Kalliopi Zachou, Pinelopi
Arvaniti, Nikolaos K. Gatselis, Kalliopi Azariadis, Georgia Papadamou,
Eirini Rigopoulou and George N. Dalekos
Department of Medicine and Research Laboratory of Internal Medicine, School of Medicine, University of Thessaly, Larissa, Greece
Corresponding
author: George N. Dalekos, MD, PhD. Professor of Medicine. Head,
Department of Medicine and Research Laboratory of Internal Medicine.
University of Thessaly, School of Medicine. Biopolis, Larissa, 41110
Larissa, Greece. Tel: ++30-2410-682285; Fax: ++30-2410-671863. E-mail:
georgedalekos@gmail.com
Published: January 1, 2017
Received: August 14, 2016
Accepted: November 11, 2016
Mediterr J Hematol Infect Dis 2017, 9(1): e2017003 DOI
10.4084/MJHID.2017.003
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background & objectives:
In the past, patients with haemoglobinopathies were at high risk of
acquiring hepatitis C virus (HCV) due to multiple transfusions before
HCV screening. In these patients, the coexistence of haemochromatosis
and chronic hepatitis C (CHC) often leads to more severe liver disease.
We assessed the HCV prevalence, clinical characteristics and outcome in
this setting with particular attention to the response to treatment
including therapies with the new direct acting antivirals (DAAs).
Methods: The medical records of 81 consecutive patients followed the last 15 years were reviewed retrospectively.
Results:
43/81 (53%) patients were anti-HCV positive including 31/43 (72.1%)
with CHC (HCV-RNA positive; age 25±7 years; 45.2% with genotype 1b;
19.4% cirrhotics; baseline ferritin 887 ng/ml; range: 81-10.820).
Thirty patients received IFN-based therapy with or without ribavirin
with sustained virological response (SVR) in 14/30 (46.7%). Eleven
patients (9 non-responders to IFN-based therapies, one in relapse and
one naïve) received treatment with DAAs (SVR: 100%). 3/11 patients
increased their transfusion needs while 1/11 reported mild arthralgias.
No drug-drug interactions between DAAs and chelation agents were
observed as attested by the stability of ferritin levels during
treatment.
Conclusions:
More than 1/3 of patients with haemoglobinopathies suffered from CHC.
Response rates to IFN-based treatment seem to be similar to other
patients with CHC, while most importantly, treatment with DAAs was
excellent and safe even in difficult to treat patients (most null
responders with severe fibrosis) suggesting that this group of HCV
patients should no longer be regarded as a difficult to treat.
|
Introduction
Hepatitis
C virus (HCV) infection is one of the leading causes of chronic liver
disease worldwide. Indeed, the most recent estimates of disease burden
show an increase in seroprevalence over the last 15 years to 2.8%,
resulting in >185 million infections worldwide.[1,2]
In
the past, patients with haemoglobinopathies represented a population at
high risk of acquiring HCV as before 1992 there was no blood screening
for HCV. As a result, the prevalence of antibodies to HCV (anti-HCV) in
patients with thalassemia varies depending on the population studied
between 12% and 85%.[3]
As chelation therapy with
new drugs seems to prevent cardiac damage and improve survival, the
chronic liver disease has emerged as a critical clinical issue in this
setting. The ‘hit’ to the liver is at least dual: high frequency of
chronic viral infections, especially HCV, and secondary hemochromatosis
of the liver due to multiple transfusions and dyserythropoiesis.[4]
Furthermore, chronic hepatitis C (CHC) and iron overload were proved to
be independent risk factors for liver fibrosis progression and their
concomitant presence results in a striking increase of the risk.[5]
So, it is essential for patients with haemoglobinopathies to have a
multidisciplinary approach, in order to achieve both effective
chelation therapy and treatment of CHC, with a view to preventing liver
complications and improve morbidity and mortality.[3,4,6]
Clinical
care for patients with the HCV-related liver disease has improved
considerably during the last two decades, thanks to the enhanced
understanding of the pathophysiology of the disease, and the
therapeutic advances.[7] Until 2011, interferon-α
(IFN-α) with or without ribavirin was the approved treatment for
patients with thalassemia, resulting in sustained virological response
(SVR) rates of 40-60%.[8-10] The recent license of
Direct Acting Antivirals (DAAs) opened new venues in the treatment of
CHC regarding extremely high SVR rates and better compliance due to
their oral route of administration and the rarity of significant side
effects.[7] In addition, the new treatment modalities
for HCV infection permit avoiding ribavirin use in the majority of
cases, which is well-known to increase the transfusion needs by a
median of 30–40% as well as side effects in these patients.[9,11-17] The recent EASL treatment recommendations on hepatitis C,[7]
