Kayıhan Pala, Harika Gerçek, Tuncay Aydin Taş, Rukiye Çakir, Sedef Özgüç And Timur Yildiz
1 Public
Health Department, Uludag University Faculty of Medicine, Bursa, Turkey
2 Nilufer
Tuberculosis Control Association Dispensary, Bursa, Turkey
Corresponding
author: Tuncay AYDIN TAŞ, MD, Public Health Department, Uludag
University Faculty of Medicine, Bursa, Turkey. Tel: +90 02242954281,
E-mail:
taydintas@uludag.edu.tr
Published:
November 1, 2016
Received: June 6, 2016
Accepted: October 23, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016059, DOI
10.4084/MJHID.2016.059
This article is available on PDF format at:

This is
an Open Access article distributed under the terms of the Creative
Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Objective: The
aim of this study is to describe the epidemiological and clinical
aspects of patients who applied to the Bursa Nilufer Tuberculosis
Dispensary by investigating the trends in epidemics over three decades.
Method:
In this retrospective observational study, the records of all
tuberculosis cases (1630 patients) treated in the last 30 years
(1985-2014) at the Bursa Nilufer Tuberculosis Dispensary were examined
and statistically analyzed.
Results:
Males comprised 65.2% of the patients. The ages of the patients ranged
from 1 to 87 years, and the mean age was 37.4 (95% CI: 36.6-38.2).
Among the cases, 86.7% were new infections and 74.1% were pulmonary
tuberculosis. In the last decade, the education level, the percentage
of patients who had received a BCG vaccination, the proportion of women
and active employees among them increased (p<0.05), while it
decreased among men (p<0.05). Clinical symptoms accompanying TB
such
as weakness, anorexia, weight loss, and cough, decreased to a
statistically significant degree (p<0.05). In the last decade,
the
mortality rate was 3.6% and increased compared with previous decades
(p<0.05). Mortality was higher among patients who were elderly,
male, did not have a BCG scar or had a chronic disease (p<0.05).
Conclusion: This
study adds information about the change of TB epidemics in Turkey in
the last 30 years. Further studies are needed to determine the risk
factors associated with tuberculosis mortality and to evaluate the
effectiveness control programs of this disease.
|
Introduction
Tuberculosis
(TB) is a disease that primarily affects the lungs, but it can spread
to extrapulmonary organs through lymphogenic and hematogenous routes.1
Approximately one-third of the world population has an asymptomatic and
non-infectious latent infection. About 10% of these asymptomatic
patients progress to active disease and approximately 45% of
individuals with the active disease die if it is not treated.[1,2]
The
World Medical Association emphasizes that poverty fuels the spread of
tuberculosis by causing limited access to primary health care services,
inducing malnutrition, and inadequate living conditions; therefore,
tuberculosis should be considered as a disease of poverty and
inequality.[3]
Despite notable progress in the past decade,
tuberculosis is still a public health concern in most of the countries
within the World Health Organization (WHO) European Region. Countries
outside of the European Union (EU) and European Economic Area (EEA)
still suffer from high rates of TB and multidrug-resistant TB, while
EU/EEA countries have a significant number of TB cases among vulnerable
population groups, such as people of foreign origin and prisoners. In
2014, an estimated 340 000 incident cases of TB (range 320 000–350 000)
occurred in the WHO European Region, equivalent to 37 cases (35–38) per
100 000 population. This number represents about 3.6% of the total
burden in the world. About 83% of incident TB cases in 2014 occurred in
the 18 high-priority countries.[4]
Turkey is one of the 18
high-priority countries.
The first data regarding the
epidemiological situation of tuberculosis in Turkey pertained to the
year 1950. TB mortality, which was 204/100.000 in 1950, decreased to
8.8/100.000 in 1980 and 1.6/100.000 in 2000. While the tuberculosis
incidence was 177/100.000 according to the values for 1960, it dropped
to 24/100.000 in 2002.[5] In
Turkey, the estimated TB prevalence was
22/100.000, the incidence was 18/100.000, and the mortality was
0.61/100.000 for 2014.[6]
In Turkey, the TB control program began in
1918 under the guidance of Tuberculosis Control Associations, which
were voluntary organizations, and have been maintained via vertical
structuring within the Ministry of Health and provincial
organizations.[7] Public Health Law
(No. 1593, 1930) designated
tuberculosis as a notifiable disease and made its treatment free of
charge. In Turkey, information regarding the patients comes primarily
from the records of Tuberculosis Control Dispensaries (TCDs). Valuable
information resources are also the general death records and the
results of epidemiologic studies. Since 2007, the Department of
Tuberculosis Control has collected information regarding patients
registered at the TCDs and has published them as reports.[8]
TCDs
are the health institutions that provide diagnosis, treatment,
follow-up and control, patient notification, registration, archiving
and statistics, immunizations, screening, drugs, training, health
education activities, social welfare, coordination, and consultancy.[9]
The TCD follows the guidelines of "Stop TB Strategy" and the
"International Standards for Tuberculosis Care" adopted by the WHO.[10]
Bursa
Nilufer Tuberculosis Control Dispensary was established as the Bursa
Tuberculosis Control Association in 1948 and was included among public
interest associations in 1949.[11]
Since its service building moved
from the city center to the Nilufer district in 2003, the Association
has provided services as the Nilufer Tuberculosis Control Association
Dispensary.[12]
The dispensary case series are relevant for determining the current
situation of tuberculosis in Turkish.
In
this study, we analyzed socio-demographic characteristics, the clinical
findings, diagnosis and treatment processes and treatment results of
the patients who applied to Bursa Nilüfer
Tuberculosis Control
Dispensary (NTCD) in a period of thirty years.
Material
and Method
Bursa
is the fourth most populous city (1.8 million) in Turkey and is located
in Northwestern Anatolia in Turkey. Nilufer is one of the three central
districts of Bursa which was established on the western side of the
city. Nilufer is the newest and most planned and organized district of
Bursa; it is the most rapidly urbanizing area of the city, and its
population is slight over 300.000. The Nilufer ranks the first place
amongst the districts of Bursa significantly contributing to the
economy of Turkey and Bursa. The first Organized Industrial Zone of
Turkey has been established within the district of Nilufer in 1961.
There are twenty-three Family Health Centre, one Community Health
Center, one Tuberculosis Control Dispensary and two public hospitals in
Nilufer.
This descriptive study was carried out between June 2014
