Francesca Gulli1, Stefano Angelo Santini2, Cecilia Napodano2, Patrizia Bottoni2, Krizia Pocino2, Gian Ludovico Rapaccini3 and Umberto Basile2
1 Department of Laboratory Medicine, Madre Giuseppina Vannini Hospital, Rome, Italy.
2
Department of Laboratory Medicine, Catholic University of the Sacred
Heart, Rome,
Italy.
3 Institute of Internal Medicine, Catholic University of the Sacred Heart, Rome, Italy.
Corresponding
author: Umberto Basile. Department of Laboratory Medicine - Catholic
University of the Sacred Heart, L.go A Gemelli 8 00168 Rome,
Italy. E-mail:
umberto.basile@policlinicogemelli.it
Published: January 1, 2017
Received: October 1, 2016
Accepted: December 12, 2016
Mediterr J Hematol Infect Dis 2017, 9(1): e2017007 DOI
10.4084/MJHID.2017.007
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Cryoglobulins are immunoglobulins that precipitate in serum at
temperatures below 37°C and resolubilize upon warming. The clinical syndrome of
cryoglobulinemia usually includes purpura, weakness, and arthralgia, but the
underlying disease may also contribute other symptoms. Blood samples for
cryoglobulin are collected, transported, clotted and spun at 37°C, before the
precipitate is allowed to form when serum is stored at 4°C in a Wintrobe tube
for at least seven days. The most critical and confounding factor affecting the
cryoglobulin test is when the preanalytical phase is not fully completed at
37°C. The easiest way to quantify cryoglobulins is the cryocrit estimate.
However, this approach has low accuracy and sensitivity. Furthermore, the
precipitate should be resolubilized by warming to confirm that it is truly
formed of cryoglobulins. The characterization of cryoglobulins requires the
precipitate is several times washed, before performing immunofixation, a
technique by which cryoglobulins can be classified depending on the
characteristics of the detected immunoglobulins. These features imply a
pathogenic role of these molecules which are consequently associated with a
wide range of symptoms and manifestations. According to the Brouet
classification, Cryoglobulins are grouped into three types by the
immunochemical properties of immunoglobulins in the cryoprecipitate. The aim of
this paper is to review the major aspects of cryoglobulinemia and the
laboratory techniques used to detect and characterize cryoglobulins, taking
into consideration the presence and consequences of cryoglobulinemia in
Hepatitis C Virus (HCV) infection.
|
Easy Definition and Classification
Cryoglobulins
(CGs) are immunoglobulins (Igs) which undergo reversible precipitation
or gelling when exposed to temperatures below 37°C and re-dissolve upon
re-warming.
They were first described in a patient with Multiple
Myeloma (MM),[1] but the term “cryoglobulin” was coined later on to
describe the phenomenon of cold-precipitable serum Igs.[2] CGs remain
soluble when specific conditions are applied, mostly dependent on
temperature. The specific reason for their cold-insolubility is still
unclear and may depend on a variety of factors, although low
temperatures seemingly trigger reversible cryoprecipitation, possibly
by inducing steric modifications in the molecules which can return to
the initial conformation at 37°C.[3,4] These features imply a
pathogenic role of these molecules which are consequently associated
with a wide range of symptoms and manifestations.[5,6,7]
In 1974
Brouet et al. proposed a classification which correlated the
immunochemical characteristics of cryoglobulinemias with clinical
features of patients.[5]
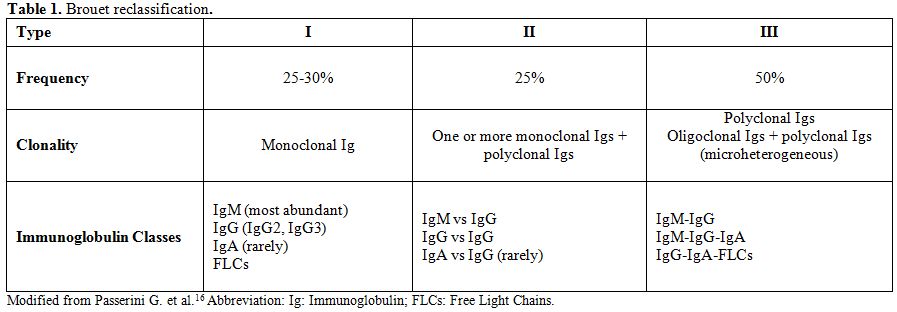
Type I monoclonal CGs are due to a
lymphoproliferative process and almost exclusively found in the context
of malignancies such as multiple myeloma and Waldenström
macroglobulinemia. Their precipitation at low temperatures may be due
to variations in amino acid composition or carbohydrate content of
involved monoclonal Igs.
Type I cryoglobulinemia patients present
a prevalence of clinical signs typical of the underlying
lymphoproliferative disease, so cryoglobulinemia is often a casual
encounter; nevertheless, vascular occlusion in association with
hyperviscosity syndrome and purpuric/dystrophic lesions of the skin
(usually affecting the lower limbs) are not uncommon findings.
Type
II and type III have generally mixed CGs (MCs), the precipitation
phenomenon being due to interactions occurring between involved Igs
other than to specific characteristics of single Igs themselves. The
IgM component of MCs has rheumatoid factor (RF) activity.[8] This system
is still widely accepted since it offers good correlations between the
associated disease state and clinical manifestations, although other
authors have described the presence of atypical CGs, also in the serum
of HCV infected patients.[8,9]
The employment of more sensitive
methodologies such as immunofixation, immunoblotting, and
two-dimensional polyacrylamide gel electrophoresis has enabled the
identification of patients affected by microheterogeneous CGs. The
concept of microheterogeneity is an innovative taxonomic element that
consists in the presence of two or more oligoclonal bands in MCs. This
form is considered as an intermediate stage between type II and type
III cryoglobulinemia.[10,11,12]
In 1997, a novel immunochemical
profile was described. It was observed in a patient affected by
Gougerot-Sjogren syndrome, which consisted of a biclonal IgM component
and a polyclonal IgG.[13]
The authors, therefore, suggested a further division of the type II group of CGs into the following two subgroups:
• Type IIa: characterized by the presence of only one monoclonal component
• Type IIb: characterized by the presence of several monoclonal components
In
another study, the definition of class IIb CGs to oligoclonal CGs
previously described was extended.[14] As a whole, technological