suggest that patients with haemoglobinopathies should be treated with
an IFN-free regimen, without ribavirin. However, patients with
haemoglobinopathies and CHC have been excluded from the major clinical
trials that led to the approval of DAAs. Indeed, so far, only some case
reports have been published showing favourable results after DAAs
administration in CHC patients with concurrent haemoglobinopathies.[18,19]
Hence, at present, no experience is available regarding the safety and
efficacy of DAAs in this population which is traditionally considered
difficult to treat, because of the coexistence of liver
hemochromatosis, the more advanced liver disease and the usual
nonresponse or relapse to previous IFN-based therapies.
Accordingly, the aims of our study were:
1°
to assess the prevalence of CHC in patients with haemoglobinopathies in
Central Greece a region with low anti-HCV prevalence (0.34%) in the
general population;[20]
2° to report the
clinical characteristics and the outcome in this setting giving
particular attention to the response to treatment after utilizing the
new DAAs and a strong emphasis on the safety and efficacy of use in
this specific population.
Patients and Methods
The
medical records of 81 consecutive patients with haemoglobinopathies (70
with β-thalassemia major and 11 with sickle cell anaemia/β-thalassemia)
followed by our Department between 2000 and 2015 were retrospectively
analysed. For patients who had received IFN-based treatment, sustained
virological response (SVR) was defined according to the European
Association for the Study of the Liver (EASL) clinical practice
guidelines published in 2011 (serum HCV-RNA<50 IU/ml 24 weeks after
treatment withdrawal).[8] For patients who received
DAAs, SVR was defined according to the EASL clinical practice
guidelines published in 2015 (undetectable serum HCV-RNA 12 weeks after
stopping treatment).[7]
Anti-HCV was determined
using a second- or third-generation enzyme immunoassay at least twice
within six months while active virus replication was defined by the
detection of HCV-RNA using a sensitive commercially available
quantitative real-time PCR kit (COBAS Taqman HCV Test; cut-off of
detection: 25 IU/ml).
The histological evaluation was assessed using the Knodell histologic/activity index score.[21] According to previous publications of our group,[22-24]
for statistical reasons patients were divided into two groups according
to inflammation: minimal-mild (score:0-8) and moderate-severe
(score:9-18) and according to fibrosis: none-mild-moderate (score:0-2)
and severe fibrosis-cirrhosis (score:3-4).
In addition, fibrosis
was assessed in 11 patients who received DAAs by transient elastography
(FibroScan device powered by VCTE Echosens equipped with the standard M
probe). Liver stiffness measurements (LMS) were expressed as median
(kPa) of all valid measurements with associated interquartile range
(IQR) and success rate. LSM was considered valid if there were ten
successful acquisitions and an IQR/LSM <0.3. Patients were divided
into four groups; F0-F1, F1-F2, F2-F3 and F3-F4 according to Metavir
score.
The study was approved by the Ethics Committee of Thessaly University, Medical School.
Statistical analysis: Results are expressed as median (range) and mean ± SD where appropriate. Data were analysed by Mann-Whitney U-test (MWU), x2 (two-by-two with Yate’s correction) and Fisher’s exact test. Two-sided p
values less than 0.05 were considered as statistically significant.
95%CI were calculated with the Wilson procedure with a correction for
continuity. Results
Forty-three
out of 81 patients (53%; 95%CI: 42-63.8%) tested anti-HCV positive.
Thirty-one patients had CHC (31/81; 38%; 95%CI: 27.4-48.6%) as they
were HCV-RNA positive. The baseline epidemiological, clinical,
laboratory and histologic characteristics of CHC patients with
haemoglobinopathies are shown in Table 1.
All patients but one with sickle cell anaemia/β-thalassemia, were
receiving chelation therapy to avoid iron overload, however, ferritin
levels varied (Table 1). Liver
histology before starting therapy was available in 22 patients. In
total, clinical and/or histological evidence of cirrhosis had 6/31
(19.4%; 95%CI: 5-33%) patients.
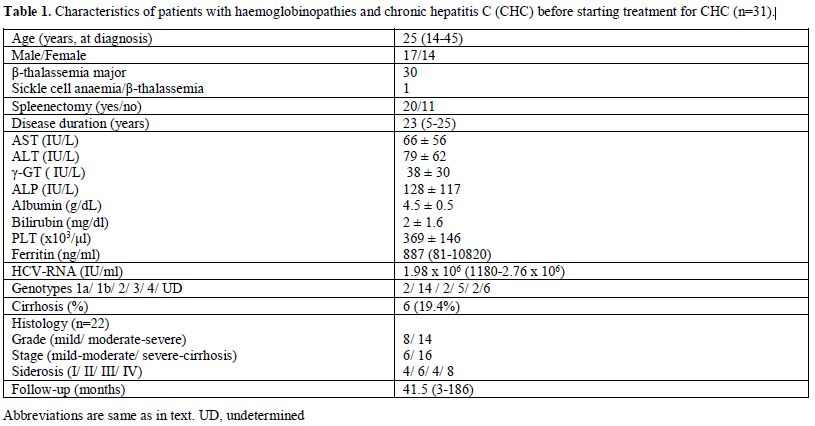 |
Table
1. Characteristics of patients with haemoglobinopathies and chronic hepatitis C (CHC) before starting treatment for CHC (n=31). |
Thirty out of 31 CHC
patients received IFN-based therapy with or without ribavirin. The SVR
rate independently of HCV-genotype, in IFN-based treated patients, was
46.7% (14/30; 95%CI: 29-64%) (Figure 1).
In fact, 22 patients had received one treatment course with IFN-based
therapy, while the remaining (8/30; 26.6%; 95%CI: 11-42%) had received