and February 2015. The files of all cases (1662 people) receiving
tuberculosis treatment in Nilufer Tuberculosis Control Dispensary
(NTCD) were reviewed, comprising the treatments of the last 30 years
(1985-2014). Thirty-two patients who had started treatment of
tuberculosis were later diagnosed carriers of another disease and then
excluded from this study. Therefore only the data of 1,630 patients
were evaluated.
TB cases were diagnosed both in Dispensary and
public hospitals. Pulmonary TB was diagnosed by X-ray, smear microscopy
of sputum and sputum culture. The most commonly used media for the
isolation of tuberculosis were solid egg-based (Löwenstein–Jensen,
Ogawa) and agar-based (Middlebrook 7H10 or 7H11) media; manual liquid
synthetic (Middlebrook 7H9) and automated liquid media (Bact/Alert 3D,
MGIT 960, VersaTREC). Non-pulmonary TB was diagnosed only in hospitals.
According to legislation in Turkey, patients diagnosed of TB in
hospitals referred to the regional dispensary for receiving TB
treatment. In 30 years there was no change in the definitive TB
diagnosis (X-ray, smear microscopy of sputum and sputum culture) in
Dispensary; but there is no information in defining TB diagnosis
methods in the hospitals in the dispenser records.
In this study,
were retrospectively examined the tuberculosis patient registers,
tuberculosis patient monitoring vouchers, patient examination forms and
computer records. The data obtained were included in a data collection
form consisting of 48 questions. The patients’ socio-demographic
information (age, gender, marital status, educational status,
profession, work conditions, social security status), the presence of
chronic disease, TB history, the history of contact with a tuberculosis
patient, the presence of a Bacillus Calmette-Guérin (BCG) scar,
symptoms accompanying the diagnosis, case description, reason for
examination, TB locations and treatment result were examined in this
form. The underlying concepts and definitions used in this study were
based on the "T. R. Ministry of Health Tuberculosis Diagnosis and
Treatment Guidelines 2011".[10]
The occupations of working patients
were classified according to the International Standard Classification
of Occupations ISCO 08.
Drug resistance has been evaluated in
patients since mid-1990’s. TB resistance was determined by drug
susceptibility testing. In the laboratory, drug susceptibility testing
of TB isolates was performed by the proportion methods (agar based MB
7H10/11, Löwenstein Jensen); automated fluid systems (MGIT 960,
VersaTREK) and molecular methods (Real-time PCR, reverse
hybridization). Multidrug-resistant TB is resistant to at least
isoniazid and rifampin. Patients with the following characteristics
were considered at risk of MDR-TB:
• Failure of retreatment regimens,
• Chronic TB cases,
• Exposure to a known MDR-TB case,
• Failure of first-line chemotherapy,
• Relapse and return after default
without recent treatment failure, and
• History of using poor or unknown
quality TB drugs.
TB
treatment has been changing for the last 30 years period in the
dispensary. In the first two decades nine-month treatment regimens were
applied (in the first two months isoniazid, rifampin,
pyrazinamide, and ethambutol or streptomycin and then for 7 months
isoniazid and rifampin). In the last decade, a six-months standard TB
treatment was applied. Standard TB treatment includes the use of 4
drugs: rifampin, pyrazinamide, isoniazid, and ethambutol given for two
months, followed by a rifampin/isoniazid continuation phase for an
additional four months.
The permission for the study was received
from Uludağ University, Faculty of Medicine, Ethics Committee (dated
June 10, 2014, and numbered 2014-12/3).
The
research data were evaluated using the SPSS 18.0 software package. The
descriptive statistics, chi-square test, chi-squared test for trend and
Fisher's exact test were employed in the data analysis. A p-value less
than 0.05 was considered significant.
Results
The
total number of 1630 TB cases received tuberculosis treatment in NTCD
from 1985 through 2014. When the patients were grouped into three
periods of ten years (classified according to when the patient file was
opened), 627 patients were diagnosed from 1985-1994, 490 from
1995-2004, and 513 from 2005-2014. 65.2% of the patients were male, and
the male/female ratio was 1.88. The patients’ ages ranged from 1 to 87
years; the mean age was 37.4 (95% G.A.: 36.6-38.2). The gender and age
distribution of the cases are shown in Table 1 and
socio-demographic characteristics of the cases according to sex and
ten-year periods are shown in Table 2.
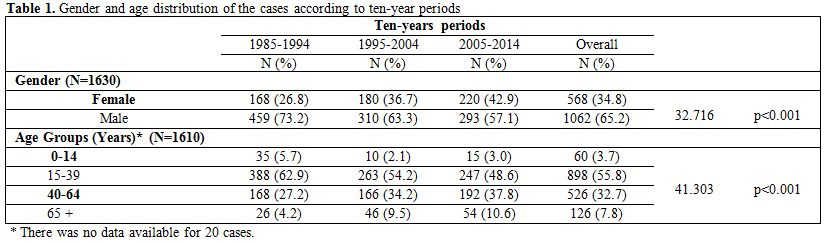 |
Table 1. Gender and age distribution of the
cases according to ten-year periods |
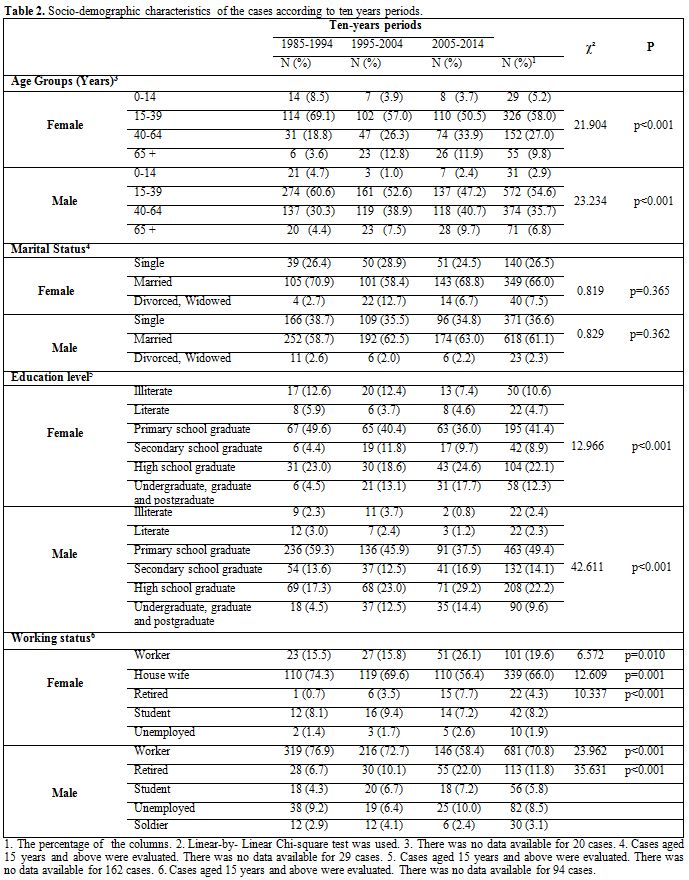 |
Table 2. Socio-demographic characteristics of
the cases according to ten years periods. |
According
to Dispensary records, 1572 cases were born in Turkey (96.4%), the rest
of them were born in eleven different countries (Bulgaria, Greece,
Yugoslavia, Turkmenistan, Georgia, Macedonia, Saudi Arabia, Uzbekistan,
Ethiopia, Azerbaijan, Iraq) except Syria. There were no refugees from
Syria receiving TB treatment in the NTCD.
The most frequently
systemic symptom observed was coughing (72.1%). Night sweating was
observed in 49.6%, sputum in 46.3%, hemoptysis in 14.9%, fever in 7.9%,
and side pain in 3.1%. Allegations of malaise, anorexia, weight loss
and coughing decreased in the last decade compared with the previous
ten-year periods; however, complaints of back pain increased in the
years (Table 3).
The main characteristics of TB cases related to diagnosis and treatment
are shown in Table 3.
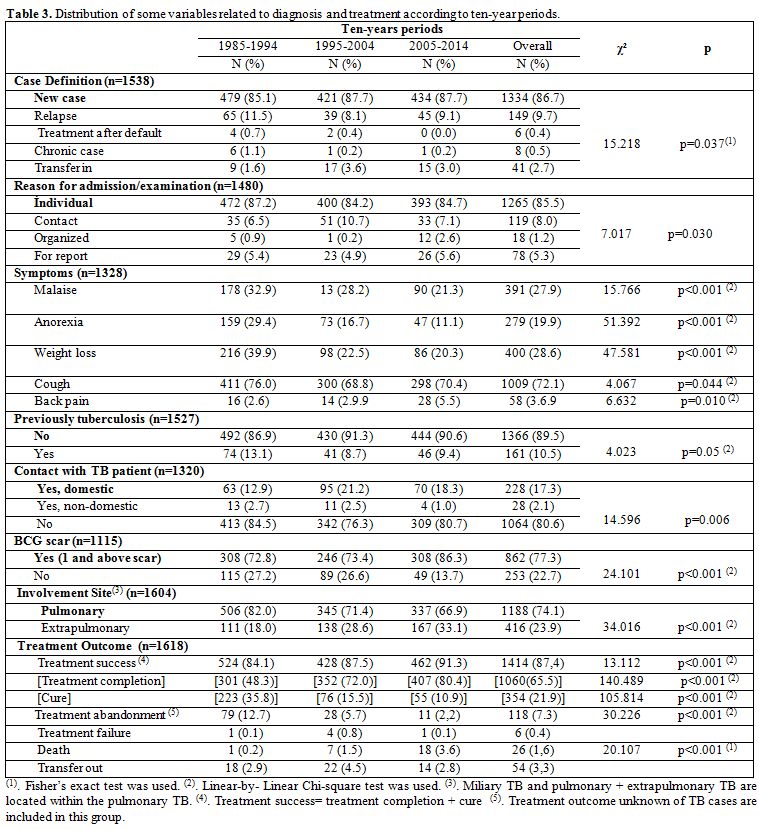 |
Table 3. Distribution of some variables related
to diagnosis and treatment according to ten-year periods. |
The
relapse rate was 1.7% in the 0 -14 year age group compared with 15.4%
in the 40-64 year age group (p<0.001); it was 11.8% in pulmonary
TB cases compared with 3.8% in extra pulmonary TB cases
(p<0.001).
The relapse rate, BCG scar presence, and contact history are shown
according to gender, age group and involvement site in Table 4.
Domestic
contact was determined for all children (0-14 year age group). Among
all adult females, the non-contact was 77.1%, domestic contact was 21%,
and the non-domestic contact was 1.9 %; among all adult males, the
non-contact was 83.6, the domestic contact was 14.4 %, and the
non-domestic contact was 2% in men (p=0.014).
The site involvement in relation to gender, age group, and treatment
success is shown in Table 5.
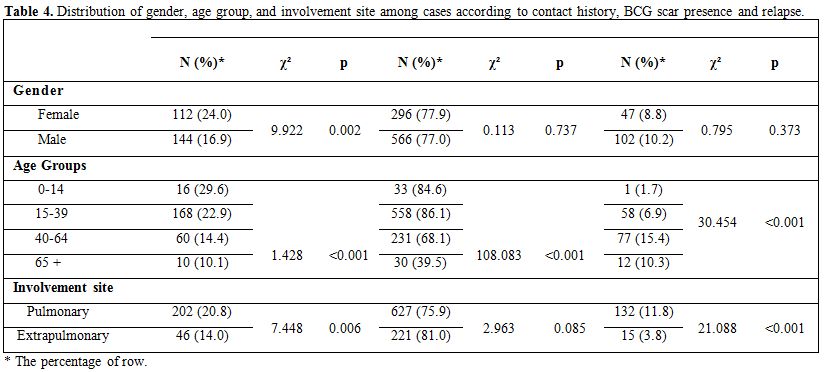 |
Table
4. Distribution of gender, age group, and involvement site among cases
according to contact history, BCG scar presence and relapse. |
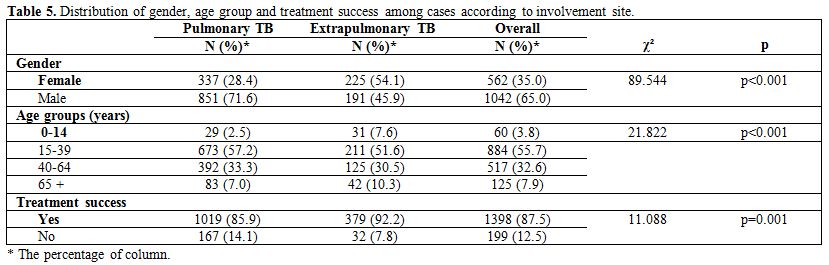 |
Table 5. Distribution of gender, age group and
treatment success among cases according to involvement site. |
Involvement
of the pleura was found in 11.5% of the cases, lymph node involvement
in 9.2%, bone involvement in 1.6% and genitourinary system involvement
