Vincenzo De Sanctis1, Ashraf T. Soliman2, Heba Elsedfy3, Alice Albu4, Soad Al Jaouni5, Salvatore Anastasi6, Maria Grazia Bisconte7, Duran Canatan8, Soteroula Christou9, Shahina Daar10, Salvatore Di Maio11, Mohamed El Kholy3, Doaa Khater12, Mohamed Elshinawy13 ,Yurdanur Kilinc14, Roberto Mattei15, Hala H. Mosli16, Alessandra Quota17, Maria Grazia Roberti18, Praveen Sobti19, Saif AL Yaarubi20, Saveria Canpisi21 and Christos Kattamis22
1 Pediatric and Adolescent Outpatient Clinic, Quisisana Hospital, Ferrara, Italy.
2
Department of Pediatrics, Division of Endocrinology, Hamad General
Hospital Doha, Qatar and Department of Pediatrics, Division of
Endocrinology, Alexandria University Children's Hospital, Alexandria,
Egypt.
3 Department of Pediatrics, Ain Shams University, Cairo, Egypt.
4 Endocrinology and Diabetes Department of Elias Hospital, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania.
5
Head Division of Pediatric Hematology Oncology, Deputy Chair of
Hematology & Head Section of Hematology Research Lab, King Fahd
Medical Research Center Department of Hematology Faculty of Medicine,
King Abdulaziz University Jeddah, Kingdom of Saudi Arabia.
6 Thalassemia Unit, Maternal and Child Department, Garibaldi Hospital, Catania, Italy.
7 Thalassemia Unit, Cosenza, Italy.
8 Director of Thalassemia Diagnosis Center of Mediterranean Blood Diseases Foundation Antalya, Turkey.
9 Thalassemia Unit, Nicosia, Cyprus.
10
Department of Haematology, College of Medicine and Health
Sciences, Sultan Qaboos University, Sultanate of Oman & Visiting
Scholar, Stellenbosch Institute for Advanced Study (STIAS), Wallenberg
Research Centre at Stellenbosch University, Stellenbosch 7600, South
Africa.
11 Emeritus Director in Pediatrics, Children's Hospital "Santobono-Pausilipon", Naples, Italy.
12
Department of Pediatrics, Endocrinology Unit, Alexandria University
Children's Hospital, Egypt and Child Health Department, Sultan Qaboos
University Hospital, Muscat, Sultanate of Oman.
13
Department of Pediatrics, Hematology Unit, Faculty of Medicine,
University of Alexandria, Egypt and Child Health Department, Sultan
Qaboos University Hospital, Muscat, Oman.
14 Çukurova University, Medical Faculty, Department of Pediatric Hematology, Adana, Turkey.
15 Pediatric Unit, Adria, Italy.
16
Internal Medicine, Endocrinology and Metabolism, Department of Medicine
King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia.
17 Thalassemia Unit, Gela, Italy.
18 Immunohematology and Blood Bank Unit, OORR Foggia, Italy.
19 Pediatric Hemato-Oncology Unit , Christian Medical College and Hospital, Ludhiana Punjab, India.
20 Head of Pediatric Endocrine Unit, Department of Child Health, Sultan Qaboos University Hospital, Al-Khoud, Sultanate of Oman.
21 Thalassemia Unit, Umberto 1° Hospital, Siracusa, Italy
22 First Department of Paediatrics, University of Athens, Athens, Greece.
Corresponding
author: Vincenzo De Sanctis MD, Pediatric and Adolescent Outpatient
Clinic, Quisisana Hospital, 44100 Ferrara, Italy; Tel.: +39 0532
770243; E-mail:
vdesanctis@libero.it
Published: January 1, 2017
Received: August 20, 2016
Accepted: November 14, 2016
Mediterr J Hematol Infect Dis 2017, 9(1): e2016060, DOI
10.4084/MJHID.2017.001
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Multi-transfused thalassemia major (TM) patients frequently develop
severe endocrine complications, mainly due to iron overload, anemia,
and chronic liver disease, which require prompt diagnosis, treatment
and follow-up by specialists. The most common endocrine
complication documented is hypogonadotropic hypogonadism which
increases with age and associated comorbidities. It is thus important
for physicians to have a clear understanding of the pathophysiology and
management of this disorder. Also to be aware of the side effects,
contraindications and monitoring of sex steroid therapy. In this paper,
practical ICET-A recommendations for the management of hypogonadism in
adult females with TM are addressed. Methods:
In March 2015, the Coordinator of the International Network of
Clinicians for Endocrinopathies in Thalassemia and Adolescent Medicine
(ICET-A) conducted a two-step survey to assess the attitudes and
practices of doctors in the ICET-A network taking care of adult female
TM patients with hypogonadism. They were clinically characterized by
the absence of pubertal development or discontinuation or regression of
the maturation of secondary sex characteristics, and biochemically by
persistent low FSH, LH and estradiol levels. Recently a supplementary
survey on adult female hypogonadism in TM was undertaken within the
ICET-A network. Results:
The completed questionnaires were returned by 16 of 27 specialists
(59.2%) following 590 female TM patients over the age of 18 years; 315
patients (53.3%) had hypogonadism, and only 245 (74.6%) were on hormone
replacement therapy (HRT). Contraceptive oral pills (COC) were the
first treatment choice in 11 centers (68.7%). A wide range of COCs was
used with different progestin contents. In general, the patients’
compliance to treatment was reported as good in 81.2% of centers. The
frequency of required tests for follow-up HRT, in addition to the
regular check-up for thalassemia, was variable in the participating
centers. Conclusions:
Doctors taking care of TM patients should have sound knowledge of the
pathophysiology of hypogonadism in adult females with TM. They should
know the potential effects of HRT including advantages and
disadvantages of estrogen and progestins. Moreover, they should keep in
consideration the emotional needs of these patients dreaming of
attaining a full pubertal development.
|
Introduction
Multi-transfused
thalassemia major (TM) patients frequently develop severe endocrine
complications mainly due to iron overload, anemia, and chronic liver
disease, which require prompt diagnosis, treatment and close follow-up