more than one course. In Figure 2, the type of treatment response to IFN-based therapy in respect to the number of treatment courses is shown. In Figure 3, the type of treatment response according to the IFN-based treatment schedule is also shown.
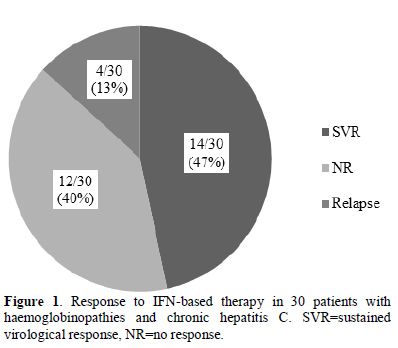 |
Figure 1. Response to IFN-based therapy in
30 patients with haemoglobinopathies and chronic hepatitis C.
SVR=sustained virological response, NR=no response. |
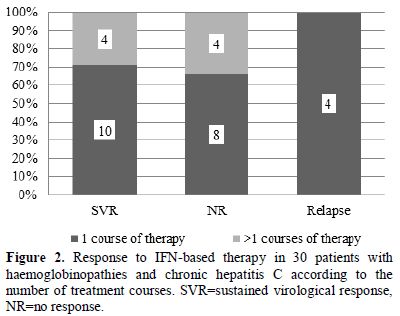 |
Figure 2. Response to
IFN-based therapy in 30 patients with haemoglobinopathies and chronic
hepatitis C according to the number of treatment courses. SVR=sustained
virological response, NR=no response. |
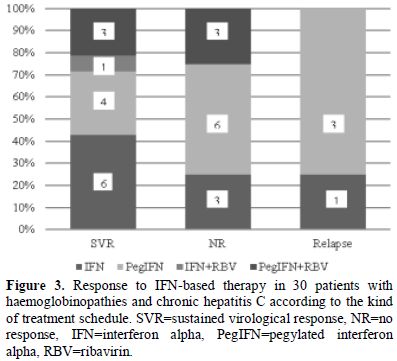 |
Figure 3. Response to IFN-based therapy in
30 patients with haemoglobinopathies and chronic hepatitis C according
to the kind of treatment schedule. SVR=sustained virological response,
NR=no response, IFN=interferon alpha, PegIFN=pegylated interferon
alpha, RBV=ribavirin. |
The SVR rate
in patients who received monotherapy with IFN or PegIFN (10/23; 43%;
95%CI: 23-63%) did not significantly differ compared to that observed
in patients who received combination therapy with ribavirin (4/7; 57%;
95%CI: 20-94%). Two out of 7 patients who received ribavirin increased
their transfusion needs. In total, 4/30 (13.3%; 95%CI: 1-25%) developed
neutropenia during treatment but treatment was not discontinued while
one patient presented anxiety and severe myalgias which led to
treatment discontinuation.
Regarding outcome (except three
patients who were lost to follow-up), 6 out of 22 (27%; 95%CI: 8-45%)
patients who were not cirrhotic at initial presentation, developed
cirrhosis. Two out of 6 patients who developed cirrhosis achieved an
SVR with IFN-based therapies whereas 10/16 patients, who did not
develop cirrhosis achieved SVR during follow-up (p>0.05). Of note,
no patient died during the follow-up of the study.
Eleven
patients (9 non-responders to previous treatment with IFN-based
therapies, one in relapse and one naïve patient) received treatment
with DAAs according to the recent EASL guidelines[7] (Tables 2 and 3). The baseline epidemiological, clinical, laboratory and histologic characteristics of these 11 patients are shown in Table 2. Seven patients had MRI T2* measurements during the last six months and had mild (n=5) to moderate liver hemochromatosis (n=2; Table 2).
Regarding chelation therapy, three patients were receiving
deferoxamine, two deferasirox, one deferiprone and four a combination
of deferoxamine with deferiprone. The estimation of liver disease stage
was based on transient elastography and 7/11 (63.6%; 95%CI: 35-92%)
patients had severe fibrosis (F3-F4) (Table 2).
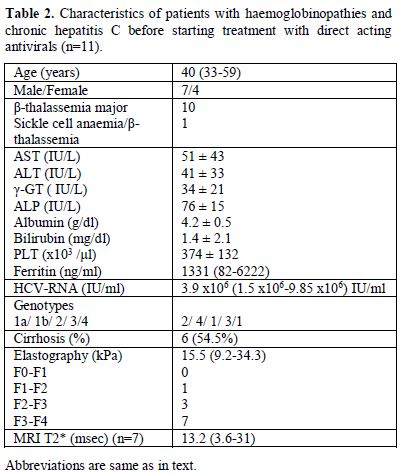 |
Table 2. Characteristics of patients with
haemoglobinopathies and chronic hepatitis C before starting treatment
with direct acting antivirals (n=11). |
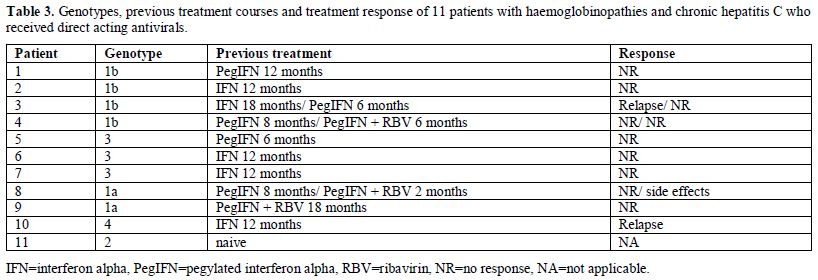 |
Table 3. Genotypes, previous treatment
courses and treatment response of 11 patients with haemoglobinopathies
and chronic hepatitis C who received direct acting antivirals. |
The treatment schedules with DAAs are shown in Table 4.
Regarding safety, only one patient mentioned arthralgias which,
however, did not lead to treatment discontinuation. Three patients
increased the transfusion needs (two of them were receiving ribavirin).
As far as ferritin levels are concerned, there was no significant
elevation during treatment with DAAs (Figure 4). All 11 patients treated with DAAs achieved SVR at week 12 post treatment.
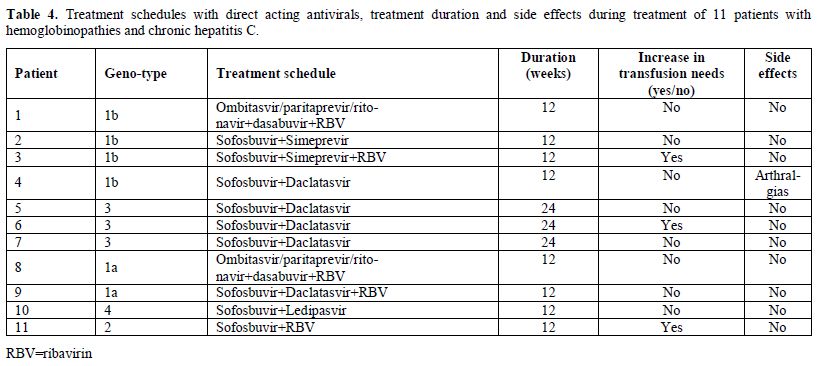 |
Table 4. Treatment schedules with direct
acting antivirals, treatment duration and side effects during treatment
of 11 patients with hemoglobinopathies and chronic hepatitis C. |
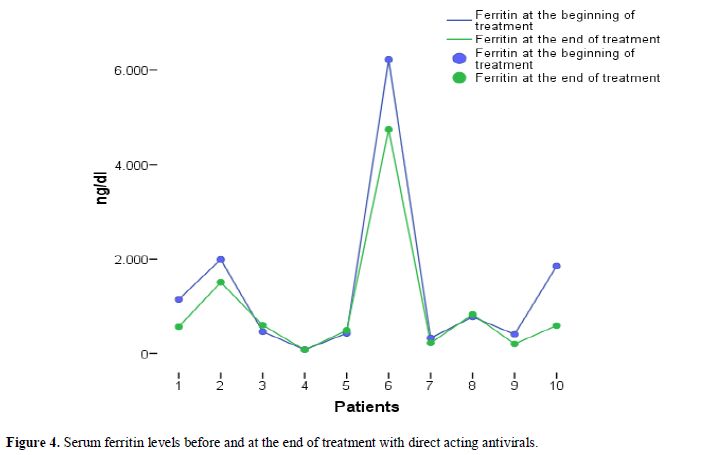 |
Figure 4. Serum ferritin levels before and at the end of treatment with direct acting antivirals. |
Discussion
The
current study assessed the prevalence of anti-HCV positivity and that
of CHC among patients with haemoglobinopathies, the characteristics of
the patients with CHC and haemoglobinopathies, and their response to
treatment with emphasis to the preliminary results of the treatment
responses to DAAs in this patient group. The following three major
points have risen:
a) the prevalence of anti-HCV and CHC in patients with haemoglobinopathies is still increased;
b)
HCV infection is diagnosed in young age, however 20% of the patients
had already cirrhosis before starting treatment and almost 50% were
infected with HCV genotype 1b;
c) the response rates to IFN-based
therapies were the same as in other patient groups, while most
importantly, treatment with DAAs was very effective (SVR 100%) and safe
even in difficult to treat HCV patients (most null responders with
severe fibrosis/cirrhosis).
In our retrospective study, we found a
prevalence anti-HCV positivity of 53% in patients with
haemoglobinopathies, while more than one-third of the patients (38%)
