Received: November 14, 2016
Accepted: September 27, 2016
Mediterr J Hematol Infect Dis 2017, 9(1): e2017005 DOI 10.4084/MJHID.2017.005
This article is available on PDF format at:

Department of Internal Medicine, College of Medicine, University of Duhok, Iraq.

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
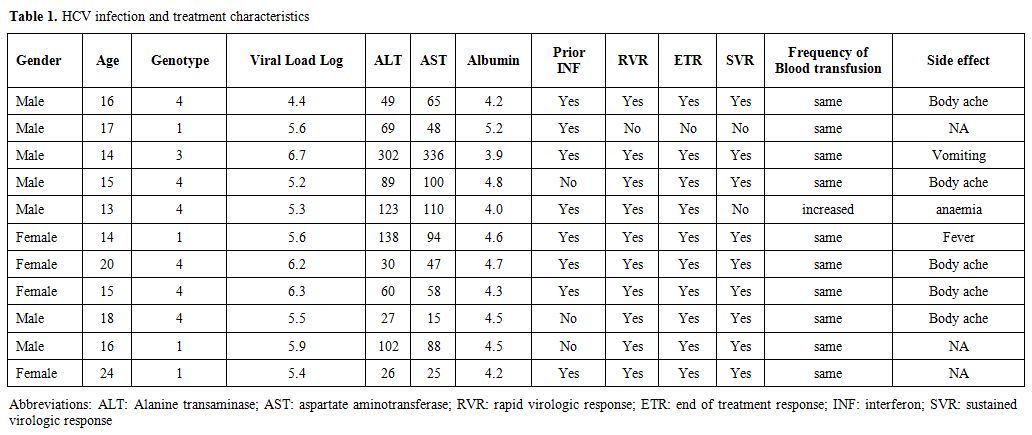 |
Table 1. HCV infection and treatment characteristics |
References