progress has allowed CGs typing in a more specific and sensitive
manner: as a result, Brouet’s classification has been completed
although without substantial modifications (Table 1).[15,16]
 |
Table
1. Brouet reclassification. |
•
Type III comprises immune complexes containing polyclonal rheumatoid
factor (RF), but does not show a monoclonal component.
HCV
could play an important role both in the induction and persistence of
cryoglobulinemia, as well as affecting the evolution of such a
condition from type III CG to type II CG as has been confirmed by other
authors as well.[7,17,18]
The inclusion of new subtype of CG,
transitional step between type II CG and type III CG, represents
progress towards greater attention on clinical, histopathological and
follow-up aspects as well as on a more adequate diagnostic and
therapeutic indication, which differ along each and every moment of the
evolutionary pathway of the disease. For this reason, and in light of
current knowledge, the CGs classification may be integrated into the
Brouet classification.[16]
Molecular Basis of Cryoprecipitation
The
solubility of a protein depends on numerous factors such as the
concentration, the temperature, the pH, the ionic strength of the
solution and the net charge that depends on the amino acids and
residues from the carbohydrate content. However, the biochemical
mechanisms at the basis of this process are not fully understood.[4]
Cryoprecipitation
in type I CG can be considered a simple phenomenon of solubility that
is derived from the unfavorable interaction between CGs and solvent at
low temperatures.[19] The aggregation is often the result of
electrostatic interactions, which in turn depend on the structural
characteristics of CGs as an altered glycosylation with reduction in
sialic acid content.[4,20] Levo assumed that the existence of an
impoverishment of sialic acid would make immunogenic Igs favor
cryoprecipitation, especially during persistence and intense immune
stimulation. This stimulation would lead to the manifestation of a
secretory defect with production of Ig without sialic acid.[21] The
absence of sialic acid in the structure of Ig generates an immune
response to the epitope exposure before being masked; the immune
complex thus formed would acquire the capacity to precipitate. The
author extended his theory by assuming that hepatocellular damage
favoring the permanence of Ig without sialic acid reduced the capacity
of hepatocyte deputies to remove them.[21]
Cryoprecipitation in
monoclonal CGs appears to be characterized by particular amino acid
sequences that may create a structural change at the level of the
quaternary structure of the protein, causing an autoaggregation. This
phenomenon starts with a slow phase (lag phase) and the formation of
small aggregates of monoclonal Igs followed by rapid and extensive
aggregation, due to a combination of weak non-ionic and hydrophobic
interactions which culminate in the precipitation.[22] A study of IgG
CGs structure showed that it can produce amorphous, gelatinous and or
crystalline precipitates.[23] Most of the factors that influence the
cryoprecipitation of monoclonal CGs are also present in MCs,
where, however, the lag phase is absent. MC precipitation is the
consequence of the rapid and progressive increase in the size of
IgG-IgM immune complexes at low temperatures also in the absence of lag
phase. MCs also show typical biological properties of immune complexes,
such as the ability to activate complement.[24] Rheumatoid Factor
MCs
are immune complexes that contain the rheumatoid factor (RF), although
the cause that induces a shift to abnormal proliferation of a single
clone of B cells that produces monoclonal IgM-κ RF is not clearly
understood. The RF is a monoclonal or polyclonal IgM, although other Ig
may be found.[10] There is a higher prevalence of Immunoglobulin G3
(IgG3) responses to HCV antigens in those patients who are HCV- and
MC-positive rather than in those who are HCV-positive and
MC-negative.[41] IgG3 fixes complement most efficiently among the
subclasses, thereby leading to activation of the classical pathway.[41]
The presence of IgG3 in cryoprecipitates of HCV- and ANA-positive
patients constitutes the decisive factor for the possible activation of
autoimmune mechanisms over the long term.[30] In addition, IgG3
positive patients are also positive for IgG-RF,[30] known to be
autoreactive clones and their capacity to activate several cell clones
is confirmed by many clinical studies.[42,43] Thus, the presence of
IgG3 in cryoprecipitates may suggest a more highly activated immune
system, which is then more exposed to the mechanisms of autoimmune
diseases. These markers may constitute a prognostic factor for
autoimmune diseases in HCV-affected individuals, as opposed to
ANA-negative and IgG3- and IgG RF- negative subjects.[30] In naïve,
asymptomatic CG- and HCV-positive patients, the presence of IgG RF and
serum free light chains suggests their use as biomarkers, in order to
identify the transition between a silent state of probable autoimmune
lymphoproliferative disease and frank illness. The possibility of
identifying subpopulations among HCV-positive patients may open new
scenarios to targeted treatment strategies in extremely early phases
(sub-clinical).[44]
Laboratory Testing for the Detection and Typing of CGs
The
laboratory workup for detection and typing of CGs can be divided into 3
phases: the preanalytical phase, the analytical phase, and
characterization of the cryoprecipitate phase (Figure 1).
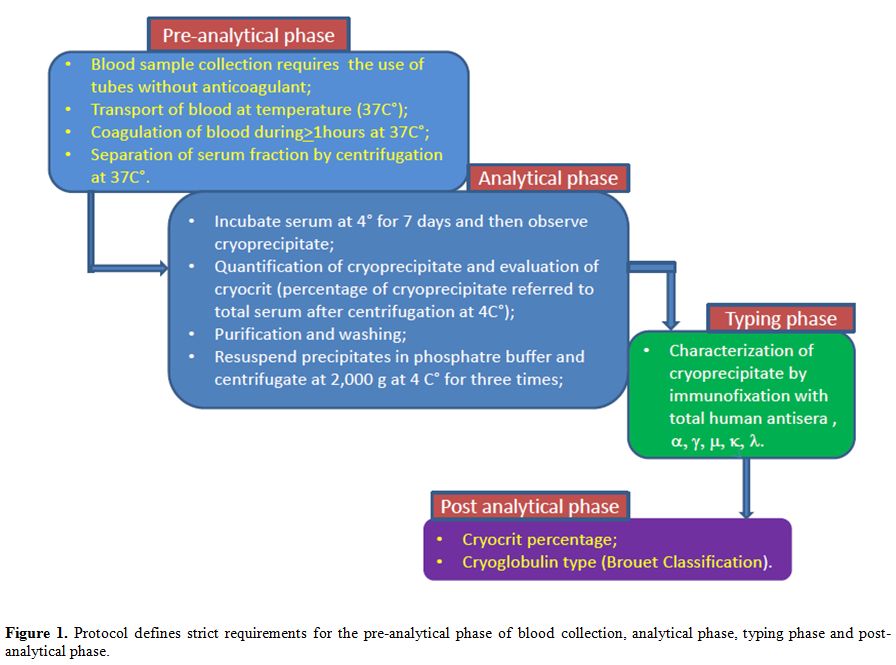 |
Figure 1. Brouet reclassification. |
Several
methods have been proposed to reduce precipitation time.[45,46] One of
these is based on a quick spectrophotometric measurement, at 350 nm, of
serum immunocomplex aggregates that form at 10°C. A critical evaluation