had CHC. This datum is in accordance with previous studies from Greece[25,26] and other European countries.[27]
As far as HCV genotype is concerned, almost half of our patients were
infected with genotype 1b, which is the most prevalent genotype among
multitransfused patients [3,28]. In
addition, nearly 20% of the patients had cirrhosis before treatment
initiation which is in accordance with previous studies in patients
with CHC and thalassemia,[3,26,29]
while the rate of progression of liver disease to cirrhosis was 27%
with a median disease duration of 23 years. The progression to
cirrhosis seems to be higher than that expected in other patients with
CHC without haemoglobinopathies.[30,31] Of note, the
achievement of SVR did not influence the progression to cirrhosis which
is rather dependent on the degree of liver iron overload.[29]
In
general, the SVR rate among patients treated with IFN-based therapies
(47%) was similar to previous reports in this population,[3,14,15] while the SVR rate among those who received IFN/Peg-IFN monotherapy was 43%, similar as in past studies.[9,12,16,17]
In a small subset of patients (n=7) who received IFN/PegIFN in
combination with ribavirin, the SVR rate was 57%, while only the
minority of them increased their transfusion needs.[3,11,13,32]
However, haemolysis was reversible after drug discontinuation, and no
significant iron overload worsening was noticed. According to our
study, the SVR rates both in patients treated with IFN monotherapy and
those treated with IFN in combination with ribavirin were similar to
other CHC patients.[33-35]
The main and most
important finding of our study, however, was the high SVR rate (100%)
achieved among HCV treatment-experienced difficult (null-responders)
patients with haemoglobinopathies who were treated with DAAs. All
patients received the appropriate treatment according to the HCV
genotype and fibrosis severity, but they considered to be difficult to
treat population, as all but one, were treatment-experienced and had
advanced liver fibrosis (stage F3-4 according to liver elastography).
Even though the number of patients was small to drawn safe conclusions,
this is one of the first studies showing quite convincingly that the
SVR rate to DAAs in multitransfused difficult-to-treat HCV patients is
similar to that observed in nonmulti-transfused naïve individuals with
CHC.[36-40]
As far as safety is concerned, none
of the 11 patients who received DAAs withdrew treatment because of side
effects while transfusion rate was elevated in 3 patients, 2 of whom
also received ribavirin. However, no significant elevation of serum
ferritin levels was observed during and after treatment. In addition,
all patients were under treatment with chelation agents. There are no
data so far about the drug-drug interactions of chelation agents and
DAAs. Although the present study was not designed to investigate
pharmacodynamics, chelation treatment does not seem to affect treatment
with DAAs.
Conclusion
More
than one-third of patients with haemoglobinopathies are still
chronically infected with CHC. Patients with haemoglobinopathies and
HCV infection seem to have similar SVR rates after IFN-based treatment
compared to other CHC patients. However, the most important and
fascinating finding of our study was the excellent virological response
rates (SVR 100%) after treatment with DAAs even in difficult to treat
HCV patients (null responders with advanced fibrosis/cirrhosis).
Side-effects related to DAAs treatment were minimal, while transfusions
rate seems to increase in some patients treated with ribavirin, without
however an elevation of ferritin levels. Therefore, our results further
support the statement of EASL and AASLD guidelines that IFN-α free
treatment with DAAs should be considered as first-line therapy in
treatment-naïve or treatment experienced patients with or without
cirrhosis due to CHC and concurrent haemoglobinopathies. Finally,
chelation therapy does not seem to affect treatment with DAAs, however,
current literature lacks sufficient evidence to give definitive support
to these preliminary results. Thus, larger studies are warranted to
ensure the safety and efficacy of DAAs in patients with
haemoglobinopathies and CHC. However, our initial results strongly
suggest that this group of HCV patients should no longer be regarded as
a “difficult” to treat when the new DAAs are used. Our study support
the need to make available the DAAs also for south-east Mediterranean
and Asian countries considering the large number of patients with
haemoglobinopathies and HCV in these areas.[41-45]
References
- Mohd Hanafiah K, Groeger J, Flaxman
AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new
estimates of age-specific antibody to HCV seroprevalence. Hepatology.
2013;57:1333–42. https://doi.org/10.1002/hep.26141 PMid:23172780

