Shahrzad Zonoozi1, Maria Barnard1, Emma Prescott2, Romilla Jones1, Farrukh T Shah2 and Ploutarchos Tzoulis1
1 Department of Diabetes, Whittington Hospital, Magdala Avenue, London, N19 5NF
2 Department of Haematology, Whittington Hospital, Magdala Avenue, London, N19 5NF
Corresponding
author: Ploutarchos Tzoulis, Department of Diabetes, Whittington Hospital, Magdala Avenue, London, N19 5NF. E-mail:
ptzoulis@yahoo.co.uk
Published: January 1, 2017
Received: October 3, 2016
Accepted: December 12, 2016
Mediterr J Hematol Infect Dis 2017, 9(1): e2017004 DOI
10.4084/MJHID.2017.004
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Sitagliptin,
a modern antidiabetic agent which is weight neutral and associated with
low rate of hypoglycaemias, is being increasingly used in type 2
diabetes mellitus (DM). However, there is a paucity of data about its
efficacy and safety in beta-thalassaemia major (β-TM).
This
retrospective case series of five patients (mean age of 45 years) is
the first study evaluating the use of sitagliptin in patients with β-TM
and DM.
Four patients responded well to sitagliptin, as evidenced
by a decrease in fructosamine by 77 and 96µmol/L (equivalent reduction
in HbA1c of 1.5% and 1.9%) observed in two patients and reduction in
the frequency of hypoglycaemia without worsening glycaemic control in
two others. One patient did not respond to sitagliptin. No patients
reported significant side effects.
This study provides evidence
that sitagliptin may be considered, with caution, for use in patients
with β-TM and DM, under the close monitoring of a Diabetologist.
|
Introduction
The
aetiology of diabetes mellitus (DM) in patients with β-TM is
multifactorial. It has been predominantly attributed to
transfusion-related pancreatic iron overload resulting in destruction
of insulin secreting β cells of the pancreas and liver haemosiderosis
leading to insulin resistance.[1] Other factors such
as hepatitis C viral infection, autoimmunity, family history of DM and
genetic factors also play an important role.[2] As
life expectancy in patients with β-TM has risen substantially, optimal
glycaemic control is becoming extremely important in order to reduce
the risk of diabetic complications.
Sulfonylureas, traditionally
considered second line glucose-lowering agents after metformin, are
associated with a poor side-effect profile, including high risk of
hypoglycaemia and weight gain. For these reasons, the 2015 position
statement for type 2 DM recommends considering the use of dipeptidyl
peptidase 4 (DPP-4) inhibitors or gliptins as second line agents when
the first line agent, metformin, has not achieved optimal glycaemic
control.[3] The most popular drug in this drug class,
sitagliptin, inhibits DPP-4, the key enzyme which inactivates glucagon
like peptide 1 (GLP-1),[4] leading to increased levels
of GLP-1 in the plasma. GLP-1 is a gut hormone which increases insulin
secretion and suppresses glucagon secretion in a glucose-dependent
manner. DPP-4 inhibitors have similar glucose-lowering efficacy as
sulfonylureas, whilst associated with lower risk of hypoglycaemia and
no weight gain.[5] In addition, some studies have
suggested that sitagliptin may have greater durability of glucose
control and better maintenance of beta-cell function in comparison with
sulfonylureas.[6]
In patients with thalassaemia
and DM there has been little to no published data supporting the
efficacy of modern oral antidiabetic agents such as sitagliptin. The
aim of this study was to evaluate the efficacy and safety of
sitagliptin in patients with β-TM and DM in a Specialist Thalassaemia
Centre in the UK.
Methods
Our
study is a retrospective case series of patients with β-TM and DM at
our institution treated with sitagliptin. All the participants attended
the Joint Diabetes Thalassaemia Clinic at our Specialised Thalassaemia
Centre serving the largest cohort of patients with thalassaemia in the
UK.
There were no pre-specified criteria for the use of
sitagliptin. For example, markers of pancreatic β-cell function, such
as serum C-peptide, and of insulin resistance, such as homoeostasis
model assessment of insulin resistance (HOMA-IR), were not evaluated
prior to treatment initiation. They were not regarded as essential
since sitagliptin is effective as an add-on glucose-lowering therapy
even in patients with insulin deficiency by suppressing glucagon
secretion in a glucose-dependent manner.[7]
Retrospective
case notes and biochemical results review was performed in order to
collect data on: demographic characteristics (age, gender, ethnic
origin), duration of diabetes, smoking status, weight, antidiabetic
treatment history, fructosamine, blood pressure, lipid profile, liver
function tests and ferritin on a 6-monthly basis starting from the time
point of sitagliptin initiation until most recent review or its
discontinuation.
Since glycated haemoglobin (HbA1c) can be
unreliable in patients with β-TM due to regular transfusions,
fructosamine levels were regularly monitored as a surrogate marker of