of such results has allowed us to propose a “functional”
classification: “fast” CGs: positive results obtained with the rapid
test, as well as with the traditional test; “slow” CGs: only the
traditional assay shows a positive result.[46]
Detection by flow
cytometry of CGs is sensitive, specific and fast. Detected CGs in
positive serum by the conventional method were derived from patients
suffering from autoimmune diseases potentially associated with low
level of CGs.[45]
The determination of circulating CGs has been
performed with these two methods, and the utility of the rapid test
might be considered in the therapeutic management of cryoglobulinemic
patients.
Preanalytical Phase:
Several recent reports have stressed the need for international
guidelines concerning CGs detection, particularly in sample handling
and transport procedures, and exhorted the necessity of standardized
protocols for global harmonization.[4,6,7,10,21,47] This potentially
life-threatening condition requires appropriate laboratory testing,
especially for those patients showing clinical symptoms associated with
such a condition. The required tests, while feasible with a simple
biochemical quantification, need strict pre-analytical protocol
adhesion to maintain the sample at a stable temperature of 37°C,
especially throughout the initial steps. Failure to ensure these
critical conditions from sample collection may result in cryoglobulin
misdetection, to the detriment of the patient. The difficulty in
ensuring the appropriate preanalytical condition and the variability in
following the procedure can entail a heterogeneity of results, thus
substantiating the perceived difficulty of performing this relatively
simple test. As a result, this analysis is often neglected by
clinicians, despite its usefulness in patient management.[4,47]
However, CGs detection still requires global harmonization, and there
are yet no internationally accepted standard protocols, although
several approaches have been described and proposed.[10,16] Most
authors agree about the necessity of keeping the sample at 37°C
following collection and transportation, as well as throughout clotting
and during the initial centrifuging stages. Sample collection is the
most critical phase, and improper collection and transport accounts for
the most common reasons for undetected cryoglobulins and
false-negatives.[10,48] Samples should be kept warm from the moment of
collection, but not kept in hand, to prevent contamination. Up to now,
a cost-effective transportation means has not yet been validated,
although a variety of systems has been proposed. There is also a great
need to attain strict hygienic procedures (roughly 80% of Mixed
Cryoglobulinemia (MC) affected patients are HCV-positive) so a
disposable system would offer an ideal compromise.[48]
Sample Collection:
Blood sample collection requires the use of tubes without
anticoagulant, which should be kept at 37°C both before and after
sample collection for at least 30 min until complete clotting.[49,50]
Tubes with separating gel are highly discouraged due to the risk of
interfering substances which may be released by the gel during
incubation at 37°C to enable clotting. Should non-gel tubes be
unavailable, it is desirable to ask the manufacturer for information
concerning the gel composition and its specific characteristics. Blood
samples should reach 10 mL.[50] A smaller volume might mean missing
detection of low concentration CGs that could potentially be associated
with severe pathologies.[8] Some authors emphasize the necessity of
spinning the blood sample at 37°C, and alternatively suggest to
separate the serum from the clot without using a centrifuge if this is
not preheated.[48,49] Other authors assert that blood should be kept at
37°C until serum separation only for patients with type I CG, whereas
samples from patients affected by MC may be handled at room
temperature.[47] Kallemuchikkal suggests that clotted blood should be
spun at 2000x g for 10 min at 37°C.[50] Musset suggests 2000x g for 30
min at 37°C;[11] Brouet and Dammacco recommend spinning at 37°C but do
not specify either time or speed.[5,51] Since these are not critical
factors for analysis they could be performed downstream
Analytical Phase:
• Sample
observation. Following centrifugation, the supernatant serum sample
should be transferred into Wintrobe tubes and incubated at 4°C. The
precipitation process manifests itself in a variety of ways in samples,
depending on the concentration of CGs present, and may require either a
few hours (when CGs-often type I-become insoluble at room temperature)
or longer periods of time, particularly in the case of low
concentrations of type III CGs. Observation at 4°C is a critical
parameter in this analysis and should be established in an adequate
manner to achieve detection of even extremely low levels of CGs in the
serum.[50,47] For a correct performance of the CGs search analysis, the
serum sample should be kept at 4°C for at least seven days: during this
time, the sample should never be frozen or warmed to avoid significant
variations in immunoglobulin solubility.[11,50]
• Artefact
verification. By definition, CGs precipitate or gel in a reversible
manner at temperatures below 37°C. Therefore, after the quantification,
it is necessary to confirm thermo-reversibility of the precipitate by
re-dissolving it at 37°C for one hour or by setting aside an aliquot of
serum to be stored at 37°C for the same amount of time (7
days).[5,11,50] In particular, patients undergoing anticoagulant
therapy may present with cryoprecipitates composed of
heparin-fibronectin complexes or by fibrinogen-fibrin which are
morphologically similar to CGs. In such cases, immunotyping of
cryoprecipitate is necessary to confirm the presence of immunoglobulins
and exclude false-positivity due to artifacts.[50,52]