by specialists.[1-3]
The most common endocrine
complication documented in adult TM patients is hypogonadotropic
hypogonadism which increases with age and the associated comorbidities.[3]
In adult females TM patients, hypogonadism is clinically diagnosed by
the absence of pubertal development, or discontinuation or regression
of the maturation of secondary sex characteristics due to pituitary
dysfunction and/or gonadal damage, secondary to iron overload.[4]
The incidence rate of hypogonadism, in both sexes, varies considerably
between countries and much more between specialized centers, ranging
from around 50% and may even approach 100%.[1-4]
Evidence
suggests that more severe defects are related to a higher rate of iron
loading possibly due to increased vulnerability to free radical
toxicity.[1-4]
Hormone replacement therapy (HRT)
in females with hypogonadism aims to alleviate symptoms of estrogen
deficiency and prevent long-term complications such as osteoporosis.[5]
However, HRT has been linked to various risks and the debate regarding
its risk-benefit ratio continues. The principal risks of HRT are
thromboembolic disease, stroke, cardiovascular events, gallbladder
disease, breast cancer and endometrial hyperplasia or endometrial
cancer.[6]
In March 2015, the Coordinator (VDS)
of the International Network of Clinicians for Endocrinopathies in
Thalassemia and Adolescent Medicine (ICET-A) conducted a two-step
survey to assess the attitudes and practices of doctors taking care of
adult TM with hypogonadism. In this report, we present the results of
the study and the practical recommendations for hypogonadism in adult
females with TM based on literature review and the experience of
specialists of ICET-A network. Where possible, the recommendations are
based on and linked to, the evidence that supports them, unless
good-quality evidence is absent.
Materials and Methods19196677
The first step survey was held on the 19th and 20th of March 2015, in Rome, during the 10th
International Workshop of ICET-A. A questionnaire was distributed
before the beginning of sessions to participants with relevant
experience in thalassemia care. The answers were collected and
discussed at the end of the session. The aim of the study was to
investigate the attitudes and prescription habits of doctors concerning
HRT in TM patients. Exclusion survey criteria included patients with
thalassemia intermedia.
The second step survey was administered online to the ICET-A members on July 4th,
2016. An introductory letter explained the purpose of the study. The
questionnaire consisted of 23 questions, namely: personal doctors’
data, place of work, specialization, number of female patients with TM
followed over the age of 18 years, number of TM patients on HRT, type/s
of HRT used, the patients' compliance to treatment, the speciality of
the physician recommending HRT, opinions on indications and
contraindications for HRT use among doctors and types and number of
tests used during patients' follow-up.
After collection and analysis of data, the ICET-A Steering Committee (VDS, ATS, HE, MEK, SDM, CK) prepared (third step)
practical recommendations for the management of these patients. In
making these recommendations, experts considered the differences in
countries’ facilities, general cost of tests and recommended management
of hypogonadism. The ICET- A members were asked to provide comments on
the accuracy, feasibility, and approval of the recommendations.
Results
First step:
Twenty-five questionnaires were distributed, and 24 (96%) were
answered. The participants included ten pediatricians, four
endocrinologists, and ten hematologists. They were following a total of
2326 females and males with TM.
Twelve different formulations and
three routes of administration for HRT were used. The majority of
respondents (33.3%) used ethinyl estradiol 30 µg/drospirenone 3 mg as
first-line treatment choice, (25%) ethinyl estradiol 20 µg/drospirenone
3 mg. Ethinyl estradiol 35 µg/cyproterone acetate 2 mg (41.6%) and
ethinyl estradiol 20 µg/drospirenone 3 mg (29.1%) were reported as
second-line treatment choice. Transdermal patch, estradiol transdermal
plus progesterone, and etonogestrel/ethinyl estradiol vaginal ring were
used and recommended by 16.6%, 4.1%, and 4.1%, respectively.[7]
Second step:
The questionnaires were returned by 16 of 27 specialists: 6 pediatric
endocrinologists, 2 endocrinologists; 3 pediatric hematologists, 3
hematologists, 1 pediatrician and 1 general practitioner [the majority
(75%) were female doctors] following 590 female TM patients over the
age of 18 years; 315 (53.3%) had hypogonadism, and 245 (74.6%) were on
HRT.
The reported most common contraindications to treatment
were: elevation of liver enzymes - from 3 to 6 times the normal values
(62.5%), thrombophilia (43.7%), insulin dependent diabetes (25%),
insulin dependent diabetes associated with vascular complications
(6.2%), patient non-compliance to treatment (25%). HRT was recommended
by endocrinologists in 9 thalassemia centers, by endocrinologists and
gynecologists in 6, and by endocrinologist and hematologist in 1
center. Responders were asked to select the three commonest compounds
used as HRT. Contraceptive oral pills (COC) were the first choice of
treatment in 11 centers. A wide range of COCs was used with different
progestin contents, such as drospirenone, dydrogesterone, norgestrel,
norethisterone, gestodene, desogestrel, medroxyprogesterone acetate,
micronized progesterone soft gelatin capsules. In 6 Centres transdermal
estrogen patch in combination with oral progesterone was given as the
first choice of treatment. In general, patients' compliance to
treatment was reported as good in 81.2% of Centres.
The
frequency and number of required tests during follow-up, in addition to