in 1.6%.
All of the 26 deaths occurred in patients aged 40 years and
older. The mortality rate was significantly higher among elderly (over
65) people compared to 40-64 age groups (p<0.001), males
(p=0.038),
patients without a BCG scar (p=0.012), patients with multi-drug
resistant TB (p=0.033) and those with chronic diseases (p<0.001).
When
the BCG scar was analysed according to ten-year periods, the proportion
of patients with a BCG scar of the 15-39 year age group decreased from
72.9% in the first ten years to 55.7% in the most recent decade, and
the proportions among those aged 40-64 years and over 65 years
increased from 19.8% to 34.1% and from 1.0% to 6.9%, respectively
(p<0.001).
Twenty-four percent (1035) of the TB patients had at
least one chronic disease (No information about chronic diseases were
available for 595 patients). The presence of chronic diseases in TB
cases have risen from 11,3% in the first decade to 26,6% in the second
decade and 37,6% in last decade respectively (p<0.001). The
accompanying chronic diseases were: diabetes mellitus in 31.3%;
hypertension in 16.9%; chronic obstructive pulmonary disease (COPD),
asthma and respiratory illness including chronic bronchitis in 14.1%
and heart disease in 9.2%.
Culture
and/or microscopic examinations could not be performed for 22.7% of the
patients with pulmonary TB. In these cases, TB was diagnosed with
clinical suspicion, X-rays, and ex juvantibus therapy. The proportion
of pulmonary TB cases with positive bacteriology significantly
increased (p<0.001) over the most recent decade (Table 6).
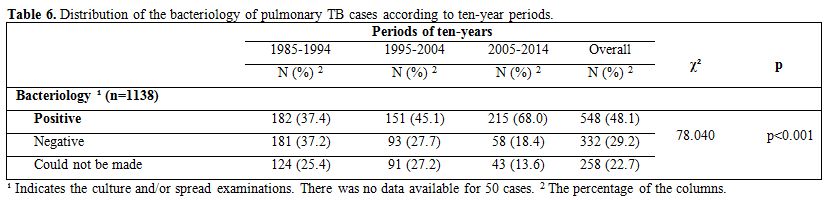 |
Table 6. Distribution of the bacteriology of
pulmonary TB cases according to ten-year periods. |
Among
the new pulmonary TB cases with positive bacteriology, treatment
success was experienced by 80.7% during the first decade, 86.8% during
the second decade and 92.1% during the third decade (p=0.003).
Drug
resistance was examined in 198 cases, and multi-drug resistant TB was
positive in 6.1%. There is no significant difference between ten-year
periods regarding multi-drug resistant TB (p=0.666).
Discussion
In
the most recent decade, the number of patients diagnosed with
tuberculosis at the NTCD was lower than in the first 10 years but
higher than in the second decade. In the 1980s, the incidence of TB in
our country was greater compared to the second decade as well as other
developing countries. TB incidence has decreased in second decade due
to impact of the fight against tuberculosis and socio-economic
development. However, the fight against TB has declined in the last
decade in Turkey; for example, some dispensaries have been closed by
Ministry of Health. Both closure of a Dispensary in Bursa and the
increase in population due to internal migration in City may have led
to increasing the number of patients in last decade.
Social Security
Institution (SSI) patients were registered at TCDs with the transfer of
SSI hospitals to the Ministry of Health.[8]
These changes of this
study, although parallel the rises and falls throughout all the Turkey,
may be related to the patient registration and notification system, the
related screening programs, patients' applications to the TCDs and the
registering of SSI patients in the national TB program.
Similar to
other studies carried out in Turkey and throughout the world, this
study found a higher proportion of males than females among TB patients
(throughout Turkey, 58.6%; in the USA, 62%).[13-18]
Men are more
likely than women to participate in social and working life and
therefore more often exposed to infection. In addition to male- and
female-specific biological and epidemiological differences, the low
number of female tuberculosis case notifications can be explained with
to the difficulties that woman face in access to health services due to
various socio-economic and cultural factors.[19-21]
In this study, the
proportion of female patients was significantly higher in the most
recent decade compared with the previous ten-year periods. This datum
can be explained by an increase of females’ participation in social
life and work life and improved access to health services as well as
with a real increase in incidence of TB due to worsening economic and
living conditions.
The age distribution of tuberculosis patients is
also an important indicator in determining the changes in TB's
epidemiology. In developed countries, tuberculosis is most common among
the elderly and mostly results from the reactivation of a previous
primary infection; however, in developing countries, TB affects all age
groups, especially youth and young adults.[22-24]
Throughout Turkey,
5.4% of those who received treatment between 2005 and 2011 were in the
0-14 year age group, 58.3% were in the 15-44 year age group, and 11.1%
were in the 65 and over age group.[17]
In the USA, 5% of tuberculosis
patients are below the age of 15 years, 40% of them are in the 15-44
year age group, and 24% of them are over 65 year-old.[18]
In our study,
although the majority of the patients were in the 15-44 year age group,
in line with nationwide studies of Turkey and other studies, the
proportion of patients who were younger than 15 years was
lower.[14,17,25] When ten-year periods were
compared, the proportion of
patients who were below the age of 15 years was significantly lower in
the first decade than in the most recent decade whereas that of the
45-64 year age group and the over 65 age was higher. Although this
indicates that the disease moved from the young age group towards an
older age group, the change may have resulted from the ageing of the
population, the fact that elderly people are more prone to infections
and the difficulty of diagnosing tuberculosis infections in childhood
in the past.[23,24,26]
A low level of education is a risk factor for
TB at the individual level.[27] In
studies carried out in Van and
Isparta in Turkey, the proportions of TB cases with an elementary
educational attainment or below are 83.3% and 66.3%, respectively
higher than in this study (54.9%).[28,29]
The proportion of patients
with nine or more years of education increased linearly between
ten-year periods for females (p=0.005) and males (p<0.001). This
increase may have been related to the increase in the general education
level of the patients who visited the NTCD and of the country’s
population in general; furthermore, it indicates that tuberculosis does
not affect only people with lower educational levels.
The
percentages of occupation, business and working status along and the
indicators related to the working lives of the patients varied across
the studies. In this study, the percentage of unemployed patients was
6.2%, which was lower than in other studies. The percentage of employed
patients was 53.0%, which was higher than in other studies.[14,19]
In a
study carried out in North America, TB was associated with working in
non-professional jobs and with unemployment.[27]
In this study, the
percentage of those working in jobs that did not require qualification
