cytarabine (HDAC).[59] We observed that the SDAC regimen was associated with a greater MRD-negativity frequency. In 178 patients, who achieved CR after intensive induction, the MRD level assessed at days 16-18 after induction, was associated with outcome. A cutoff of 0.15% was used to identify cases MRD positive. The 5-year RFS was 16% for MRD-positive patients and 43% for patients with no evidence of residual disease (p<0.001).[60] Thus, a rapid decline in MRD levels after induction therapy may reflect a highly chemo-sensitive disease with a “per se” favorable prognosis.[61] Early MRD clearance was also prognostic within the intermediate cytogenetic risk group (5-year RFS 15% vs 37%, P=0.016) as well as for patients with normal karyotype and NPM1 mutations (5-year RFS 13% vs 49%, P=0.02) or FLT3-ITD (3-year RFS rates 9% vs 44%, P=0.016).[60] The prognostic impact of flow MRD determined post induction[52,62] and post consolidation was subsequently confirmed in several studies. In a large cohort of younger patients, low MRD values distinguished patients with a relatively favorable outcome from those with a high relapse rate, short RFS, and OS. Either in the whole group or in the subgroup with intermediate-risk karyotype, MRD was an independent prognostic factor. Multivariate analysis after cycle 2 confirmed that high MRD values (>0.1% of WBC) were associated with a greater risk of relapse.[63] These data were confirmed in a large cohort of older patients treated within UK-NCRI protocols. MPFC-MRD negativity, which was achieved in 51% of patients after cycle 1 (C1) (n=286) and 64% of patients after cycle 2 (C2) (n=279), conferred a significantly better 3-year survival from CR (C1: 42% vs 26% in MRD-positive patients, P=0.001; C2: 38% vs18%, respectively; P<0.001).MPFC-MRD negativity was also associated with a lower relapse rate (C1: 71% vs 83% in MRD-positive patients, P=0.001; C2: 79% vs 91%, respectively; P<0.001), being the higher risk of early relapse observed in MRD-positive patients (median time to relapse, 8.5 vs 17.1 months, respectively).[64] The authors concluded that post-induction MRD assessment was able to predict disease outcome better than the post-consolidation evaluation. However, also diverging opinions have been published supporting the hypothesis that delayed time-points may be even more informative as compared to earlier ones. Our group has demonstrated[65] that levels of MRD ≥ 3.5x10-4 as measured after consolidation therapy were associated with a high probability of relapse and a short duration of OS and RFS. The prognostic role of MRD positivity after consolidation was confirmed in multivariate analysis. This observation was further challenged in two extended series of 100 and 147 patients[66,67] confirming that the persistence of ≥ 3.5x10-4 residual leukemic cells, at the end of consolidation therapy, discriminated between high and low-risk categories. In line with our experience, Kern et al.[55] reported that the 75th percentile of the MRD log-difference between day 1 and post-consolidation time-point was the sole variable dividing the patients into two groups with significantly different OS. Moreover, Walter et al.[68] found that MRD assessment at the pre-ASCT time-point correlated with outcome. In 253 consecutive patients receiving myeloablative (MA) ASCT, a three-year estimate of OS were 73% and 32% in MRD negative and MRD positive patients, respectively. The level of residual disease ≥0.1% was considered as MRD positivity. The pre-ASCT time-point and the 0.1% threshold were more recently confirmed in a series of 241 patients who received either non-myelo-ablative(NMA) or MA ASCT. Three-year relapse estimates were 28% and 57% for MRD negative and MRD positive NMA patients, and 22% and 63% for MA patients.[69] The prognostic significance of peri-transplant MRD dynamics was recently confirmed in a series of 279 adults patients who received MA ASCT in first or second remission. Ten-color multiparametric flow cytometry analyses of marrow aspirates were performed before and 28±7 days after transplantation. The 214 MRD negative patients had excellent outcomes, whereas those with MRD positivity before or after ASCT had a high risk of relapse and poor survival.[70] In order to improve the prediction power of MRD approach, Zhao et al.[71] exploited a combination of LAIP and WT1. They defined a positive MRD combination as two consecutive positive findings of WT1, MPFC or both, in the same sample, within a year post transplantation. With this dual approach, a higher sensitivity than the single approach was achieved, without loss of specificity. Several studies confirmed a good correlation between MRD detection by MPFC and WT1 analysis, after ASCT.[72-73] In line with this, Rossi et al.[74] observed comparable results at day +30 post-transplant. However, at day +90 WT1 analysis showed a significantly superior prediction power than MPFC, suggesting that WT1 expression may be more reliable in a long-term MRD follow up.