- Papatheodoridis
G, Thomas HC, Golna C, Bernardi M, Carballo M, Cornberg M, Dalekos G,
Degertekin B, Dourakis S, Flisiak R, Goldberg D, Gore C, Goulis I,
Hadziyannis S, Kalamitsis G, Kanavos P, Kautz A, Koskinas I, Leite BR,
Malliori M, Manolakopoulos S, Maticic M, Papaevangelou V, Pirona A,
Prati D, Raptopoulou-Gigi M, Reic T, Robaeys G, Schatz E, Souliotis K,
Tountas Y, Wiktor S, Wilson D, Yfantopoulos J, Hatzakis A. Addressing
barriers to the prevention, diagnosis and treatment of hepatitis B and
C in the face of persisting fiscal constraints in Europe: report from a
high level conference. J Viral Hepat 2016;23 (Suppl 1):1-12. https://doi.org/10.1111/jvh.12493 PMid:26809941

- Di
Marco V, Capra M, Angelucci E, Borgna-Pignatti C, Telfer P, Harmatz P,
Kattamis A, Prossamariti L, Filosa A, Rund D, Gamberini MR, Cianciulli
P, De Montalembert M, Gagliardotto F, Foster G, Grangè JD, Cassarà F,
Iacono A, Cappellini MD, Brittenham GM, Prati D, Pietrangelo A, Craxì
A, Maggio A; Italian Society for the Study of Thalassemia and
Haemoglobinopathies; Italian Association for the Study of the Liver..
Management of chronic viral hepatitis in patients with thalassemia:
recommendations from an international panel. Blood. 2010;116:2875-83. https://doi.org/10.1182/blood-2009-11-248724 PMid:20551378