the regular check-up for thalassemia, varied in the participating
Centres (even in centers within the same country). The results for each
Centre participating in the survey are reported in Table 1.
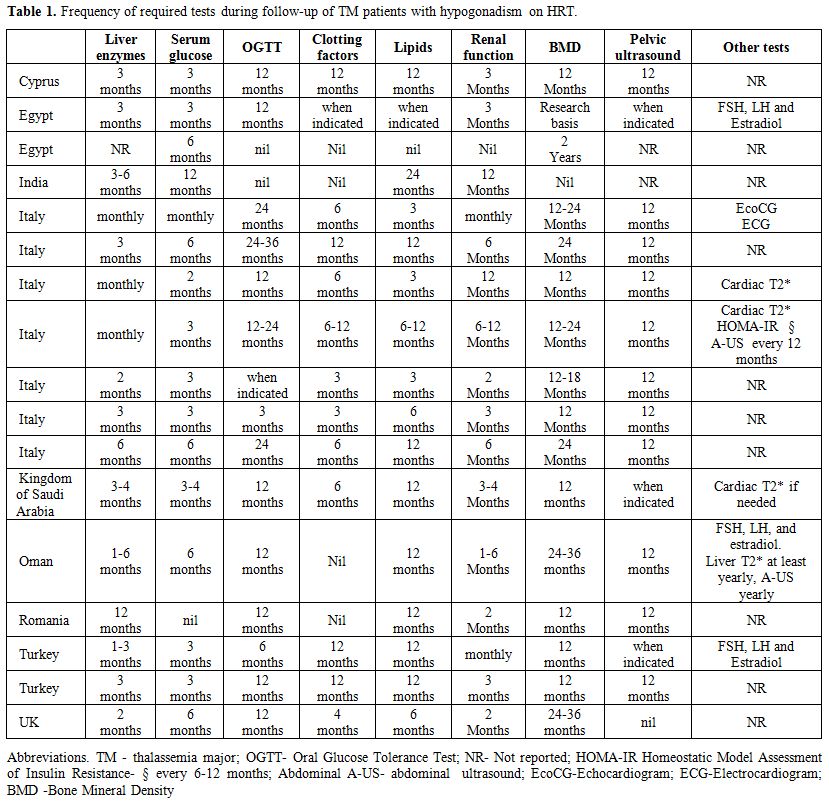 |
Table
1. Frequency of required tests during follow-up of TM patients with hypogonadism on HRT. |
Third step:
The ICET-A recommendations for hypogonadism in adult females with TM
were based on published, peer-reviewed scientific evidence, expert
opinion, and accumulated professional knowledge and experience of
ICET-A network specialists. Recommendations from published guidelines
were used when available and appropriate. Original articles for the
evidence-based recommendations were obtained following a computer
search for ‘hormone replacement’ as a keyword and also in combination
with ‘venous thrombosis’ (VTE) or ‘deep venous thrombosis’ (DVT) or
‘pulmonary embolism’ or ‘thrombophilia’ or "chronic liver disease" or
"diabetes" applied to Medline.
The ICET-A Network also issued
expert consensus opinions on topics for which limited or low-level
evidence was available in the literature. Since not all published
references were based on randomized controlled trials, the
recommendations have been scored according to the following criteria:
A. High confidence indicates that further research is unlikely to change the confidence in the estimate of effect (●●●)
B. Moderate confidence indicates that further research may change the confidence in the estimate of effect (●●○)
C.
Low confidence indicates that further research would likely have a
significant impact on the confidence in the estimate of effect (●○○)
D. Insufficient indicates that the evidence is unavailable or does not permit a conclusion (○○○)
Discussion
The
goals of substitutive therapy in adult female patients with
hypogonadism are to maintain secondary sexual characteristics, to
optimize the accrual of bone mineral content and to promote physical
and social well-being.
Few trials of the effects and
complications of estrogen therapy in primary and secondary hypogonadism
of women at premenopausal age have been published and none of TM
patients. As a consequence of the scanty evidence, recommendations for
HRT in thalassemia are based on publications on the effects and
complications of COC used for contraception and postmenopausal hormone
replacement in healthy women.
The three forms of estrogen produced
in the human body are estrone (E1), estradiol (E2) and estriol (E3).
The estrogen composition in the female body is approximately 3%
estrone, 7% estradiol, and 90% estriol. The potencies of these hormones
vary, with estradiol being the most potent followed by estrone and
estriol.[6,8]
Sequential
estrogen-progestogen replacement therapy is the mainstay of treatment
for women with hypogonadism. Estrogen may be replaced using oral,
micronized, vaginal, or transdermal preparations. Subcutaneous implants
and more recently, nasal sprays and injectable estrogen preparations
are also available.
There are three types of estrogen available
for hormone replacement: estradiol, ethinylestradiol (a synthetic
estrogen, EE) and conjugated equine estrogens (derived from pregnant
mare urine, CEE). Major characteristics that differentiate one
formulation from another include the form of estrogen used and its
dosage, and the generation of the progestin.
The formulations of
COC have changed over the past 50 years. The dose of the EE component
has decreased from the original 100-150 μg to 15 to 30 μg. These
changes were made to lower the risk of thromboembolic complications
associated with the use of oral contraceptive pills.[6]
In
the absence of a consensus regarding the ideal hormonal replacement
regimen for women facing a premature cessation of ovarian function, the
estroprogestative substitution commonly involves either HRT or COC
prescription.
Several studies compared estrogen
preparations in adult females, but the adolescent and young adult
population are relatively understudied.[9-12] A recent
report in girls with Turner syndrome demonstrated more physiologic
estrogen concentrations with the use of the transdermal estrogen
preparation versus oral preparations.[13] Ninety
percent of the EE is absorbed from the upper gastrointestinal tract in
1 to 2 hours, then exposed to oxidation. Following absorption, EE is
metabolized during passage through the enterohepatic circulation. EE
has a strong hepatic impact related to its 17a-ethinyl group. This
group prevents the inactivation of the EE and results in a slow
metabolism and prolonged tissue retention. EE is much more potent than
the naturally secreted estrogens because it remains in the blood for a
longer time after administration and has a greater effect on the liver.[8]
In
our survey, the majority of specialists (11 centers) preferred COCs as
the first line of treatment COCs of convenience, efficacy and patients’
preference and availability.
COC are classified into different
generations (first, second, third and fourth), depending on the time of
introduction into the market. They vary regarding the dose of estrogen
and the type of progestin. Progestins are needed to avoid an unopposed
estrogen effect and maintain endometrial health. Progestins can be
administered via the oral, transdermal (as a patch), or intra-uterine
routes. Micronized progestogens are available to use orally, vaginally
and as transdermal (cream) preparations.
Progestins have no
selectivity for the various steroid receptors. The first progestins
developed were medroxyprogesterone acetate (MPA) and norethisterone
enanthate (NET-EN). Shortly after, these were followed by its first
derivative norethisterone acetate (NET-A). Many more synthetic
progestins have been developed in the following years. We now have
second, third and fourth-generation progestins. Examples are