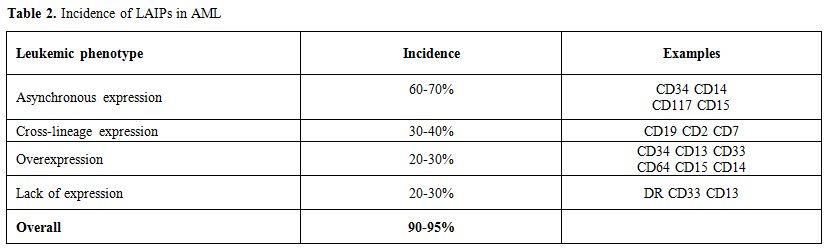 |
Table 2. Incidence of LAIPs in AML |
Selecting an early or delayed time-point might entail the choice of different therapeutic options: the early time-point option may prove useful to identify as soon as possible high risk patients for whom a fast allocation to very intensive treatments is required. For these patients, approaches such as dose dense schedule[75] and/or ASCT could be incorporated into the upfront treatment strategy.[76] On the other hand, opponents to this hypothesis raise concerns of potential over-treatment for patients showing a slow blast clearance which can cause MRD to be still positive after induction and negative after consolidation. In our experience[65,66] approximately 30% of patients who are MRD positive after induction, become negative at the end of consolidation; this underlines the impact of a standard consolidation course in rescuing into an MRD negative status a significant proportion of patients. The clinical outcome of these “slow responders” is not significantly different from that of patients who test MRD negative soon after induction. Based on these observations, we hypothesized that the final outcome will rely on the overall debulking effect produced by the whole [induction-consolidation] upfront therapy.[65,66] In our experience, the prognostic significance of post consolidation flow MRD is also maintained in elderly patients. Comparing 149 young and 61 elderly adults we observed that elderly patients reached a post-consolidation MRD negative status less frequently than younger ones (11% vs 28%, p=0.009). However, once attained, MRD negativity resulted in a longer 5-year disease-free survival (DFS) both in elderly (57% vs 13%, p=0.0197) and in younger patients (56% vs 31%, p=0.0017). Accordingly, 5 year CIR of both elderly (83% vs 42%, p=0.045) and younger patients (59% vs 24% p=NS) who were MRD positive doubled that of MRD negative ones. Nevertheless, CIR of MRD negative elderly patients was almost twofold higher than that of younger MRD negative ones (42% vs 24%, p=NS).[77]
In the light of the prognostic
relevance of MRD detection by MPFC, we tried to optimize
risk-assessment of patients with AML by integrating the evaluation of
pre-treatment prognosticators and MRD amount at the post-consolidation
time-point.[78,79] Of 143 adult patients, those with favorable and
intermediate-risk karyotype who were MRD negative had 4-yrs RFS of 70%
and 63%, and OS of 84% and 67%, respectively. Patients with favorable
and intermediate-risk karyotype who were MRD-positive had 4-yrs RFS of
15% and 17%, and OS of 38% and 23%, respectively (p<0.001 for all
comparisons). Likewise, FLT3 wild-type patients achieving a
MRD-negative status had a better outcome than those who remained
MRD-positive after consolidation (4-yrs RFS 54% vs 17% p<0.0001, OS
60% vs 23% p=0.002). Therefore, patients with favorable risk karyotype,
intermediate-risk or FLT3 wild-type had a very different outcome
depending on MRD status at the end of consolidation. Doing so, we
demonstrated that patients with favorable-risk karyotype or unmutated
FLT3, whose course of the disease is conventionally classified as
favorable, show a very different outcome depending on MRD status at the
end of consolidation.