- Hershko C. Pathogenesis and management of iron toxicity in thalassemia. Ann N Y Acad Sci. 2010;1202:1-9. https://doi.org/10.1111/j.1749-6632.2010.05544.x PMid:20712765

- Angelucci
E, Muretto P, Nicolucci A, Baronciani D, Erer B, Gaziev J, Ripalti M,
Sodani P, Tomassoni S, Visani G, Lucarelli G. Effects of iron overload
and hepatitis C virus positivity in determining progression of liver
fibrosis in thalassemia following bone marrow transplantation. Blood.
2002;100:17-21. https://doi.org/10.1182/blood.V100.1.17 PMid:12070002

- Di
Marco V, Capra M, Gagliardotto F, Borsellino Z, Cabibi D, Barbaria F,
Ferraro D, Cuccia L, Ruffo GB, Bronte F, Di Stefano R, Almasio PL,
Craxì A. Liver disease in chelated transfusiondependent thalassemics:
the role of iron overload and chronic hepatitis C. Haematologica.
2008;93:1243-6. https://doi.org/10.3324/haematol.12554 PMid:18556410

- EASL recommendations on treatment of Hepatitis C 2015. J Hepatol. 2015;63:199-236. https://doi.org/10.1016/j.jhep.2015.03.025 PMid:25911336

- European
Association for the Study of the Liver. EASL Clinical Practice
Guidelines: management of hepatitis C virus infection. J Hepatol.
2011;55:245–264. https://doi.org/10.1016/j.jhep.2011.02.023 PMid:21371579

- Donohue
SM, Wonke B, Hoffbrand AV, Reittie J, Ganeshaguru K, Scheuer PJ, Brown
D, Dusheiko G. Alpha interferon in the treatment of chronic hepatitis C
infection in thalassaemia major. Br J Haematol. 1993;83:491-497. https://doi.org/10.1111/j.1365-2141.1993.tb04676.x PMid:8387324

- Hussein
NR, Tunjel I, Basharat Z, Taha A, Irving W. The treatment of HCV in
patients with haemoglobinopathy in Kurdistan Region, Iraq: a single
centre experience. Epidemiol Infect. 2016;144:1634-40. https://doi.org/10.1017/S0950268815003064 PMid:27125573

- Telfer
PT, Garson JA, Whitby K, et al. Combination therapy with interferon
alpha and ribavirin for chronic hepatitis C virus infection in
thalassaemic patients. Br J Haematol. 1997;98:850-855. https://doi.org/10.1046/j.1365-2141.1997.2953112.x PMid:9326177

- Di
Marco V, Lo Iacono O, Almasio P, Ciaccio C, Capra M, Rizzo M, Malizia
R, Maggio A, Fabiano C, Barbaria F, Craxi A. Long-term efficacy of
alpha interferon in beta-thalassemics with chronic hepatitis C. Blood.
1997;90: 2207-12. PMid:9310471

- Li
CK, Chan PK, Ling SC, Ha SY. Interferon and ribavirin as frontline
treatment for chronic hepatitis C infection in thalassaemia major. Br J
Haematol. 2002;117:755-8. https://doi.org/10.1046/j.1365-2141.2002.03491.x

- Butensky
E, Pakbaz Z, Foote D, Walters MC, Vichinsky EP, Harmatz P. Treatment of
hepatitis C virus infection in thalassemia. Ann N Y Acad Sci 2005;
1054: 290-299. https://doi.org/10.1196/annals.1345.038 PMid:16339677

- Alavian
SM, Tabatabaei SV. Treatment of chronic hepatitis C in polytransfused
thalassaemic patients: a meta-analysis. J Viral Hepat. 2010;17:236-244.
https://doi.org/10.1111/j.1365-2893.2009.01170.x PMid:19638104

- Vafiadis
I, Trilianos P, Vlachogiannakos J, Karagiorga M, Hatziliami A,
Voskaridou E, Ladas SD. Efficacy and safety of interferon-based therapy
in the treatment of adult thalassemic patients with chronic hepatitis
C: a 12 years audit. Ann Hepatol. 2013;12:532-8. PMid:23813130

- Kalafateli
M, Kourakli A, Gatselis N, Lambropoulou P, Thomopoulos K, Tsamandas A,
Christofidou M, Zachou K, Jelastopoulou E, Nikolopoulou V,Symeonidis A,
Dalekos GN, Lambropoulou-Karatza C, Triantos C. Efficacy of interferon
a-2b monotherapy in b-thalassemics with chronic hepatitis C. J
Gastrointestin Liver Dis. 2015;24:189-96. PMid:26114179