• Cryoprecipitate
quantification. CGs quantification may be expressed in the following
ways: as cryocrit (CRT), as a measurement of total proteins, as an
immunonephelometric quantification of immunoglobulins or as the area
under the curve in the gamma region following electrophoresis of
resolubilized cryoprecipitate (performed at 37°C). The CRT is a
semi-quantitative parameter in common use, as it is simple and cheap,
although it is affected by a great number of variables which discourage
its use as a comparable parameter among patients, or as an indicator of
associated pathologies. The CRT expresses the percentage ratio between
cryoprecipitate volume and serum volume obtained by centrifugation at
4°C for 15 min at 1700x g.[50] The evaluation is performed by the
operator; that implies CGs quantification is fairly inaccurate,
unspecific and rather insensitive. The limiting factors include the
test tube type used for measurement, the spinning conditions employed
for centrifugation, the necessity of incubating large volumes of serum
to achieve reliable data and the assumption of an underlying
correlation between CRT protein concentration and the sedimented volume
of material. Moreover, as CRT measurement is not performed on washed CG
material, CRT values may be strongly affected, to a certain extent, by
the presence of serum proteins trapped within the
cryoprecipitate.[8,50] Nevertheless, CRT values are often used in
published clinical case reports and persist as recommended quantitative
data used in the literature.
• Cryoprecipitate
washing techniques. Removal of serum proteins previous to
cryoprecipitate characterization is a fundamental step which guarantees
a correct interpretation of the immunoelectrophoretic profile.
Cryoprecipitates may be washed with a physiological saline solution,
with PBS (phosphate buffered solution), or with polyethylene glycol
6000 3% in PBS.[5,11,50] In all cases, the washing solution must be
kept at 4°C, and CGs should be resuspended by agitation in a volume of
solution corresponding to the amount of supernatant discarded after
spinning of the sample at 4°C. Washed cryoprecipitates should then be
centrifuged (at 4°C) to separate once again the precipitate from the
washing solution/buffer. Spinning conditions and washing cycles vary in
the literature. Consensus can be reached by establishing a minimum of 3
wash cycles, which should be increased for CRT values >4%. When
cryoprecipitates are low (<1%) it is advisable to recuperate samples
after each wash by incubating the sample at 4°C for 72h, before moving
on to the next washing step. When CGs do not dissolve in the wash
solution by agitation, they should be resuspended by incubating them at
37°C until complete resuspension. The sample should then be recuperated
by following 72h of incubation at 4°C before carrying on with the
washes.10 In rare cases, the common wash solutions may dissolve the
cryoprecipitate in an irreversible fashion so CGs cannot be recuperated
to perform their characterization.[53] Washed cryoprecipitate should be
dissolved by incubation at 37°C. CGs may also be treated with reducing
solutions, such as 10% acetylcysteine, or 1% β-mercaptoethanol or 0.5
mmol dithiothreitol.[4]
Cryoprecipitate Typing
Immunocharacterization
of cryoprecipitates, initially performed by Brouet using
immunoelectrophoresis, is now carried out with the use of more
sensitive methods such as agarose gel immunofixation techniques
(considered the “gold standard”), immuno-subtraction by capillary
electrophoresis, immunoblotting and two-dimensional polyacrylamide gel
electrophoresis.[12] These procedures not only confirm the presence of
immunoglobulins but also enables classification into types I-III. As
mentioned, typing of the cryoglobulin provides direction toward
identification of a possible underlying disease. The rather subjective
reading of the results means that two independent specialist
laboratories should be used. A minimum competency-based standard is
required for those who review and interpret CG patterns. Protein
Laboratories are encouraged to have an educational module suitable for
continuing professional development.[54]
Other Quantification Methods
Total protein
quantification is a method alternative to CRT measurement, although it
is still awaiting validation. It permits evaluation of CGs
concentration, although it is strongly affected by the presence of
other proteins contained in cryoprecipitates such as albumin,
fibronectin, C1q and other complement factors. Total protein
quantification requires accurate washing of cryoprecipitates as well as
complete re-suspension of CGs. It offers the advantage of greater
sensitivity as opposed to CRT since it evaluates cryoprecipitates that
adhere to the bottom of Wintrobe tubes and may, therefore, escape
visual inspection. Musset et al. quantify total proteins in
cryoprecipitates by spectrophotometric analysis at 280nm following CGs
solubilization in 0.1nmol/L NaOH.[11] Brouet et al. re-suspend CGs in
0.1mol/L of acetic acid and perform a colorimetric quantification of
cryoprecipitate content of total proteins using either Pyrogallol Red
or Coomassie Blue staining:[5] 1mL of serum is stored at 4°C for 3 days
and subsequently centrifuged at 5000 rpm for 5 min at 4°C. CGs are
separated from supernatant serum, washed three times with 3mL of cold
water and re-dissolved physiological solution at 37°C. Nephelometric
quantification of albumin may detect contamination from residual serum
proteins. Literature reports indicate that the reference serum
cryoprecipitate total protein content values should be <20 mg/L.[47]
Other experimental quantification data may be obtained by calculating
the difference between the nephelometric measurement of the total serum
immunoglobulin concentration at 37°C and supernatant immunoglobulin