was significantly higher among workers, and the increase in this
category in the most recent decade was statistically significant. The
sociocultural characteristics of the patients, poor working conditions
in jobs that did not require qualification, subcontracted labor,
crowded living conditions and screening programs in workplaces are
considered important reasons for this increase.
The relapsed cases
can be related to insufficient previous treatment, re-exposure to
infections, endogenous reactivation of pulmonary or extrapulmonary
focus of infection among TB patients and having multidrug-resistant TB.
In a study carried out in Brazil, the relapse rate was reported as 5.5%
and was associated with high mortality.[30]
This study had a lower
percentage of new cases than other studies, but a higher relapse
rate.[14,29]
The relapse rate decreased in the second decade, tended to
increase in the third decade and was significantly higher among
patients aged 40 years and older. The high relapse rate may have been
due to the high rate of treatment abandonment or happens because the
directly observed treatment could not be implemented effectively, or
because of inadequate or inappropriate treatment regimens, drug
resistance, or advanced age.
The most contagious patients produce
living TB bacilli with sputum. The contact time with a patient
producing Koch bacilli and the intensity of the bacilli are the risk
factors in the development of infection.[31]
The reported history of
contact is 8.5%, 13.5% and 38.8% in various studies.[14,15,29] The
present study found that the history of contact was high in females and
children with pulmonary tuberculosis, similar to other
studies.[26,32,33]
In this study, the patients’ contact rate with
tuberculosis patients was higher in the last decade than in the first
decade and was lower than in the second decade (p=0.006). Among the
patients as a whole, TB contact history rate was 19.4%, but diagnosed
by contact examination for TB rate was 8% and this rate was higher in
the last decade than in the first decade but lower than in the second
decade (p=0.030). The contact examinations are important for
identifying hidden TB patients who do not visit the dispensary or who
wait until late presenter patients and children who are infected as a
result of close contact with infectious adult and adolescent TB
patients. The high contact rate in the NTCD region paired with a low
contact examination rate means that it is necessary to improve contact
examinations. In Turkey, the percentage of extrapulmonary TB cases was
reported to be 27.0% in 2005, 36.8% in 2011 and 36.0% in 2014.[6,17]
In
2011, 27.3% of the male patients and 50.2% of the female patients had
extrapulmonary involvement.[17] In
the study by Özkara et al. in 108
dispensaries, the rate of extrapulmonary TB was reported to vary
between 14.8% and 28.4% between provinces.[34]
In this study, although
the rate of extrapulmonary TB was significantly lower in males, relapse
cases and patients with a history of contact in the last decade, it was
significantly higher in females[14,35,36] and those younger than 15
years[35] and most frequently
showed pleura and lymph node
involvement,[30,37]
similar to some domestic and foreign studies. In
studies carried out in the United States and Brazil, drug resistance
was reported to be low in extrapulmonary TB patients.[30,36]
Extrapulmonary tuberculosis can be affected by comorbidities that cause
immunodeficiency, such as HIV infection; patient characteristics;
endocrine factors; immunological and genetic susceptibilities; and
differences in endemic tuberculosis strains by population and
geographical region, and these factors should be identified.[30,35,36]
Extrapulmonary tuberculosis had different epidemiological
characteristics and risk factors from pulmonary tuberculosis; it can
become infectious if it spreads to the lungs, but it is otherwise not
infective. Therefore, it is important to determine the risk factors of
extrapulmonary tuberculosis for early diagnosis and treatment.
In
this study, similar to others, TB cases were most often accompanied by
coughing symptoms upon admission in 25.4% to 80% of patients (in
Isparta in Turkey 25.4%, in Los Angeles 72.7%, in Samsun in Turkey 80%
of patients).[29,38,39]
In different studies, the other symptoms upon
admission were weight loss in 44.5% to 62.9%, anorexia in 11.7% to
59.7%, night sweating in 16% to 54.0% and fever in 52.3%.[29,38,39]
In
the study carried out in Los Angeles, the presence of coughing, fever,
weight loss and hemoptysis was associated with health insecurity and a
negative tuberculin skin test.[38]
The complaints of malaise
(p<0.001), anorexia (p<0.001), weight loss
(p<0.001) and
coughing (p=0.044) decreased in the most recent decade compared with
the previous decades. These findings may indicate that diagnosing TB
based on classic symptoms is less possible.
The
meta-analysis by Colditz et al. reported that the BCG vaccine decreased
the tuberculosis infection risk by an average of 52.0-74.0% and also
decreased the deaths from lung tuberculosis, meningitis, miliary and
tuberculosis in newborns.[40]
However, the BCG vaccine does not protect
from primary infection or reactivation of latent pulmonary
tuberculosis, which is the main source for the spread of the bacilli in
the community.[41] In a study
carried out in Izmir, the percentage of
0-14- year-olds with a BCG scar was determined to be 74.6%.[33] In the
present study, the proportion of patients with a BCG scar was 84.6%
among the 0-14 age group and was significantly lower among those aged
40 years and older. The overall percentage of patients with a BCG scar
and the percentage of patients aged 40 years and older with a BCG scar
have increased at a statistically significant rate in the most recent
decade. This increase may also occur due to vaccination of some cases
in BCG campaigns across the country that took place between 1953 and
1967.[42] There was no significant
difference between those with a BCG
scar and those without a BCG scar regarding pulmonary and
extrapulmonary organ involvement. Among those with BCG scar, the
mortality rate was significantly lower. Along with the need for further
studies that demonstrate the protectiveness of the BCG vaccine and its
effect on mortality, these results suggest the need for other, more
effective vaccines to prevent latent tuberculosis as well.
The WHO
aims to identify at least 70% of smear-positive tuberculosis patients
bacteriologically and to treat at least 85% of them successfully. The
percentages of treatment success and case identification are the
outcomes used to measure the effectiveness of tuberculosis
control.[43,44] In this study,
among patients with pulmonary TB, the
percentages with examined bacteriologically and positive bacteriology
were 77.3% and 62.3%, respectively, which are consistent with the
values determined in other studies.[34,39]
In the study by Özkara et
al.,[34] the treatment success of
new pulmonary TB cases with positive