levonorgestrel (LNG, 2nd generation), gestodene (GES, 3rd
generation), and drospirenone (DRSP), dienogest (DNG) and trimegestone
(TMG), all fourth generation. The third-generation progestins have
minimal impact on blood glucose levels, plasma insulin concentrations,
and the lipid profile. Thus, they are suitable for use in patients with
lipid disorders or diabetes.[9-11]
Contrary to
menopausal women, adolescents and young female adults with hypogonadism
due to other pathologic mechanisms as in TM patients, the HRT treatment
is extremely complex because of associated comorbidity (iron overload,
the presence of thrombophilic status, chronic liver disease, impaired
glucose tolerance or diabetes and cardiovascular disease). In addition,
the long- term duration of chelation treatment and psychosocial
patients’ needs enhance the difficulty of the management.[1-4,14-18]
Taher et al.[17]
reported in a retrospective multicentre study, that thromboembolic
events (TE) occurred in a clinically relevant proportion (1.65%) of
8,860 thalassemia patients (75.3% with TM). Thromboembolic events were
4.38 times more frequent in thalassemia intermedia (TI) than in TM
patients (p < 0.001). More venous events occurred in TI and more
arterial events took place in TM.
A survey, done in 9 Italian
thalassemia Centres, disclosed that 32 patients out of a total of 735
(683 with TM and 52 TI), had VTE episodes corresponding to an incidence
of 3.95% and 9.61%, respectively. Localization of TE varied; the main
one (16/32) involved the central nervous system.[15]
Patients
with TE events presented a higher incidence of associated organ
dysfunction, such as cardiomyopathy, diabetes, liver function
anomalies, and hypothyroidism than those without TE events (50 vs.
13.8%, p <0.05).[15]
Haghpanah and
Karimi conducted an electronic search on PUBMED (MEDLINE), SCOPUS, and
Google Scholar databases up to January 2011. Out of 152 thalassemic
patients with cerebral thromboembolic events; 48% were splenectomized.
Nine TM patients had diabetes. Activated protein C resistance,
decreased protein C or protein S or plasminogen level were detected in
8 patients.[18] Inadequate transfusion was reported
to increase the risk of thrombosis secondary to increased release of
pro-coagulant red cell particles.
Oral administration of EE
leads to pharmacologic concentration of the hormone in the portal vein
before it is metabolized by the liver. This first-pass reaction results
in an increased hepatic production of several hormone binding
globulins, clotting factors, lipoproteins and angiotensinogen. This
increase in VTE risk is highest during the first year of use. It may
vary according to the different characteristics of COCs, such as
estrogen dose, molecule, and type of progestins. Whether the type of
estrogen molecule is associated with different degree of risk for
venous thrombosis remains controversial.[19,20]
Based
on the Women’s Health Initiative (WHI) trials, oral conjugated equine
estrogen and 2.5 mg MPA increased VTE compared with placebo (RR, 2.06;
CI, 1.57–2.70). These findings, however, require confirmation.[21,22]
Newer generation formulations of hormonal contraceptives seem to be more thrombogenic than those of second-generation.[19,20,22]
Using LNG as the reference , VTE rate ratios for other progestins were:
NET-EN0.98, desogestrel 1.82, GES 1.86, DRSP 1.64 and cypropterone
(CPA) 1.88.[19,20,22]
Some
observational studies assessed the risk of VTE associated with
transdermal estrogen therapy in non-thalassemic population. The
pro-thrombotic effects seem to be circumvented by transdermal
administration of estrogen and, therefore, have significant clinical
implications.[22]
The VTE risk for vaginal ring or patch is as high as for COCs of third or fourth generation.[22]
Liver dysfunction in thalassemics is mainly attributable to liver siderosis and chronic HCV infection (chronic hepatitis C).[23,24]
Furthermore, chronic hemolysis in TM favours the development of
bilirubin gallstones. The incidence of gallstones varies considerably
in clinical studies and is related to age and the efficiency of
transfusion treatment of studied cohorts. Thirty percent of 858
consecutive Italian TM patients had chololithiasis diagnosed by
abdominal ultrasonography or a history of cholecystectomy.[25]
In addition, chronic application of third generation progestogens as
contraceptives or HRT could influence the serum lipid profile, and
consequently increase the risk of biliary lithogenicity.[26]
Although
elevation of liver enzymes - from 3 to 6 times the normal values was
the commonest contraindication to hormonal treatment reported by 10/16
Centres, further studies including liver imaging and LIC assessment are
needed to clarify the role of HRT on liver enzyme levels, metabolic
variables and liver fat content.
Insulin dependent diabetes (IDDM)
and impaired glucose tolerance (IGT) are relatively common
complications in thalassaemia major (ΤΜ) patients with iron overload
and sub-optimal chelation therapy. The prevalence of IDDM and IGT in
adolescents and young adults with TM mainly treated with
desferrioxamine mesylate (DFO) varies considerably in 2 studies ranging
from 0 to 21% and from 9.3 to 24.3%, respectively.[27,28]
Even higher differences exist in other studies depending on the age
composition and on the efficiency of chelation of the
studied TM cohorts.
Currently, there appear to be wide
variations in the way that professionals evaluate the risk-benefit
equation in subjects with IDDM, and significant differences in
prescribing practice have been identified. In women with
insulin-dependent or non–insulin-dependent diabetes COCs use have
limited effect on daily insulin requirements and no effect on long-term
diabetes control or progression to retinopathy, if clinical and
metabolic monitoring can be ensured. COCs must be avoided in case of,