- Papadopoulos
N, Deutsch M, Georgalas A, Poulakidas H, Karnesis L. Simeprevir and
sofosbuvir combination treatment in a patient with HCV cirrhosis and
HbS beta 0-thalassemia: Efficacy and safety despite baseline
hyperbilirubinemia. Case Rep Hematol. 2016;2016:7635128. https://doi.org/10.1155/2016/7635128

- Hussein
NR. Sofosbuvir-containing regimen for the treatment of hepatitis C
virus in a patient with sickle-thalassemia: The first case report. Int
J Infect. 2016 (In press), doi: 10.17795/iji-38077. https://doi.org/10.17795/iji-38077

- Gatselis
NK, Rigopoulou E, Stefos A, Kardasi M, Dalekos GN. Risk factors
associated with HCV infection in semi-rural areas of central Greece.
Eur J Intern Med. 2007;18:48-55. https://doi.org/10.1016/j.ejim.2006.09.008 PMid:17223043

- Knodell
RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW,
Wollman J. Formulation and application of a numerical scoring system
for assessing histological activity in asymptomatic chronic active
hepatitis. Hepatology. 1981;1:431-35. https://doi.org/10.1002/hep.1840010511 PMid:7308988

- Gatselis
NK, Zachou K, Norman GL, Tzellas G, Speletas M, Gabeta S, Germenis A,
Koukoulis GK, Dalekos GN. IgA antibodies against deamidated gliadin
peptides in patients with chronic liver diseases. Clin Chim Acta 2012;
413:1683-8. https://doi.org/10.1016/j.cca.2012.05.015 PMid:22643316

- Kaffe
ET, Rigopoulou EI, Koukoulis GK, Dalekos GN, Moulas AN. Oxidative
stress and antioxidant status in patients with autoimmune liver
diseases. Redox Rep 2015;20:33-41. https://doi.org/10.1179/1351000214Y.0000000101 PMid:25117650

- Norman
GL, Gatselis NK, Shums Z, Liaskos C, Bogdanos DP, Koukoulis GK, Dalekos
GN. Cartilage oligomeric matrix protein: A novel non-invasive marker
for assessing cirrhosis and risk of hepatocellular carcinoma. World J
Hepatol 2015;7:1875-83. https://doi.org/10.4254/wjh.v7.i14.1875 PMid:26207169 PMCid:PMC4506945

- Kountouras
D, Tsagarakis NJ, Fatourou E, Dalagiorgos E, Chrysanthos N, Berdoussi
H, Vgontza N, Karagiorga M, Lagiandreou A, Kaligeros K, Voskaridou
E,Roussou P, Diamanti-Kandarakis E, Koskinas J. Liver disease in adult
transfusion dependent beta-thalassaemia patients: investigating the
role of iron overload and chronic HCV infection. Liver Int
2013;33:420-7. https://doi.org/10.1111/liv.12095 PMid:23402611

- Triantos
C, Kourakli A, Kalafateli M, Giannakopoulou D, Koukias N, Thomopoulos
K, Lampropoulou P, Bartzavali C, Fragopanagou H, Kagadis GC,
Christofidou M, Tsamandas A, Nikolopoulou V, Karakantza M,
Labropoulou-Karatza C. Hepatitis C in patients with ß-thalassemia
major. A single-centre experience. Ann Hematol 2013;92:739-46. https://doi.org/10.1007/s00277-013-1692-6 PMid:23412560

- Prati
D, Poli F, Farma E, Picone A, Porta E, Mattei C, Zanella A, Scalamogna
A, Gamba A, Gronda E, Faggian G, Livi U, Puricelli C, Viganò M, Sirchia
G. A multicenter prospective study on the risk of acquiring liver
disease in anti-hepatitis C virus negative patients affected from
homozygous beta-thalassemia. Blood. 1998;92:3460-64. PMid:9787188

- Christofidou
M, Lambropoulou-Karatza C, Dimitracopoulos G, Spiliopoulou I.
Distribution of hepatitis C virus genotypes in viremic patients with
beta-thalassemia. Eur J Clin Microbiol Infect Dis. 2000;19:728-30. https://doi.org/10.1007/s100960000342 PMid:11057513

- Lai
ME, Origa R, Daniou F, Leoni GB, Vacquer S, Anni F, Corrias C, Farci P,
Conqiu G, Galanello R. Natural history of hepatitis C in thalassemia
major: a long-term prospective study. Eur J Hematol. 2013;90:501-7. https://doi.org/10.1111/ejh.12086 PMid:23414443

- Thein
HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis
progression rates in chronic hepatitis C virus infection: a
meta-analysis and meta-regression. Hepatology. 2008;48:418-31. https://doi.org/10.1002/hep.22375 PMid:18563841

- Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin North Am. 2015;44:717-34. https://doi.org/10.1016/j.gtc.2015.07.003 PMid:26600216