bacteriology was 82.5%, which is lower than the value determined in the
present study (87.1%). In the present study, while treatment success
was significantly higher in the most recent decade than in the previous
decades, the percentages of patients who were cured and those who
abandoned treatment were significantly lower. “Treatment success” means
the total of treatment completion and cure rates. The increase of the
treatment success may result from decrease in treatment abandonment
along with the increase in treatment completion. The lack of request
for bacteriological examination in the end of therapy and the patients
who were unable to sputum specimens could be the reason for low cure
rates.
A study
carried out in Brazil found that being over the age of 40 years,
concomitant HIV infection, illiteracy, severe extrapulmonary TB and
re-treatment after relapse were risk factors associated with
mortality.[30] In this study, all
of the 26 observed deaths occurred in
patients aged 40 years and older. There was no significant difference
in mortality between the pulmonary and extrapulmonary TB patients. The
overall mortality rate was 3.6% in the most recent decade, which was
significantly higher than that of the previous decades
(p<0.001).
The fact that the mortality rates were significantly lower in the past
decades may arise from an inability to determine deaths because of high
rates of treatment abandonment and low presence of chronic diseases.
The mortality rate was high among elderly people (p<0.001),
males
(p=0.038), patients without a BCG scar (p=0.012), patients with
multi-drug resistant TB (p=0.033) and patients with accompanying
chronic diseases (p<0.001). All of the deaths were accompanied
by at
least one chronic illness. The increase in mortality rates among
patients treated at the NTCD may have resulted from the growth in the
elderly population. This age group has a higher risk of contracting TB,
but more frequently presents the masking of the symptoms for
comorbidities, a late diagnosis arising from a lack of sufficient care,
and a low compliance and the occasional use of drugs because of their
side effects. Further studies are needed to determine risk factors and
the reasons for the recent increase of mortality to reduce the spread
of TB and the deaths.
Strengths
and Limitations of the Study
The
fact that the study covers an extensive period of 30 years and the high
number of cases reported is the strength of this survey, which analyzes
only the data and risk factors that were defined in the records. The TB
patients treated at the NTCD were not only residents of the Nilufer
district of Bursa but also of some other districts that the dispensary
serves. Therefore, the TB prevalence and incidence for the Nilufer
district could not be calculated in this study.
Conclusion
The
fact that the study covers an extensive period of 30 years and the high
number of cases reported is the strength of this survey, which analyzes
only the data and risk factors that were defined in the records. The TB
patients treated at the NTCD were not only residents of the Nilufer
district of Bursa but also of some other districts that the dispensary
serves. Therefore, the TB prevalence and incidence for the Nilufer
district could not be calculated in this study.The number of patients
treated for tuberculosis in the NTCD was lower in the most recent
decade than in the first decade of the survey and higher than in the
second decade. In the last ten years, the proportion of females,
elderly people and patients with nine years of education or more
increased significantly, and of workers among female patients. However,
low income countries should further reduce the obstacles related to
gender in the diagnosis and treatment of tuberculosis by making
structural and social changes, such as increasing women's socioeconomic
circumstances and education levels, reducing discrimination and
implementing strategies to remove gender inequalities in health.
Unfortunately, the mortality rate also significantly increased probably
determined by higher number, pulmonary tuberculosis with positive
bacteriology, of elderly people, with accompanying chronic diseases,
resistance to antibiotic could also have a role.
It is necessary to increase the efficiency of the control programs and
laboratories to detect patients with active pulmonary tuberculosis and
efficiently treat them avoiding their contact with risk groups,
infants, older and immunodeficient people. These procedures could
reduce the spread of infection within the community. The effectiveness
of tuberculosis control programs can improve by continuous training of
health workers and population, and by functioning of a registration
system should to ensure the reliability of patient data. It is
necessary to design extensive epidemiological studies that examine the
effectiveness of the fight against tuberculosis also in low-income
countries, the risk factors and patient mortality and reveal the causal
factors. It must not be forgotten that tuberculosis is a social disease
that is affected by the inequality in health, and efforts should be
made to bring services to patients who cannot reach healthcare services
on their own.
References
- World Health
Organization. WHO tuberculosis. Available from: http://www.who.int/mediacentre/factsheets/fs104/en/
(Accessed 02/11/2015).
- World
Health Organization. Global Tuberculosis Report 2015. Available from: http://apps.who.int/iris/bitstream/10665/191102/1/9789241565059_eng.pdf?ua=1
- World
Medical Association. Physicians advised to treat
tuberculosis as a disease of poverty. Available from: http://www.wma.net/en/40news/20archives/2015/2015_43/index.html
(Accessed 25/12/2015).
- European
Centre for Disease Prevention and Control/WHO Regional Office
for Europe. Tuberculosis surveillance and monitoring in Europe 2016.
Stockholm: European Centre for Disease Prevention and Control, 2016.
Available from: http://ecdc.europa.eu/en/publications/Publications/tuberculosis-surveillance-monitoring-Europe-2015.pdf
(Accessed 29/08/2016)
.
- Vidinel
I. A historical view to tuberculosis in Turkey In: Bilgic H,
Karadag M, eds. Toraks Kitaplari Tiberkoloz. Istanbul, AVES Yayincilik.
2010; 17-24. (in Turkish)
- World
Health Organization. WHO tuberculosis. Available from:
<https://extranet.who.int/sree/Reports?op=Replet&name=/WHO_HQ_Reports/G2/PROD/EXT/TBCountryProfile&ISO2=TR&outtype=html
(Accessed: 29/11/2015).
- �zkan
S. Basic Institution of fight against tuberculosis in our
country: Tuberculosis Dispensary In: Bilgic H, Karadag M, eds. Toraks
Kitaplari T�berk�loz. Istanbul, AVES Yayincilik. 2010; 680-686. (in
Turkish).
- �zkara
S. Tuberculosis Epidemiology in Turkey. In: Bilgic H, Karadag M,
eds. Toraks Kitaplari Tiberkoloz. Istanbul, AVES Yayincilik. 2010;
36-47. (in Turkish)
- �zdemir
T, Torkkani MH, Yilmaz Aydin L , Balci o, Danacibas R, Bilgin
A. Provincial assessment in the scope of the tuberculosis control
program. Tuberk Toraks. 2014;62(3):183-190. DOI: 10.5578/tt.7899. Epub
2014 August 7. http://www.tuberktoraks.org/linkout.aspx?pmid=25492815