cardiovascular disease or severe microvascular complications such as
nephropathy with proteinuria or active proliferative retinopathy.[29-33]
The
safety of prescription of COCs to women with type II diabetes is
unclear, but a supervised program similar to that of IDDM patients is
recommended.
Conclusions and Recommendations
Despite
the large number of patients for whom HRT is prescribed, there are no
prospective studies of treatment and/or recommendations to guide
clinicians in the application of the optimal treatment regimens in
patients with TM presenting with hypogonadism and complications
influenced by HRT. Therefore, there is an urgent need to develop
guidelines based on solid research in order to optimize the care of
this group of women.
The United States Medical Eligibility Criteria (US MEC) for Contraceptive Use, in July 2016,[33]
recommended the following medical eligibility criteria categories for
estrogen/progestin pill, hormonal patch and combined vaginal ring:
1 = A condition for which there is no restriction for the use of the contraceptive method.
2 = A condition for which the advantages of using the method generally outweigh the theoretical or proven risks.
3 = A condition for which the theoretical or proven risks usually outweigh the advantages of using the method.
4 = A condition that represents an unacceptable health risk if the contraceptive method is used.
No restrictions are reported for estrogen/progestin pill, patch or vaginal ring in TM patients (category 1).
Regarding some other pathologies, the US MEC for the contraceptive use,
reported the following risk categories: an increased risk in the
presence of family history (1st-degree relatives) for venous thrombosis (category 2)
and a high risk in presence of past TE and known thrombogenic mutations
(e.g., factor V Leiden; prothrombin mutation; and protein S, protein C,
and antithrombin deficiencies) (category 4);
in women with chronic hepatitis, COC use does not increase the rate or
severity of cirrhotic fibrosis, nor does it increase the risk for
hepatocellular carcinoma (category 1); a small increased risk for asymptomatic gallbladder disease (category 2), in subjects with gallbladder disease treated medically the risk is higher (category 3);
in women with insulin-dependent or non–insulin-dependent diabetes COC
use have limited effect on daily insulin requirements and no effect on
long-term diabetes control or progression to retinopathy (category 1), in presence of associated nephropathy, retinopathy, or neuropathy the risk is high (category 4);
for atherosclerotic cardiovascular diseases (e.g. smoking, diabetes,
hypertension, low HDL, high LDL, or high triglyceride levels) the risk
is high (category 3/4); in subjects with migraine without aura or with aura the risk category is 2 and 4, respectively.
On
deciding to treat a hypogonadal TM woman with estrogen and progestin,
consideration must be given to the general condition of the patient,
current chelation therapy and the presence of associated complications.[34-36]
To
minimise the potential risks of treatment, excessively high sex
hormonal concentrations should be avoided.[36] The aim is to achieve,
in regularly menstruating women the typical mean serum estradiol levels
of approximately 100 pg/ml (400 pmol/l).[37]
Transdermal administration of 25-50 µg 17β estradiol generally produces
in TM patients a plasma E2 value in the early to mid-follicular phase
range (100-300 pmol/l).[36] Progesterone is usually
given at for 12-14 days each month to bring on a menstrual withdrawal
bleed. Micronized progesterone is composed of smaller particles that
may aid in absorption. It was proposed as first-line progestin because
there are reasons to believe that natural progesterone might be safer
for the cardiovascular system (no adverse lipid effects) and possibly
the breast, although the strongest evidence for endometrial protection
is for oral cyclical combined treatment.[34,35,37-39]
The
potential effects of HRT demand that doctors taking care of TM patients
have a sound knowledge of the benefits and disadvantages of estrogens
and progesterone. They must also possess comprehensive knowledge of
female reproductive biology and particular sensitivity to the emotional
needs of these patients. Current guidelines in patients with premature
ovarian failure suggest that therapy should be continued until the
average age of menopause (age 50 to 51 years) to prevent premature bone
loss, coronary heart disease, and stroke.[40,41]
Because
HRT in patients with chronic diseases is a complex task, the ICET-A
prepared some relative recommendations for HRT in TM patients and its
monitoring (Tables 2 and 3),
based on the data reported in the literature for adolescent and young
women without TM, for HRT with sex steroids. Further research consortia
are needed to investigate these important questions, and to assist
clinicians in making the best possible health care approach for the
adolescents and young women with TM and hypogonadism.
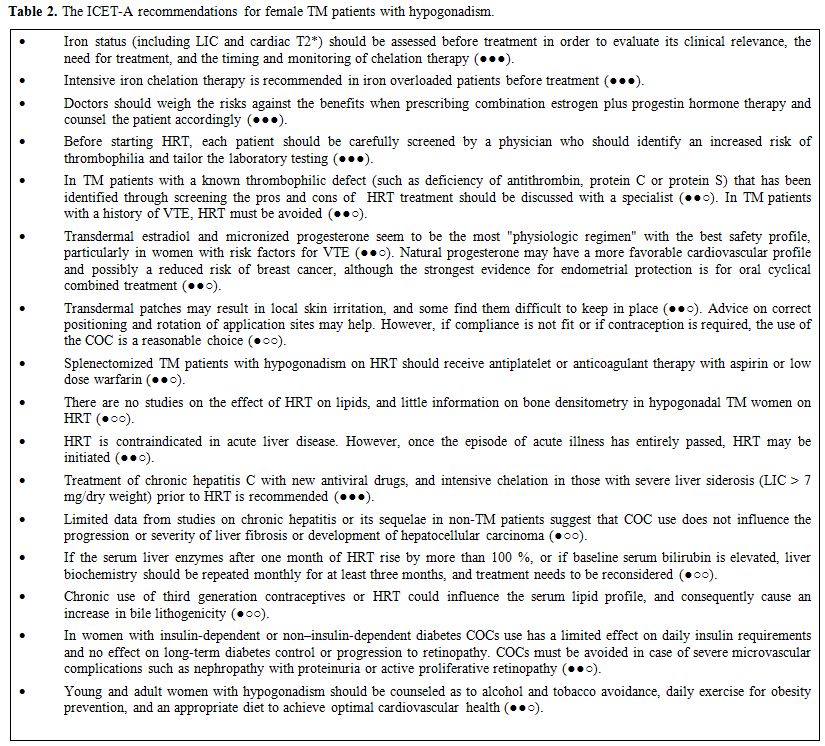 |
Table 2. The ICET-A recommendations for female TM patients with hypogonadism. |
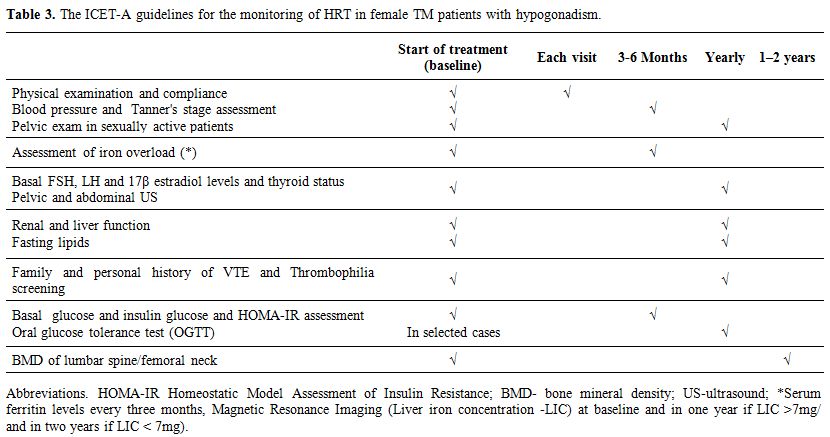 |
Table 3. The ICET-A guidelines for the monitoring of HRT in female TM patients with hypogonadism. |
Acknowledgements
We wish to express
our sincere thanks to dr. Ploutarchos Tzoulis, Department of
Endocrinology, Whittington Hospital, University College London, London,
UK for taking part in the second step survey promoted by ICET-A.References
- De Sanctis V, Elsedfy H, Soliman AT, Elhakim IZ,
Soliman NA, Elalaily R, Kattamis C. Endocrine profile of ß-thalassemia
major patients followed from childhood to advanced adulthood in a
tertiary care center. Indian J Endocrinol Metab. 2016;20:451-459 https://doi.org/10.4103/2230-8210.183456 PMid:27366710 PMCid:PMC4911833