Open Issues
Optimization of molecular MRD monitoring:
At the current time, optimized molecular monitoring of AML should be
carried out taking into account several technical and practical
aspects, such as the patient age and treatment objectives (e.g. disease
eradication), best source of sampling (bone marrow or peripheral
blood), chosen biomarkers, assay sensitivity (indicated by level of
expression of leukemic transcripts relative to the control gene), and
kinetics of disease preceding relapse. As to sampling source, Ivey et
al. recently demonstrated that the presence of MRD, as determined by
quantitation of NPM1muttranscripts in
peripheral blood, provided significant information on prognostic
outcome. BM evaluation, therefore, remains an important adjunct to
peripheral blood analysis in patients with AML.[7]
LAIP reliability:
Aberrant phenotypes include LAIPs which some authors claim to be
expressed even on normal cells, therefore compromising LAIPs
reliability for MRD monitoring. Actually, Rossi et al., in a six-color
assay, demonstrated that CD15+/CD117+
positive cells could also be detected in BM of healthy donors.[80] In
our opinion, the chance to efficiently distinguish leukemic from normal
cells increases proportionally with the number of fluorochromes in the
assay. In the AML1310 GIMEMA prospective trial, recruiting more than
500 hundred young patients with de novo AML, we detected reliable LAIPs
in 91% of the cases, using an 8-color assay (data unpublished).
Statistical methods for MRD evaluation by MPFC:
The statistical method used for the choice of the best cut-off and
time-point is a subject of debate and solutions adopted are quite
heterogeneous. Some authors, such as Al Malawi et al.,[60] used the
receiver operating characteristic (ROC) analysis to select cut-offs and
time-points. However, this approach requires that time-dependent
endpoints (survival estimates) are transformed into binary end points,
clinically relevant. Based on this, others prefer to use the maximally
selected log-rank test.[52,78,79] In our opinion, the latter has some
important advantages over ROC analysis. First, there is no need to
transform the time-dependent end points. Second, the test calculates an
exact cut-off point and provides a P value to substantiate its
discrimination power.[81]
Immunophenotypic shift:
Comparison of paired presentation/relapse samples showed instances of
selective LAIP changes. These changes consist in reduction/loss or
increment/gain of antigens expression in AML. The antigens more
frequently lost are CD11b, CD14, CD15, while those more often acquired
are CD34 and CD117.[82-85] Our and others’ opinion is that changes
between diagnosis and relapse might depend on outgrowth of
therapy-resistant sub-clones characterized by immunophenotypic
aberrancies distinct from those belonging to the original clone.[86] The
outgrowth of such minor subpopulation(s) until overt relapse, might
theoretically be anticipated since diagnosis, if such subpopulations
are identified. In this view, appears critical, once again, the number
of fluorochromes in the assay. Moreover, these immunophenotypic
“shifts” may be correlated with particular molecular and/or cytogenetic
“shifts”. Seven patients whose mutational status at diagnosis was
determined in cell-sorted sub-fractions, experienced a relapse
characterized by changes in the mutation pattern. Actually, the
mutations observed at relapse were already present at low frequencies
in the primitive CD34+CD38- populations.[86]
In line with this, Angelini et al.[87] evaluated a possible correlation
between specific LAIPs and the presence of mutations of FLT3 and NPM1.
BM samples from 132 newly diagnosed AML patients were analyzed by
9-color MPFC. Within the CD34+ population, a small fraction of CD123+CD99+CD25+ cells was identified. The expression of this phenotype in ≥11.7% of the CD34+ cells, correlated with the presence of FLT3-ITD mutations, with a specificity and sensibility >90%. CD34+CD123+CD99+CD25+ clones were also detectable at presentation in 3 patients who had FLT3wild type/NPM1mut AML and who relapsed with a FLT3mutated/NPM1mutAML. In all of the 3 cases, RQ-PCR designed at relapse for each FLT3-ITD confirmed the presence of low copy numbers of the mutation in the diagnostic samples.