glycaemic control in the preceding 2-3 weeks.[8]
Fructosamine was measured in the serum using a Roche Modular P800
system. A fructosamine level of 285 µmol/L was considered as being
equivalent to HbA1c of 6.5% with every additional 50 µmol/L of
fructosamine being calculated as equivalent to a rise of 1% in HbA1c.[9] Fructosamine and ferritin values were calculated as a mean of 2 or 3 results around the time point of interest.
Written informed consent was obtained from all five patients for publication of this case series. Results
Amongst
36 patients with β-TM and DM at our institution, five patients (4
females, 1 male), all with strong family history of diabetes and aged
between 44 and 50 years, were commenced on sitagliptin at a dose of 100
mg once daily, as shown in Table 1.
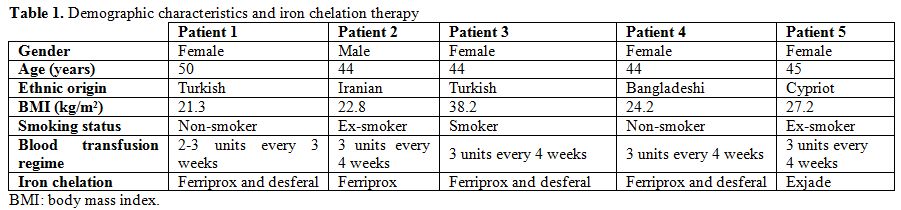 |
Table
1. Demographic characteristics and iron chelation therapy |
Sitagliptin was used
as second line agent in one patient, as add-on to metformin. Among the
other four patients on metformin and gliclazide combination therapy,
sitagliptin was added as 3rd line agent in two cases with poor glycaemic control, while it replaced gliclazide in two cases with frequent hypoglycaemias.
Review
of lipid profile and blood pressure during the time period of
sitagliptin treatment did not demonstrate any significant changes.
Patient 1.
A 50-year-old female with inadequate glycaemic control despite
lifestyle modification and metformin monotherapy was started on
sitagliptin. Fructosamine levels decreased from 340µmol/L to 323µmol/L
at 6 months and 263µmol/L at 12 months (equivalent reduction in HbA1c
of 0.3% and 1.2% respectively). Her weight increased by 1kg.
Patient 2.
Sitagliptin replaced gliclazide in a 44-year-old male who had poor
glycaemic control and frequent episodes of hypoglycaemia on dual
combination therapy with metformin and gliclazide. Fructosamine levels
initially decreased from 417µmol/L to 337µmol/L at 6 months (equivalent
reduction in HbA1c of 1.6%), but then increased to 353µmol/L at 12
months (equivalent increase in HbA1c of 0.3%). Around this time point,
gliclazide was reintroduced, resulting in fructosamine decrease to
343µmol/L at 24 months (equivalent HbA1c decrease of 0.2%). Episodes of
hypoglycaemia were eliminated, while weight remained stable for the
first 2 years, followed by an increase by 4kg in the final 6 months
when gliclazide was reintroduced.
Patient 3.
This 44-year-old female had frequent hypoglycaemias on dual combination
therapy of metformin and gliclazide. For this reason, gliclazide was
replaced with sitagliptin which resulted in an increase of fructosamine
levels from 246µmol/L to 333µmol/L at 6 months (equivalent 1.7%
increase in HbA1c). In light of poor response, sitagliptin was
withdrawn and substituted by gliclazide.
Patient 4.
This 44-year-old female had frequent hypoglycaemias on combination
therapy of metformin and gliclazide. As a result, sitagliptin was
initiated, and gliclazide dose was reduced. Six months after
sitagliptin introduction, there was a significant reduction in
frequency of hypoglycaemic episodes and a slight decrease of
fructosamine levels from 265µmol/L to 255µmol/L (equivalent reduction
in HbA1c of 0.2%). Her weight increased by 2.2 kg.
Patient 5.
A 45-year-old female had suboptimal glycaemic control despite
combination treatment with metformin and gliclazide. Sitagliptin was
added as 3rd line agent, leading to
significant fructosamine reduction from 354µmol/L to 258µmol/L
(equivalent reduction in HbA1c of 1.9%) and weight loss of 6 kg over 6
months.
Overall, four out of the five patients were
responders to sitagliptin therapy, as evidenced by a significant
reduction in fructosamine in two patients by 77 and 96µmol/L
(equivalent reduction in HbA1c of 1.5 and 1.9%). In the other two
patients, there was a significant reduction in the frequency of
hypoglycaemia with relatively stable glycaemic control. In one case
sitagliptin did not result in glucose lowering and was appropriately
stopped after 6 months. No patients exhibited signs/symptoms of
pancreatitis or cholecystitis, while liver function tests did not
change significantly after sitagliptin initiation. In total, no
significant side effects were reported.
As seen in Table 2, there was no relationship between fructosamine and ferritin levels which could explain changes in glycaemic control.
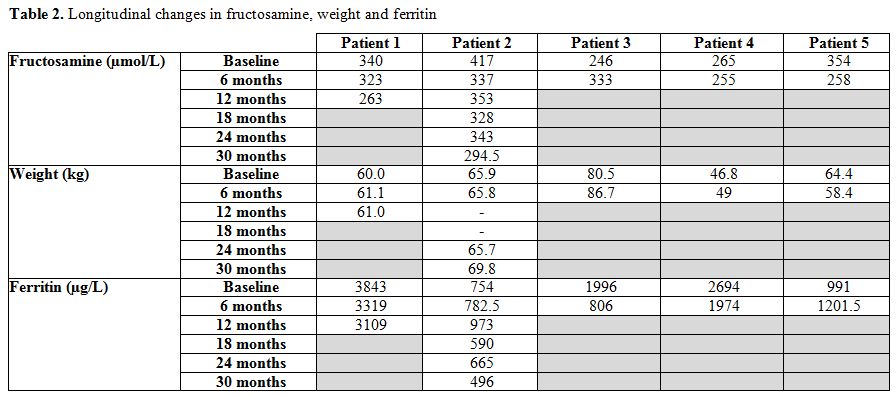 |
Table 2. Longitudinal changes in fructosamine, weight and ferritin |
Discussion
Our
case series shows that the DDP-4 inhibitor sitagliptin is an effective
glucose-lowering agent and is associated with low rate of
hypoglycaemias in patients with β-TM. Four out of five patients
initiated on sitagliptin were responders, either by achieving a
reduction in fructosamine levels or by experiencing less frequent
hypoglycaemic episodes. These findings are in keeping with RCTs in
patients with type 2 DM showing that sitagliptin improves glycaemic