- De
Sanctis V, Elsedfy H, Soliman AT, Elhakim IZ, Kattamis C, Soliman NA,
Elalaily R. Clinical and Biochemical Data of Adult Thalassemia Major
patients (TM) with Multiple Endocrine Complications (MEC) versus TM
Patients with Normal Endocrine Functions: A long-term Retrospective
Study (40 years) in a Tertiary Care Center in Italy. Mediterr J Hematol
Infect Dis. 2016 Apr 12;8(1):e2016022. doi: 10.4084/MJHID.2016.022.
eCollection 2016. https://doi.org/10.4084/mjhid.2016.022

- De
Sanctis V, Soliman AT, Candini G, Elsedfy H. The recommendation of the
International Network of Clinicians for Endocrinopathies in Thalassemia
and Adolescent Medicine for the assessment of growth hormone secretion
in thalassemia.Indian J Endocrinol Metab. 2015;19:306-307 https://doi.org/10.4103/2230-8210.149331 PMid:25729702 PMCid:PMC4319280

- Tiosano D, Hochberg Z.Endocrine complications of thalassemia. J Endocrinol Invest. 2001;24:716-723. https://doi.org/10.1007/BF03343916 PMid:11716158

- Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause 2007;14:567–571 https://doi.org/10.1097/gme.0b013e31804c793d PMid:17476146