concentration at room temperature following precipitation.[50] An
electrophoretic run of re-solubilized cryoprecipitate performed at
37°C, either using capillary electrophoresis or by agarose gel
electrophoresis, provides accurate CGs quantification. It is achieved
by calculating the area under the curve in the gamma region of the
electropherogram profile and by subtracting the equivalent amount of
co-precipitating serum globulins from this value on the basis of the
amount of residual albumin. The latter is therefore used as an internal
standard correction factor for cryoprecipitate measurement, by
performing the following calculations: γ-globulin/albumin ratio of
cryoprecipitate versus γ-globulin/albumin ratio of native serum.[55]
Cryoglobulinemia and HCV
Cryoglobulinemia is
considered to be a rare disorder, but its occurrence is strongly linked
to the prevalence of HCV infection in the general population.[25] Other
viral infections, as Hepatitis B Virus, Epstein Barr Virus , HIV can
induce, even if with but with minor frequency, mixed
crioglobulinema, that is almost always type III.[9,18,47]
The
prevalence of type MC in HCV infection depends on the stage of the
disease and the sensitivity of the analytical method. In patients with
HCV cryoglobulins of type II and III can be present at different times
in relationship with the presence of antibodies and the virus of HCV
and the emergence of clonal lymphocyte proliferation,[18] in any case,
however, the major complication, renal involvement,
is strongly associated cryoglobulinemia type II MC, mostly
in presence of IgM kappa.[32]
Chronic HCV infections are
an issue of primary interest since, according to global WHO estimates,
3% of the total world population is infected by the virus.[26] For this
reason, the development of efficacious prevention strategies and
innovative therapeutic approaches that enable a major improvement from
currently available treatments are of great importance.
The
peculiar biological characteristics of the HCV, a hepatotropic and
lymphotropic virus, may partially explain the immune and pathologic
alterations responsible for HCV-correlated disorders.
HCV-infected
patients are known to be at risk of developing liver complications. The
risks of morbidity and mortality are frequently underestimated because
they do not take into account non-liver consequences of chronic HCV
infection. Numerous extrahepatic manifestations have been reported in
up to 74% of patients, from perceived to disabling conditions. The
majority of data concern HCV-related autoimmune and/or
lymphoproliferative disorders, from mixed cryoglobulinemia vasculitis
to frank lymphomas.[32]
In particular, chronic infection of
immunocompetent cells (T and B lymphocytes, macrophages) may be
responsible for the proliferation of B lymphocytes which trigger
production of circulating immune complexes composed of CGs and
autoantibodies. To date, HCV infection is known to cause deep changes
in the immune response of the host, including the triggering of
autoimmune diseases.[27] Autoantibodies have been detected in about 40%
of HCV-positive patients, and their presence was associated with
several extrahepatic complications as well as MC.[27,28] In the MC
setting, a monotypic lymphoproliferation may often appear, and be
clinically indolent, whereas frank B-cell Non-Hodgkin’s Lymphoma
(B-NHL) may be a late complication in 10% of patients. On the other
hand, HCV may account for approximately one-third of “primitive”
B-NHL.[29]
MCs are immunocomplexes in which the antigen is usually
an IgG, and the antibody (which shows anti-IgG rheumatoid factor
activity) is either a polyclonal or monoclonal IgM.[8] In HCV-related MC,
the cold-dependent insolubility requires the presence of IgM-RF, IgG
that targets HCV core protein and the protein itself. The addition of
an irrelevant IgG to a mixture of IgM-RF and core protein was unable to
cause cryoprecipitation.[24] For the first time, cryoglobulinemia with an
IgG RF has been discovered and since then, growing evidence has
suggested that IgG subclasses could be involved in the development of
cryoglobulinemia.[30]
The search for CGs should only be performed in
subjects with suggestive clinical symptoms (asthenia, arthralgia and
purpura) or clear laboratory data (Anti-nuclear antibodies,
Anti-mitochondrial antibodies, Anti-smooth muscle antibodies,
Anti-extractable nuclear antigen antibodies, Low level of C4, Anti-HCV
antibodies ± HCV RNA), since the transient or asymptomatic observation
of CGs is often associated with a variety of pathologies that set-off a
hyper-stimulation of B-cells, such as inflammatory, neoplastic or
infectious diseases of various etiology.[31]
The presence of
cryoglobulinemia is not necessarily indicative of a disease state
(transient levels of CGs may be detected during infections, and healthy
individuals may present low levels of cryoglobulinemia), and serum
concentrations do not always correlate with the severity of symptoms.
So, some patients with apparently low levels of CGs may show severe
symptoms associated with cryoglobulinemic syndrome.[32] This potentially
life-threatening condition requires appropriate laboratory testing,
especially for those patients showing clinical symptoms associated with
such a condition.
The first classification criteria for MCs were
proposed by the Italian Group for the Study of Cryoglobulinemias in
1989. In 2002 they were revised by the inclusion of pathological and
virological findings.[8] The classification criteria included major and
minor criteria. Major Serological criteria include the type of MC, low
level of serum C4; the minors include the presence of IgM-RF and viral
diseases HCV, HBV. Major and minor pathological criteria
include respectively leukocytoclastic vasculitis, and clonal B cell
infiltrates in the liver and/or bone marrow. Major and minor clinical