Peripheral blood vs BM in MRD monitoring by MPFC:
Peripheral blood (PB) is an attractive alternative source for MRD
detection, considering that BM collection is a burden for the patients,
can be quite traumatic and, in some cases, the aspiration fails (dry
tap). Furthermore, PB MRD might have higher specificity due to the
relative absence of normal myeloid progenitors in PB. We demonstrated
that after induction and consolidation therapy, the findings in BM and
PB were significantly concordant.[88] The cut-off value of residual
leukemic cells in PB which correlated with outcome was 1.5×10-4. After consolidation, 38 of 50 patients had a level of MRD >1.5x10-4,
and 31 (82%) had a relapse. Recently, Zeijlemaker et al.[89] observed a
significant correlation between PB and BM and that MRD detection in PB
is more accurate than in BM. With MRD being assessed after induction
therapy, the 1-year cumulative incidence of relapse therapy was 29% for
PB MRD negative and 89% for PB MRD positive patients (p<0.001).
Three-year overall survival was 52% for MRD negative and 15% for
positive patients (p=0.034). Similar differences were found after
consolidation therapy.
Leukemic Stem Cell (LSC):
Finally, a lot of attention is being dedicated to the identification of
leukemic stem cell (LSC). Targeting LSC represents a very ambitious
goal not only for MRD purposes but also for the formidable therapeutic
implications. LSC resides within the CD34+CD38-
cell fraction is responsible for leukemia initiation and relapse
because of its self-renewal and repopulating capacity.[90,91] Since LSC
is more resistant to chemotherapy than the more mature CD34+CD38+
progeny, its persistence after chemotherapy may explain treatment
failure in MPFC MRD negative AML patients. The expression of
LSC-specific markers, such as CD47,[92] CD123, CD44 and C-type
lectin-like molecule 1(CLL-1)[93,94] allows to distinguish LSCs from
their normal counterpart. In particular, it was found that CLL-1
expression on CD34+CD38-
is relatively stable between diagnosis and relapse.[93,95] Using the
combination CLL-1/CD34/CD38, Van Rhenen et al.[96] demonstrated that
high percentages of residual LCS, as measured at each course of
chemotherapy, correlated with shorter patient survival. Moreover,
combining LSC and MRD frequencies, 4 patients’ groups, with different
survival, were identified. The LSC-/MRD- group had the best prognosis
while the LSC+/MRD+ the worst. In order to better quantify LSC both at
diagnosis and follow-up, Zeijlemaker et al.[97] designed a single
8-color detection tube including common markers (CD45, CD34 and CD38),
specific markers (CD45RA, CD123, CD33, CD44) and a marker cocktail
(CLL-1/TIM-3/CD7/CD11b/CD22/CD56) in one fluorescence channel. The LCS
detection tube allows recognizing not only residual cells with an
immunophenotype established at diagnosis but also those with emerging
immunophenotypes. Additionally, this tube is lower in costs and
requires fewer BM materials as compared with a multiple-tubes approach.
Future Directions
MRD detection may help refine risk-assessment of AML and, therefore, “customize” the therapeutic decision-making process. In this view, a comprehensive risk-stratification, generated by integrating the prognostic role of pre-treatment (cytogenetics/genetics) and post-treatment parameters (MRD), might help allocate the majority of patients in a more realistic category of risk. The adjusted risk-allocation might implement selection of a more appropriate post-remission strategy, particularly in regard to ASCT. In conclusion, the current treatment strategy of patients with AML must rely on a rigorous biological characterization at diagnosis to allow high risk patients to be treated intensively and timely submitted to ASCT. For the remainders, estimation of MRD status appears appropriate in order to extrapolate patients at high risk of relapse (MRD positive) for whom ASCT is required to pursue a survival advantage and low risk patients (MRD negative) for whom standard treatments may be adopted, avoiding excessive toxicity that may jeopardize an otherwise favorable clinical outcome.
References

































































































[TOP]