- No
author listed. The Writing Group for the Women's Health Initiative.
Risks and benefits of estrogen plus progestin in healthy postmenopausal
women. JAMA 2002; 288: 321-333. https://doi.org/10.1001/jama.288.3.321 PMid:12117397

- De
Sanctis V, Solimam AT, Elsedfy H, Di Maio S. Current practice in
treating adult female thalassemia major patients with hypogonadism: An
International Network of Clinicians for Endocrinopathies in Thalassemia
and Adolescence Medicine survey from Italy. Indian J Endocrinol
Metab.2016 Nov-Dec;20(6):880-881. https://doi.org/10.4103/2230-8210.192905 PMid:27867897 PMCid:PMC5105578

- Andersson
KK, Kappas A. Hormones and liver function. In: Schiff L, Schiff ER,
editors. Diseases of the liver. Philadelphia: JB Lippincott; 1982. p.
167–235.
- Batur
P, Bowersox N, McNamara M. Contraception: Efficacy, Risks, Continuation
Rates, and Use in High-Risk Women. J Womens Health (Larchmt). 2016
Aug;25(8):853-6. doi: 10.1089/jwh.2016.5942. https://doi.org/10.1089/jwh.2016.5942

- De
Leo V, Musacchio MC, Cappelli V, Piomboni P, Morgante G. Hormonal
contraceptives: pharmacology tailored to women's health Hum Reprod
Update. 2016 Sep;22(5):634-46. doi: 10.1093/humupd/dmw016. https://doi.org/10.1093/humupd/dmw016

- Nelson AL. An update on new orally administered contraceptives for women. Expert Opin Pharmacother. 2015;16: 2759-2772. https://doi.org/10.1517/14656566.2015.1100173 PMid:26512437

- Divasta AD, Gordon CM. Hormone replacement therapy and the adolescent Curr Opin Obstet Gynecol. 2010;22:363-368. https://doi.org/10.1097/GCO.0b013e32833e4a35 PMid:20724926

- Torres-Santiago
L, Mericq V, Taboada M, Unanue N, Klein KO, Singh R, Hossain J, Santen
RJ, Ross JL, Mauras N: Metabolic effects of oral versus transdermal
17beta-estradiol (E2): a randomized clinical trial in girls with Turner
syndrome. J Clin Endocrinol Metab. 2013, 98:2716–2724. https://doi.org/10.1210/jc.2012-4243 PMid:23678038

- Moratelli
S, De Sanctis V, Gemmati D, Serino ML, Mari R, Gamberini MR, Scapoli
GL. Thrombotic risk in thalassemic patients. J Pediatr Endocrinol
Metab. 1998;11 (Suppl 3): 915-921. PMid:10091165

- Borgna
Pignatti C, Carnelli V, Caruso V, Dore F, De Mattia D, Di Palma A, Di
Gregorio F, Romeo MA, Longhi R, Mangiagli A, Melevendi C, Pizzarelli G,
Musumeci S. Thromboembolic events in beta thalassemia major: an Italian
multicenter study. Acta Haematol. 1998; 99:76-79. https://doi.org/10.1159/000040814 PMid:9554453

- Michaeli
J, Mittelman M, Grisaru D, Rachmilewitz EA. Thromboembolic
complications in beta thalassemia major. Acta Haematol. 1992;87:71-74. https://doi.org/10.1159/000204720 PMid:1585774

- Taher
A, Isma'eel H, Mehio G, Bignamini D, Kattamis A, Rachmilewitz EA,
Cappellini MD. Prevalence of thromboembolic events among 8,860 patients
with thalassaemia major and intermedia in the Mediterranean area and
Iran. Thromb Haemost. 2006; 96:488-491. https://doi.org/10.1160/th06-05-0267

- Haghpanah
S, Karimi M. Cerebral thrombosis in patients with ß-thalassemia: a
systematic review. Blood Coagul Fibrinolysis. 2012;23:212-217. https://doi.org/10.1097/MBC.0b013e3283502975 PMid:22322139

- Hugon-Rodin
J, Gompel A, Plu-Bureau G. Epidemiology of hormonal
contraceptives-related venous thromboembolism. Eur J Endocrinol.
2014;171:R221-230. https://doi.org/10.1530/EJE-14-0527 PMid:25012200

- Maitrot-Mantelet
L, Hugon-Rodin J, Canonico M. Hormonal contraceptives and venous
thromboembolism: an epidemiological update. Best Pract Res Clin
Endocrinol Metab. 2013;27:25-34. https://doi.org/10.1016/j.beem.2012.11.002 PMid:23384743

- Cushman
M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, Sidney
S, Rosendaal FR; Women's Health Initiative Investigators. Estrogen plus
progestin and risk of venous thrombosis. JAMA. 2004;292:1573-1580. https://doi.org/10.1001/jama.292.13.1573 PMid:15467059

- Santen
RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, Burger HG,
Colditz GA, Davis SR, Gambacciani M, Gower BA, Henderson VW, Jarjour
WN, Karas RH, Kleerekoper M, Lobo RA, Manson JE, Marsden J, Martin KA,
Martin L, Pinkerton JV, Rubinow DR, Teede H, Thiboutot DM, Utian WH;
Endocrine Society. Postmenopausal hormone therapy: an Endocrine Society
scientific statement. J Clin Endocrinol Metab. 2010;95(7 Suppl
1):s1-s66 https://doi.org/10.1210/jc.2009-2509 PMid:20566620