- T.
R. Ministry of Health Tuberculosis Diagnosis and Treatment
Guidelines 2011. In: Akdag R, eds. Ankara, Basak Matbaacilik ve Tanitim
Hizmetleri Ltd. Sti. 2011;1. (in Turkish)
- Turkish
Republic Official Newspaper. Available from: http://www.resmigazete.gov.tr/main.aspx?home=http://www.resmigazete.gov.tr/arsiv/7110.pdf&main=http://www.resmigazete.gov.tr/arsiv/7110.pdf
(Accessed : 01/04/2016).
- Turkish
Rebuplic Ministry of Health Department of Tuberculosis 16.09.2003 tarih
ve 7546 sayili yazisi. (in Turkish).
- Ko�akoglu
S, Simsek Z, Ceylan E. Epidemiologic characteristics of the
tuberculosis cases followed up at Sanliurfa central tuberculosis
control dispensary between 2001 and 2006 years. Turkish Thoracic
Journal. 2009;10:9-14 http://www.turkishthoracicjournal.com/FullText/416/epidemiologic_characteristics_of_the_tuberculosis_cases_fol
l

- Orman
A, �nl� M, Cirit M. Assessment of 627 tuberculosis cases observed
in Afyon tuberculosis dispensary between 1990 and 2000. Respiratory
Diseases. 2002;13 (4):271-276. http://www.respiratorydiseasesjournal.org/text.php3?id=184