control and reduces the rate of hypoglycaemias. The lack of association
between changes in fructosamine and ferritin indicates that
intensification of glycaemic control could not be attributed, at least
in our small cohort, to changes in iron chelation therapy.
Whilst
previous studies have reported sitagliptin to be weight neutral in
patients with DM, a mixed response was recorded in our cohort.[10]
Two patient maintained stable weight, one achieved reduction and two
others had weight gain. Besides the effect of sitagliptin, weight gain
might be attributed to factors such as poor adherence to dietary
advice, concomitant use of sulfonylureas and psychosocial factors.
Data
review of this case series did not raise any safety concerns. Previous
studies in participants with type 2 DM have shown possible association
of DPP-4 inhibitors with pancreatitis, increased risk of infections and
arthralgias,[11,12] although the overall incidence of serious adverse events is extremely low.[13] In view of conflicting evidence about whether sitagliptin increases the risk of pancreatitis,[14-16] current consensus is that DPP-4 inhibitors should be avoided in patients with a history of pancreatitis.[15]
However gallstones, commonly seen in patients with β-TM, are not a
contraindication for the use of sitagliptin. The European spontaneous
reporting database recently published 65 reports of cholecystitis in
patients with type 2 DM treated with sitagliptin, suggesting
sitagliptin may increase the risk of cholecystitis.[17]
However a large population-based study showed that DPP-4 inhibitors
were not associated with an increased risk of bile duct and gallbladder
disease.[18] In contrast to initial reports showing
an increased incidence of nasopharyngeal and upper respiratory tract
infections in association with sitagliptin, contemporary data do not
suggest any increased risk of infection.[19] A recent FDA warning reported sitagliptin has been rarely associated with severe joint pain through an unknown mechanism.
The
main strength of our study is that this is the first study ever
conducted reporting real-life use of DPP-4 inhibitors in patients with
β-TM. Limitations of this study include its retrospective nature, the
very small number of patients and the lack of comparator group.
At
present sitagliptin as well as other modern antidiabetic agents such as
s GLP-1 agonists and SGLT-4 inhibitors are increasingly used with great
success in patients with type 2 diabetes, offering optimal glycaemic
control and reducing the rate of hypoglycaemic episodes without
associated weight gain. However the lack of evidence on efficacy and
safety of these agents in patients with thalassaemia restricts access
of these patients to potentially valuable therapeutic options. While
findings from studies in the general population may apply to patients
with β-TM, these patients have a different pathophysiological basis of
diabetes and also have multiple comorbidities and complex needs. These
reasons highlight the urgency to generate high quality evidence in this
field.
Whilst our data supports the potential for sitagliptin
use as add-on therapy to metformin and sulfonylurea combination therapy
in patients with β-TM, we recommend considering also the use of
sitagliptin as second line agent to metformin in agreement with
international guidelines for patients with type 2 DM.[3]
The rationale behind this is that sulfonylureas, which are commonly
used nowadays as second-line agents, are associated with high rate of
hypoglycaemia and significant weight gain. Specifically, optimal
candidates for switching from sulfonylurea to sitagliptin in order to
decrease significantly symptomatic hypoglycaemia are patients with
dominant insulin resistance.[20] Taking into account
that very little to no data exist on sitagliptin use in patients with
β-TM, the benefits and risks of therapy should be carefully considered
and always discussed with the patient. If patient and doctor make a
decision to start sitagliptin, they should specify the goals of this
therapy. For example, sitagliptin should be usually discontinued if the
patient does not achieve fructosamine reduction of at least 25µmol/L
(equivalent HbA1c reduction of 0.5%) within 6 months of initiation or
does not experience a significant reduction in the frequency of
hypoglycaemic episodes. Finally, initiation of sitagliptin should take
place only under the guidance, supervision and close monitoring of a
Diabetologist with experience in management of patients with β-TM and
DM.
Conclusion
Our
study provides some evidence that sitagliptin could be considered for
use in selected patients with β-TM and DM. To the best of our
knowledge, this is the first study demonstrating the efficacy and
safety of a modern oral antidiabetic agent in patients with β-TM and it
could facilitate access of this population to sitagliptin.
References
- Barnard M, Tzoulis P. Diabetes and thalassaemia. Thalassemia Reports, 2013. 3(1s): p. 18. https://doi.org/10.4081/thal.2013.s1.e18