- Sherker
AH, Senosier M, Kermack D. Treatment of transfusion-dependent
thalassemic patients infected with hepatitis C virus with interferon
alpha-2b and ribavirin. Hepatology. 2003;37:223. https://doi.org/10.1053/jhep.2003.50037 PMid:12500209

- Poynard
T, Marcellin P, Lee SS, NiederauC, Minuk GS, Ideo G, Bain V, Heathcote
J, Zeuzem S, Trepo C, Albrecht J. Randomised trial of interferon
alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon
alpha2b plus placebo for 48 weeks for treatment of chronic infection
with hepatitis C virus. International Hepatitis Interventional Therapy
Group (IHIT). Lancet. 1998;352:1426-32. https://doi.org/10.1016/S0140-6736(98)07124-4

- Fried
MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Haussinger
D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J.
Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus
infection. N Engl J Med. 2002;347:975-82. https://doi.org/10.1056/NEJMoa020047 PMid:12324553

- Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications Gut. 2006;55:1350–59. https://doi.org/10.1136/gut.2005.076646 PMid:16905701 PMCid:PMC1860034

- Sulkowski
MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I,
Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ, Schwartz H, Nelson
DR,Everson GT, Eley T, Wind-Rotolo M, Huang SP, Gao M, Hernandez D,
McPhee F, Sherman D, Hindes R, Symonds W, Pasquinelli C, Grasela DM;
AI444040 Study Group. Daclatasvir plus sofosbuvir for previously
treated or untreated chronic HCV infection. N Engl J Med.
2014;370:211–21. https://doi.org/10.1056/NEJMoa1306218 PMid:24428467

- Afdhal
N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib
R, Gitlin N, Herring R, Lalezari J, Younes ZH, Pockros PJ, Di Bisceglie
AM, Arora S, Subramanian GM, Zhu Y, Dvory-Sobol H, Yang JC,Pang PS,
Symonds WT, McHutchison JG, Muir AJ, Sulkowski M, Kwo P; ION-2
Investigators. Ledipasvir and sofosbuvir for previously treated HCV
genotype 1 infection. N Engl J Med 2014;370:1483–93. https://doi.org/10.1056/NEJMoa1316366 PMid:24725238

- Kowdley
KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman
ML, Schiff E, Ghalib R, Ryan M, Rustgi V, Chojkier M, Herring R, Di
Bisceglie AM, Pockros PJ, Subramanian GM, An D, Svarovskaia E, Hyland
RH, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Pound D, Fried MW;
ION-3 Investigators.. Ledipasvir and sofosbuvir for 8 or 12 weeks for
chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–88. https://doi.org/10.1056/NEJMoa1402355 PMid:24720702

- Feld
JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O,
Aguilar H, Xiong J, Pilot-Matias T, DaSilva-Tillmann B, Larsen L,
Podsadecki T,Bernstein B.. Treatment of HCV with ABT-450/r-ombitasvir
and dasabuvir with ribavirin. N Engl J Med 2014;370:1594–1603. https://doi.org/10.1056/NEJMoa1315722 PMid:24720703

- Ferenci
P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho
RT, Tsai N, Nyberg A, Box TD, Younes Z, Enayati P, Green S, Baruch
Y,Bhandari BR, Caruntu FA, Sepe T, Chulanov V, Janczewska E, Rizzardini
G, Gervain J, Planas R, Moreno C, Hassanein T, Xie W, King M,
Podsadecki T, Reddy KR; PEARL-III Study; PEARL-IV Study..
ABT-450/rombitasvir and dasabuvir with or without ribavirin for HCV. N
Engl J Med. 2014;370:1983–92. https://doi.org/10.1056/NEJMoa1402338 PMid:24795200

- Din
G, Malik S, Ali I, Ahmed S, Dasti JI. Prevalence of hepatitis C virus
infection among thalassemia patients: a perspective from a multi-ethnic
population of Pakistan. Asian Pac J Trop Med. 2014;7S1:S127-33.

- Rafiei
A, Darzyani AM, Taheri S, Haghshenas MR, Hosseinian A, Makhlough A.
Genetic diversity of HCV among various high risk populations (IDAs,
thalassemia, hemophilia, HD patients) in Iran. Asian Pac J Trop Med.
2013;6:556-60. https://doi.org/10.1016/S1995-7645(13)60096-6

- Shah
N, Mishra A, Chauhan D, Vora C, Shah NR. Study on effectiveness of
transfusion program in thalassemia major patients receiving multiple
blood transfusions at a transfusion centre in Western India. 4. Asian J
Transfus Sci. 2010;4:94-8. https://doi.org/10.4103/0973-6247.67029 PMid:20859507 PMCid:PMC2937304

- Yazaji
W., Habbal W., Monem F. Seropositivity of hepatitis b and c among
Syrian multi-transfused patients. Mediterr J Hematol Infect Dis 2016,
8(1): e2016046, DOI: http://dx.doi.org/10.4084/MJHID.2016.046

- Hussein
E. Evaluation of infectious disease markers in multitransfused Egyptian
children with thalassemia. Ann Clin Lab Sci. 2014 Winter;44(1):62-6
PMid:24695476

[TOP]




















