manifestations include purpura and chronic hepatitis,
membranoproliferative glomerulonephritis, peripheral neuropathy, skin
ulcers respectively. MC syndrome was defined by the presence of typical
triad (first described by Meltzer and hence known as Meltzer’s
triad),[33] including low level of C4, purpura, and leukocytoclastic
vasculitis or the presence of MC (low C4 plus two minor clinical
symptoms plus two minor serological/pathological findings).
The
classification criteria have been used for epidemiological studies in
patients with MC syndrome, but they have not been validated in
clinically well-defined patient cohorts and therefore lack appropriate
statistical support.[34]
Gene cluster variants of Human leukocyte
antigen (HLA) in specific alleles could be a condition determining
susceptibility to the development of MC and NHL during chronic HCV
infection.[6,35,36] The Multicenter Genome-Wide Association Study (GWAS)
reported an association between two particular polymorphisms on
chromosome 6 and HCV-related MC vasculitis compared to HCV controls
without evidence of lymphoproliferative disorders.[37] The first one is a
single nucleotide polymorphism (SNP) (rs2071286) located in an intronic
region of the NOTCH4 gene; the second one is a SNP (rs9461776) located
between HLA-DRB1 and HLA-DQA1 gene segments of the major
histocompatibility complex (MHC). Although the biological and
functional meaning of these associations is unknown, a wide cohort of
HCV patients with MC vasculitis present a genetic background
predisposing to this kind of disorder.[38]
Genetic factors and
impairment of the epigenetic regulation could make an extremely
important contribution to the pathogenesis of HCV-related
lymphoproliferative disorders.
The role of small, non-coding
RNA, called microRNAs (miRNA), acting as post-transcriptional
epigenetic regulators, has been suggested. miRNAs can modulate a wide
variety of genes by either preventing the translation or inducing the
cleavage of complementary mRNAs.
Deregulation of specific miRNAs
seems to be involved in the pathogenesis of lymphoma, including some
types typically found to be associated with HCV infection.[38,39,40]
HCV
infection can represent the cause of MC in 80% of cases in
regions with high incidence of HCV.[25] On the other hand low levels of
circulating mixed cryoglobulins can be detected in over 50% of HCV
infected individuals, while overt cryoglobulinemic syndrome develops in
about 5%.[18] The diffusion of HCV infection is variable in the
world, a high incidence of HCV-related MC is found in
Mediterranea basin and ever a higher incidence can be
expected in low income countries where HCV in the general
population is rather prevalent, and in immigrant in Europe from
Africa and Asia.[25,56,57] The disease expression is variable, and the
different symptoms arise from the involvement of various organs and
systems, namely skin, joints, kidney, nervous system, salivary and
lachrymal glands. Hence, the symptoms defining a full-blown MC can be
so multiple and severe to determine a very poor quality of life for the
patient.[32] HCV has a tremendous impact on patient-reported outcomes,
such as health-related quality of life and fatigue. These HCV-related
complications are responsible for a significant economic burden through
direct medical costs associated with managing the liver disease, as
well as the indirect costs associated with decreased work productivity.
Antiviral therapy has been indicated as first-line therapy in patients
with mild-to-moderate HCV-related MC vasculitis.[32] The importance of
this extrahepatic manifestations of HCV is nowadays officially
recognized and the latest AASLD guidelines for the new direct acting
antivirals (DAA) indicated the MC among the highest priority conditions
to treat because of the risk for severe complications. [58,59] In severe
cases, or in patients intolerant/ineligible to antiviral therapy,
anti-CD20 monoclonal antibody rituximab should be considered.[58,60]
Conclusion
The possibility of
detecting even very limited amounts of CGs may offer an invaluable
resource to clinicians operating in this field. There is also a growing
demand for more efficient and rapid tests for detection of their
presence. Since even limited amounts of cryoglobulins may be both
pathogenic and significant in certain clinical contexts, their
detection at low levels may be critical for diagnosis and especially
for those patients requiring plasmapheresis. A high prevalence of
cryoglobulin ≤0.05 g/L in clinical practice may be responsible for
severe renal and neurological complications, leading to high morbidity
and mortality in these patients. Therefore both appropriate therapy and
careful follow-up is required to improve such patients' outcome.[58,61]
The
diagnosis of cryoglobulinemia syndrome is predominantly based on the
laboratory demonstration of serum CGs, with or without associated
characteristic clinical signs and symptoms. Diminished serum complement
components may reflect ongoing consumption by CG immune complexes.
Appropriate
phases of CG research are fundamental for a correct diagnosis and
adequate treatment of the associated diseases. Given the variability of
testing conditions used in different laboratories and the lack of test
standards and reference values, further investigation into
standardization of CG testing should be performed in the future. The
biological importance and activity of CGs, such as their ability to
activate proinflammatory complement proteins, needs to be defined as
well.
References
- Wintrobe MM, Buell MV. Hyperproteinemia associated
with multiple myeloma. With report of a case in which an extraordinary
hyperproteinemia was associated with thrombosis of the retinal veins
and symptoms suggesting Raynaud's disease. Bull. John Hopkins Hosp.
1933; 52 156.