- Martinez
AN, Rhee JT, Small PM, Behr MA. Sex differences in the
epidemiology of tuberculosis in San Francisco. Int J Tuberc Lung Dis.
2000;4(1):26–31. PMid:10654640

- �ift�i
F, Bozkanat E, Ilvan A, Kartaloglu Z, Sezer O, Kaliskan T, Kaya
H. The year of 2003 treatment results of soldier patients with
tuberculosis in a military hospital which has feature of the referance.
Turk Thorac J. 2006;7(1):45-50.

- Turkish
Republic Ministry of Health, Public Health Institution of
Turkey Department of Tuberculosis. Tuberculosis struggle in Turkey 2013
Report: In: Özkan S, Musaonbasioglu S, eds. Ankara, Uzman Matbaacilik,
2014. (in Turkish).
- CDC.
Reported Tuberculosis in the United States, 2014. Atlanta, GA:
U.S. Department of Health and Human Services, CDC, October 2015 .
Available from: http://www.cdc.gov/tb/statistics/reports/2014/pdfs/tb-surveillance-2014-report.pdf
- Borgdorff
MW, Nagelkerke NJD, Dye C, Nunn P. Gender and tuberculosis: a
comparison of prevalence surveys with notification data to explore sex
differences in case detection. Int J Tuberc Lung Dis. 2000
Feb;4(2):123-32. PMid:10694090

- Neyrolles
O, Murci LO. Sexual inequality in tuberculosis. PLoS Medicine
2009;6(12):1-6. http://dx.doi.org/10.1371/journal.pmed.1000199
PMid:20027210 PMCid:PMC2788129

- Hudelson
P. Gender differentials in tuberculosis: the role of
socio-economic and cultural factors. Tuber Lung Dis. 1996
Oct;77(5):391-400. http://dx.doi.org/10.1016/S0962-8479(96)90110-0

- Tatar
D, Keskin p, zacar R, Halilolar H. Similarity and differences
of tuberculosis in the young and the elderly patients. Tuberculosis and
Thorax. 2002; 50: 485-91.
 http://www.tuberktoraks.org/abstracttext.aspx?issue_id=17&ref_ind_id=246
http://www.tuberktoraks.org/abstracttext.aspx?issue_id=17&ref_ind_id=246 
- Öztop
A, tnsal I, Genay T, Özge A, kakmak R, Uoku R. The
epidemiological characteristics of tuberculosis patients registered in
Kahramanlar dispensary between 1999 and 2003. Respiratory Diseases.
2006;17(3):123-132. http://www.respiratorydiseasesjournal.org/pdf/pdf_SHD_332.pdf

- Kili�aslan
Z. Epidemiology of Tuberculosis and Tuberculosis in the
World. In: Bilgic H, Karadag M, eds. Toraks Kitaplari Tuberkoloz.
Istanbul, AVES Yayincilik. 2010; 25-35. (in Turkish).
- Kiter
G, Coskunol I, Alptekin S. Evaluation of tuberculosis patients
enrolled to Esrefpasa tuberculosis dispensary between january 1997 to
june 1998. Tuberculosis and Thorax . 2000; 48(4): 333-339. http://www.tuberktoraks.org/abstracttext.aspx?issue_id=9&ref_ind_id=111