- Monge
L, Pinach S, Caramellino L, Bertero MT, Dall'omo A, Carta Q. The
possible role of autoimmunity in the pathogenesis of diabetes in
B-thalassemia major. Diabetes Metab, 2001. 27(2 Pt 1): p. 149-54.
PMid:11353881

- Inzucchi
SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters
AL, Tsapas A, Wender R, Matthews DR. Management of Hyperglycemia in
Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a
Position Statement of the American Diabetes Association and the
European Association for the Study of Diabetes. Diabetes Care, 2015.
38(1): p. 140-149. https://doi.org/10.2337/dc14-2441 PMid:25538310

- Chen
XW, He ZX, Zhou ZW, Yang T, Zhang X, Yang YX, Duan W, Zhou SF. Clinical
pharmacology of dipeptidyl peptidase 4 inhibitors indicated for the
treatment of type 2 diabetes mellitus. Clinical and Experimental
Pharmacology and Physiology, 2015. 42(10): p. 999-1024. https://doi.org/10.1111/1440-1681.12455 PMid:26173919

- Nauck
MA, Meininger G, Sheng D, Terranella L, Stein PP, Sitagliptin Study 024
Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor,
sitagliptin, compared with the sulfonylurea, glipizide, in patients
with type 2 diabetes inadequately controlled on metformin alone: a
randomized, double-blind, non-inferiority trial. Diabetes, Obesity and
Metabolism, 2007. 9(2): p. 194-205. https://doi.org/10.1111/j.1463-1326.2006.00704.x PMid:17300595

- Seck
T, Nauck M, Sheng D, Sunga S, Davies MJ, Stein PP, Kaufman KD, Amatruda
JM, Sitagliptin Study 024 Group. Safety and efficacy of treatment with
sitagliptin or glipizide in patients with type 2 diabetes inadequately
controlled on metformin: a 2-year study. Int J Clin Pract, 2010. 64(5):
p. 562-76. https://doi.org/10.1111/j.1742-1241.2010.02353.x PMid:20456211