- Lerner AB, Watson CJ. Studies of
cryoglobulins; unusual purpura associated with the presence of a high
concentration of cryoglobulin (cold precipitable serum globulin). Am J
Med Sci. 1947 Oct;214(4):410-5. https://doi.org/10.1097/00000441-194710000-00009 PMid:20266939

- Lawson
EQ, Brandau DT, Trautman PA, Middaugh CR. Electrostatic properties of
cryoimmunoglobulins. J Immunol. 1988 Feb 15;140(4):1218-22.
PMid:3343512

- Sargur R, White P, and Egner W. Cryoglobulin evaluation: best practice? Ann Clin Biochem. 2010 Jan;47(Pt 1):8-16 https://doi.org/10.1258/acb.2009.009180 PMid:20040797

- Brouet
JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical
significance of cryoglobulins. A report of 86 cases. Am J Med. 1974
Nov;57(5):775-88. https://doi.org/10.1016/0002-9343(74)90852-3

- Ramos-Casals M, Stone JH, Cid MC, Bosch X. The cryoglobulinaemias. Lancet. 2012 Jan 28;379(9813):348-60. https://doi.org/10.1016/S0140-6736(11)60242-0

- Retamozo
S, Brito-Zerón P, Bosch X, Stone JH, Ramos-Casals M. Cryoglobulinemic
disease. Oncology (Williston Park). 2013 Nov;27(11):1098-1105,
1110-6
 .
.
- Ferri C, Zignego AL, and Pileri SA. Cryoglobulins. J Clin Pathol. 2002 Jan;55(1):4-13. https://doi.org/10.1136/jcp.55.1.4 PMid:11825916 PMCid:PMC1769573

- Ferri C, Zignego AL. Relation between
infection and autoimmunity in mixed cryoglobulinemia. Curr Opin
Rheumatol. 2000 Jan;12(1):53-60. https://doi.org/10.1097/00002281-200001000-00009 PMid:10647955

- Motyckova G, Murali M. Laboratory testing for cryoglobulins. Am J Hematol. 2011 Jun;86(6):500-2. https://doi.org/10.1002/ajh.22023 PMid:21594887

- Musset
L, Diemert MC, Taibi F, Thi Huong Du L, Cacoub P, Leger JM, Boissy G,
Gaillard O, Galli J. Characterization of cryoglobulins by
immunoblotting. Clin Chem 1992;38:798-802. PMid:1597004

- Tissot
JD, Pietrogrande M, Testoni L, Invernizzi F. Clinical implications of
the types of cryoglobulins determined by two dimensional polyacrylamide
gel electrophoresis. Haematologica. 1998;83:693-700.
PMid:9793252

- Pontet
F, Halimi C, Brocarde A, Delacour T. Biclonal immunoglobulin M
dysglobulinaemia: evolving aspects in a case of primary Sjogren
syndrome. Eur J Clin Chem Clin Biochem. 1997:35:287-90. https://doi.org/10.1515/cclm.1997.35.4.287

- Le Carrer D. Cryoglobulinemies:
proposition d'un protocole d'exploration biologique. Actualisation de
leur classification. Feuillets boil. 1998;39/221:55-65.

- Oliver M, Coton T, Ragot C, Delpy R, Moalic JL, Debonne JM. Les cryoglobulinemies. Annal Biol Clin 2004;62:521-8.

- Passerini G, Basile U. Recommendations
for a protocol to detect, quantify and characterize cryoglobulins.
Biochimica Clinica, vol. 34, no. 3, pp. 218–222, 2010.

- Trejo O, Ramos-Casals M,
García-Carrasco M, Yagüe J, Jiménez S, de la Red G, Cervera R, Font J,
Ingelmo M. Cryoglobulinaemia: study of etiologic factors and clinical
and immunologic features in 443 patients from a single center. Medicine
(Baltimore) 2001; 80: 252–62. https://doi.org/10.1097/00005792-200107000-00004

- Ferri C. Mixed cryoglobulinemia. Orphanet J Rare Dis. 2008 Sep 16;3:25. https://doi.org/10.1186/1750-1172-3-25 PMid:18796155 PMCid:PMC2569912

- Middaugh CR, Kehoe GM, Prystowsky MB,
Gerber-Jenson B, Jenson JC, Litman GW. Molecular basis of the
temperature-dependent insolubility of cryoglobulins. IV. Structural
studies of the IgM monoclonal cryoglobulin. Immunochemistry. 1987; 15:
171-87. https://doi.org/10.1016/0161-5890(78)90146-3

- Mizuochi
T, Pastore Y, Shikata K, Kuroki A, Kikuchi S, Fulpius T, Nakata M,
Fossati-Jimack L, Reininger L, Matsushita M, Fujita T, Izui S. Role of
galactosylation in the renal pathogenicity of murine immunoglobulin G3
monoclonal cryoglobulins. Blood 2001; 97: 3537–43. https://doi.org/10.1182/blood.V97.11.3537 PMid:11369648

- Levo Y. Nature of cryoglobulinemia. Lancet.1980 Feb; 1(8163):285-7. https://doi.org/10.1016/S0140-6736(80)90781-3

- Lawson EQ, Brandau DT, Trautman PA,
Aziz SE, Middaugh CR. Kinetics of the precipitation of
cryoimmunoglobulins. Mol Immunol. 1987; 24: 897-905. https://doi.org/10.1016/0161-5890(87)90001-0

- Podell DN, Packman CM, Maniloff J,
Abraham GN. Characterization of monoclonal IgG cryoglobulins:
fine-structural and morphological analysis. Blood 1987; 69: 677-81.
PMid:3801676

- Sansonno
D, Dammacco F. Hepatitis C virus, cryoglobulinaemia, and vasculitis:
immune complex relations. Lancet Infect Dis. 2005; 5: 227–36. https://doi.org/10.1016/S1473-3099(05)70053-0

- Zignego AL, Giannini C, Ferri C.
Hepatitis C virus-related lymphoproliferative disorders: an overview.
World J Gastroenterol. 2007 May 7;13(17):2467-78. https://doi.org/10.3748/wjg.v13.i17.2467 PMid:17552031 PMCid:PMC4146766

- Craxi A, Laffi G, Zignego AL.
Hepatitis C virus (HCV) infection: A systemic disease. Mol Aspects Med.
2008 Feb-Apr;29(1-2):85-95. https://doi.org/10.1016/j.mam.2007.09.017 PMid:18177700