- Sen
V, Uluca , Yilmaz S, Selimoglu Sen H, Tuncel T, Genes A, Karabel
M, Garkan MF. The evaluation of the clinical and laboratory
characteristics of children with pulmonary tuberculosi. Dicle Medical
Journal. 2014; 41(3): 552-557. http://dx.doi.org/10.5798/diclemedj.0921.2014.03.0473

- Young
BN, Rendon A, Rosas A, Baker J, Healy M , Gross JM , Long J ,
Burgos M , Hunley KL. The effects of socioeconomic status, clinical
factors, and genetic ancestry on pulmonary tuberculosis disease in
northeastern mexico. PLOS ONE 2014; 9(4): 1-8. Epub 2014 April 11, http://dx.doi.org/10.1371/journal.pone.0094303

- �zbay
B, Sezgi C, Altin�z O, Sertogullarindan B, Tokg�z N. Evaluation
of tuberculosis cases detected in our region between 1999 and 2003.
Tuberculosis and Thorax. 2008;56(4):396-404. http://www.tuberktoraks.org/abstracttext.aspx?issue_id=41&ref_ind_id=624

- Zengin
E, Kisioglu AN, S�nmez Y. The characteristics of tuberculosis
cases which registered in Isparta central district tuberculosis
dispensary and the sufficiency situation of dispensary registries: 2000
to 2007 years . SD� Tip Fak�ltesi Dergisi 2009;16(3):14-18. (in
Turkish). http://dergipark.ulakbim.gov.tr/sdutfd/article/view/1089001618/1089001643

- Gomes
NMDF, Bastos MCDM, Marins RM, et al. Differences between risk
factors associated with tuberculosis treatment abandonment and
mortality. Pulmonary Medicine, vol. 2015, Article ID 546106, 8 pages. http://dx.doi.org/10.1155/2015/546106

- �zt�rk
R. Natural Resistance in Tuberculosis and Risk Factors,
Tuberculosis Semposioum in the 21th Century and 2nd Course of
Tuberculosis Diagnosis Methods in the Laboratory Samsun: 2003.
Available from: http://www.klimik.org.tr/wp-content/uploads/2012/02/982011124221-Recep_Ozturk.pdf
(Accessed: 13/01/2016). (in Turkish)

- Aksel
N, Mertoglu A, Dogan H. Comparison of the male and female cases
with pulmonary tuberculosis. Izmir Gegos Hastanesi Dergisi.
2008;22(2):35-45. http://www.igh.dergisi.org/pdf/pdf_IGH_111.pdf

- Tatar
D, Alptekin S, Coskunol I, Aydin M, Arslangiray S. The
retrospective analysis of tuberculosis in children followed up at Izmir
Esrefpasa dispensary between 1995-2000.Respiratory Diseases.
2002;13(2):94-100. http://www.respiratorydiseasesjournal.org/text.php3?id=154

- �zkara
S, Kiliraslan Z, Oztark F, Seymenoglu S , Erdogan AR ,Tellioglu
C ,Kosan AA, Kaya B, Kologlu F ,Kibaroglu E. Tuberculosis in Turkey
with regional data. Turkish Thorasic Journal. 2002;3(2):178-87. http://www.turkishthoracicjournal.com/FullText/969/tuberculosis_in_turkey_with_regional_data

- Forssbohm
M, Zwahlen M, Loddenkemper R, Rieder HL . Demographic
characteristics of patients with extrapulmonary tuberculosis in
Germany. European Respiratory Journal 2008 Jan;31(1):99-105. Epub 2007
Sep 5. http://dx.doi.org/10.1183/09031936.00020607

- Peto
HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology
of extrapulmonary tuberculosis in the United States, 1993–2006. Clin
Infect Dis. 2009;49(9):1350-1357.
http://cid.oxfordjournals.org/content/49/9/1350.full.pdf html http://dx.doi.org/10.1086/605559

- Kolsuz
M, Ersoy S, Demircan N, Metintas M, Erginel S, Alatas F, Urgun
I. The evaluation of pulmonary tuberculosis patients enrolled to
Eskisehir Deliklitas tuberculosis control dispensary. Tuberculosis and
Thorax. 2003;51(2):163-170 http://www.tuberktoraks.org/abstracttext.aspx?issue_id=20&ref_ind_id=295

- Miller
LG, Asch SM, Emily IY, Knowles L, Gelberg L, Davidson P. A
population-based survey of tuberculosis symptoms: how atypical are
atypical presentations? Clin Infect Dis. 2000;30(2):293-299. doi:
10.1086/313651. http://cid.oxfordjournals.org/content/30/2/293.full.pdf+html
http://dx.doi.org/10.1086/313651

- Kayhan
S. Demographic and clinical characteristics of tuberculosis: A
report of 2404 cases at a referral hospital. Afr. J. Microbiol. Res.
2012;6(9):2033-2037

- Coldwitz
GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E,
Fineberg HV. The efficacy of bacillus Calmette-Guerin vaccination of
newborns and infants in the prevention of tuberculosis: meta-analyses
of the published literature. Pediatrics. 1995;96(1):29-35. http://pediatrics.aappublications.org/content/pediatrics/96/1/29.full.pdf

- World
Health Organization Geneva. WHO car Vaccine [online]. Weekly
epidemiological record, 2004;79:25-40. Available from: http://www.who.int/wer/2007/wer8221.pdf
PMid:14768304

- Kili�aslan
Z, Kologlu F. The Role of the Tuberculosis Society Fight
against TB in Turkey. In: Bilgic H, Karadag M, eds. Toraks Kitaplari
Tiberkoloz. Istanbul, AVES Yayincilik. 2010; 673-679. (in Turkish).
- World
Health Organization. Stop TB Partnership and World Health
Organization. The Global Plan to Stop TB, 2006-2015: actions for life
towards a world free of tuberculosis. WHO, Geneva, Switzerland.
Available from: http://www.stoptb.org/assets/documents/global/plan/globalplanfinal.pdf
- Jordan
TS, Davies PD. Clinical tuberculosis and treatment outcomes. Int
J Tuberc Lung Dis 2010;14(6):683-8. PMid:20487604

[TOP]
















 http://www.tuberktoraks.org/abstracttext.aspx?issue_id=17&ref_ind_id=246
http://www.tuberktoraks.org/abstracttext.aspx?issue_id=17&ref_ind_id=246 


