- Prati
D, Capelli C, Silvani C, De Mattei C, Bosoni P, Pappalettera M, Mozzi
F, Colombo M, Zanella A, Sirchia G. The incidence and risk factors of
community-acquired hepatitis C in a cohort of Italian blood donors.
Hepatology. 1997; 25:702-704. https://doi.org/10.1002/hep.510250335 PMid:9049222

- Prati
D, Zanella A, Farma E, De Mattei C, Bosoni P, Zappa M, Picone A, Mozzi
F, Rebulla P, Cappellini MD, Allain JP, Sirchia G. A multicenter
prospective study on the risk of acquiring liver disease in
anti-hepatitis C virus negative patients affected from homozygous
beta-thalassemia. Blood.1998; 92:3460-3464. PMid:9787188

- Origa
R, Galanello R, Perseu L, Tavazzi D, Domenica Cappellini M, Terenzani
L, Forni GL, Quarta G, Boetti T, Piga A. Cholelithiasis in thalassemia
major Eur J Haematol. 2009; 82:22-25 https://doi.org/10.1111/j.1600-0609.2008.01162.x PMid:19021734

- Sieron
D, Czerny B, Sieron-Stoltny K, Karasiewicz M, Bogacz A,
Seremak-Mrozikiewicz A, Kotrych D, Boron D, Mrozikiewicz P. The effect
of chronic estrogen application on bile and gallstone composition in
women with cholelithiasis. Minerva Endocrinol. 2016;41:19-27.
PMid:25413941

- Cunningham
MJ, Macklin EA, Neufeld EJ, Cohen AR. Thalassemia Clinical Research
Network. Complications of beta-thalassemia major in North America.
Blood 2004;104:34-39 https://doi.org/10.1182/blood-2003-09-3167 PMid:14988152

- De
Sanctis V, Soliman AT, Elsedfy H, Pepe A, Kattamis C, El Kholy M,
Yassin M. Diabetes and Glucose Metabolism in Thalassemia Major: An
Update. Expert Rev Hematol. 2016;9:401-408 https://doi.org/10.1586/17474086.2016.1136209 PMid:26697756

- Gourdy P. Diabetes and oral contraception. Best Pract Res Clin Endocrinol Metab. 2013;27:67-76. https://doi.org/10.1016/j.beem.2012.11.001 PMid:23384747

- Suthipongse
W, Taneepanichskul S. An open-label randomized comparative study of
oral contraceptives between medications containing 3 mg drospirenone/30
microg ethinylestradiol and 150 microg levonogestrel/30 microg
ethinylestradiol in Thai women. Contraception 2004;69:23–26. https://doi.org/10.1016/j.contraception.2003.08.014 PMid:14720615

- Godsland
IF, Gangar K, Walton C, Cust MP, Whitehead MI, Wynn V, Stevenson JC.
Insulin resistance, secretion, and elimination in postmenopausal women
receiving oral or transdermal hormone replacement therapy. Metabolism.
1993;42:846-853. https://doi.org/10.1016/0026-0495(93)90058-V

- Mueck AO. Hormone replacement therapy for internal risk patients. Gynakol Geburtshilfliche Rundsch. 2006;46:174-190. https://doi.org/10.1159/000095726 PMid:17068402

- CDC. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65 (No. RR-3):55-80.

- Bergendal
A, Kieler H, Sundström A, Hirschberg AL, Kocoska-Maras L. Risk of
venous thromboembolism associated with local and systemic use of
hormone therapy in peri- and postmenopausal women and in relation to
type and route of administration. Menopause 2016; 23:593-599. https://doi.org/10.1097/GME.0000000000000611PMid:27023862

- North
American Menopause Society. The 2012 hormone therapy position statement
of: The North American Menopause Society. Menopause 2012; 19:257-271. https://doi.org/10.1097/gme.0b013e31824b970a PMid:22367731 PMCid:PMC3443956

- Katz
M, De Sanctis V, Vullo C, Wonke B, McGarrigle HH, Bagni B.
Pharmacokinetics of sex steroids in patients with beta thalassaemia
major. J Clin Pathol. 1993;46:660-664. https://doi.org/10.1136/jcp.46.7.660 PMid:8157756 PMCid:PMC501398

- Baber
RJ, Panay N, Fenton A; IMS Writing Group. 2016 IMS Recommendations on
women's midlife health and menopause hormone therapy. Climacteric.
2016;19:109-150. https://doi.org/10.3109/13697137.2015.1129166 PMid:26872610

- Picardo
E, Mitidieri M, Minniti E, Ambroggio S, D'Addato F, Benedetto C,
Gregori G, Baù MG. The first case of breast cancer in thalassemic
patient: case report and review of literature. Gynecol Endocrinol.
2015;31:345-348. https://doi.org/10.3109/09513590.2014.998646 PMid:25578420

- Bawa
R, Matemavi P, Maizlin I, Sung KJ. Ductal carcinoma in-situ in Turner
syndrome patient undergoing hormone replacement therapy: A case report.
Int J Cancer Ther Oncol. 2016; 4(1):4113. DOI: 10.14319/ijcto.41.13 https://doi.org/10.14319/ijcto.41.13

- van
Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic
review on therapeutic interventions to restore ovarian function and
achieve pregnancy. Hum Reprod Update. 1999 ;5 :483-492. https://doi.org/10.1093/humupd/5.5.483 PMid:10582785

- Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606-614. https://doi.org/10.1056/NEJMcp0808697 PMid:19196677 PMCid:PMC2762081













