- Ishikawa
M, Takai M, Maeda H, Kanamori A, Kubota A, Amemiya H, Iizuka T, Iemitsu
K, Iwasaki T, Uehara G, Umezawa S, Obana M, Kaneshige H, Kaneshiro M,
Kawata T, Sasai N, Saito T, Takuma T, Takeda H, Tanaka K, Tsurui N,
Nakajima S, Hoshino K, Honda S, Machimura H, Matoba K, Minagawa F,
Minami N, Miyairi Y, Mokubo A, Motomiya T, Waseda M, Miyakawa M, Naka,
Y, Terauchi Y, Tanaka Y, Matsuba I. Factors Predicting Therapeutic
Efficacy of Combination Treatment With Sitagliptin and Insulin in Type
2 Diabetic Patients: The ASSIST-K Study. Journal of Clinical Medicine
Research, 2015. 7(8): p. 607-612. https://doi.org/10.14740/jocmr2149w PMid:26124906 PMCid:PMC4471747

- Cappellini M-D C. A., Eleftheriou A, Piga A, Porter J, Taher A. Guidelines for the Clinical Management of Thalassaemia. 2nd Revised edition ed. 2008: Thalassaemia International Federation.
- Juraschek
SP, Steffes MW, Selvin E. Associations of Alternative Markers of
Glycemia with HemoglobinA1c and Fasting Glucose. Clinical Chemistry,
2012. 58(12): p. 1648-1655. https://doi.org/10.1373/clinchem.2012.188367 PMid:23019309 PMCid:PMC3652236

- Ahuja
V, Chou CH. Novel Therapeutics for Diabetes: Uptake, Usage Trends, and
Comparative Effectiveness. Curr Diab Rep, 2016. 16(6): p. 47.

- Amori
RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2
diabetes: Systematic review and meta-analysis. JAMA, 2007. 298(2): p.
194-206. https://doi.org/10.1001/jama.298.2.194 PMid:17622601

- Richter
B, Bandeira-Echtler E, Bergerhoff K, Lerch C. Dipeptidyl peptidase-4
(DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst
Rev, 2008(2): p. Cd006739. https://doi.org/10.1002/14651858.cd006739.pub2

- Gooßen
K, Gräber S. Longer term safety of dipeptidyl peptidase-4 inhibitors in
patients with type 2 diabetes mellitus: systematic review and
meta-analysis. Diabetes, Obesity and Metabolism, 2012. 14(12): p.
1061-1072. https://doi.org/10.1111/j.1463-1326.2012.01610.x PMid:22519906

- Deacon
CF, Lebovitz HE. Comparative review of dipeptidyl peptidase-4
inhibitors and sulphonylureas. Diabetes, Obesity and Metabolism, 2016. https://doi.org/10.1111/dom.12610

- Scheen
AJ. Safety of dipeptidyl peptidase-4 inhibitors for treating type 2
diabetes. Expert Opin Drug Saf, 2015. 14(4): p. 505-24. https://doi.org/10.1517/14740338.2015.1006625 PMid:25630605

- Egan
AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, Rosebraugh
C. Pancreatic safety of incretin-based drugs--FDA and EMA assessment. N
Engl J Med, 2014. 370(9): p. 794-7. https://doi.org/10.1056/NEJMp1314078 PMid:24571751

- Pizzimenti
V, Giandalia A, Cucinotta D, Russo GT, Smits M, Cutroneo PM, Trifirò G.
Incretin-based therapy and acute cholecystitis: a review of case
reports and EudraVigilance spontaneous adverse drug reaction reporting
database. Journal of Clinical Pharmacy and Therapeutics, 2016. 41(2):
p. 116–118. https://doi.org/10.1111/jcpt.12373 PMid:26936090

- Faillie
J, Yu OH, Yin H, Hillaire-Buys D, Barkun A, Azoulay L. Association of
Bile Duct and Gallbladder Diseases With the Use of Incretin-Based Drugs
in Patients With Type 2 Diabetes Mellitus. JAMA Internal Medicine,
2016. 176(10): p. 1474-1481. https://doi.org/10.1001/jamainternmed.2016.1531 PMid:27478902

- Yang
W, Cai X, Han X, Ji L. DPP-4 inhibitors and risk of infections: a
meta-analysis of randomized controlled trials. Diabetes/Metabolism
Research and Reviews, 2016. 32(4): p. 391-404. https://doi.org/10.1002/dmrr.2723 PMid:26417956

- Kim
HM, Lim JS, Lee B-W, Kang E-S, Lee H C, Cha B-S. Optimal Candidates for
the Switch from Glimepiride to Sitagliptin to Reduce Hypoglycemia in
Patients with Type 2 Diabetes Mellitus. Endocrinology and Metabolism,
2015. 30(1), p. 84–91. https://doi.org/10.3803/EnM.2015.30.1.84 PMid:25325279 PMCid:PMC4384675

[TOP]






