- Haddad J, Deny P, Munz-Gotheil C,
Ambrosini JC, Trinchet JC, Pateron D, Mal F, Callard P, Beaugrand M.
Lymphocytic sialodenitis of Sjogren's syndrome associated with chronic
hepatitis C virus liver disease. Lancet 1992; 339: 321-3. https://doi.org/10.1016/0140-6736(92)91645-O

- Roccatello
D, Morsica G, Picciotto G, Cesano G, Ropolo R, Bernardi MT, Cacace G,
Cavalli G, Sena LM, Lazzarin A, Piccoli G, Rifai A. Impaired
hepatosplenic elimination of circulating cryoglobulins in patients with
essential mixed cryoglobulinemiaand hepatitis C virus (HCV) infection.
Clin Exp Immunol 1997; 110: 9-14. https://doi.org/10.1111/j.1365-2249.1997.475-ce1383.x PMid:9353142 PMCid:PMC1904798

- Bachy E, Besson C, Suarez F, Hermine O.
Hepatitis C virus infection and lymphoma. Mediterr J Hematol Infect
Dis. 2010; 31;2(1).

- Basile U, Gulli F, Torti E, De
Matthaeis N, Colacicco L, Cattani P, Rapaccini GL. Anti-nuclear
antibody detection in cryoprecipitates: Distinctive patterns in
hepatitis C virus-infected patients. Dig and Liv Dis 47 (2015) 50–56. https://doi.org/10.1016/j.dld.2014.09.010 PMid:25445409

- Ferri
C, Sebastiani M, Giuggioli D, Colaci M, Fallahi P, Piluso A, Antonelli
A, Zignego AL. Hepatitis C virus syndrome: A constellation of organ-
and non-organ specific autoimmune disorders, B-cell non-Hodgkin's
lymphoma, and cancer. World J Hepatol. 2015 Mar 27;7(3):327-43. https://doi.org/10.4254/wjh.v7.i3.327 PMid:25848462 PMCid:PMC4381161

- Cacoub P, Gragnani L, Comarmond C,
Zignego AL. Extrahepatic manifestations of chronic hepatitis C virus
infection. Dig Liver Dis 2014;46S5:S165-S73.

- Meltzer M, Franklin EC. Cryoglobulinemia: a study of 29 patients. Am J Med 1966;40:828-36. https://doi.org/10.1016/0002-9343(66)90199-9

- Quartuccio L, Isola M, Corazza L,
Ramos-Casals M, Retamozo S, Ragab GM, Zoheir MN, El-Menyawi MA, Salem
MN, Sansonno D, Ferraccioli G, Gremese E, Tzioufas A, Voulgarelis M,
Vassilopoulos D, Scarpato S, Pipitone N, Salvarani C, Guillevin L,
Terrier B, Cacoub P, Filippini D, Saccardo F, Gabrielli A, Fraticelli
P, Sebastiani M, Tomsic M, Tavoni A, Mazzaro C, Pioltelli P, Nishimoto
N, Scaini P, Zignego AL, Ferri C, Monti G, Pietrogrande M, Bombardieri
S, Galli M, De Vita S. Validation of the classification criteria for
cryoglobulinaemic vasculitis. Rheumatology (Oxford). 2014
Dec;53(12):2209-13. https://doi.org/10.1093/rheumatology/keu271 PMid:24994905

- Cacoub
P, Renou C, Kerr G, Hue S, Rosenthal E, Cohen P, Kaplanski G, Charlotte
F, Thibault V, Ghillani P, Piette JC, Caillat-Zucman S. Influence of
HLA-DR phenotype on the risk of hepatitis C virus-associated mixed
cryoglobulinemia. Arthritis Rheum. 2001 Sep;44(9):2118-24. https://doi.org/10.1002/1529-0131(200109)44:9<2118::AID-ART364>3.0.CO;2-X

- Hwang SJ, Chu CW, Huang DF, Lan KH,
Chang FY, Lee SD. Genetic predispositions for the presence of
cryoglobulinemia and serum autoantibodies in Chinese patients with
chronic hepatitis C. Tissue Antigens. 2002 Jan;59(1):31-7. https://doi.org/10.1034/j.1399-0039.2002.590106.x PMid:11972876

- Zignego
AL, Wojcik GL, Cacoub P, Visentini M, Casato M, Mangia A, Latanich R,
Charles ED, Gragnani L, Terrier B, Piazzola V, Dustin LB, Khakoo SI,
Busch MP, Lauer GM, Kim AY, Alric L, Thomas DL, Duggal P. Genome-wide
association study of hepatitis C virus- and cryoglobulin-related
vasculitis. Genes Immun. 2014 Oct;15(7):500-5. https://doi.org/10.1038/gene.2014.41 PMid:25030430 PMCid:PMC4208981

- Zignego AL, Gragnani L, Piluso A,
Sebastiani M, Giuggioli D, Fallahi P, Antonelli A, Ferri C.
Virus-driven autoimmunity and lymphoproliferation: the example of HCV
infection. Expert Rev Clin Immunol. 2015 Jan;11(1):15-31. https://doi.org/10.1586/1744666X.2015.997214 PMid:25534977

- Peveling-Oberhag
J, Crisman G, Schmidt A, Doring C, Lucioni M, Arcaini L, Rattotti S,
Hartmann S, Piiper A, Hofmann WP, Paulli M, Küppers R, Zeuzem S,
Hansmann ML. Dysregulation of global microRNA expression in splenic
marginal zone lymphoma and influence of chronic hepatitis C virus
infection. Leukemia. 2012 Jul;26(7):1654-62. https://doi.org/10.1038/leu.2012.29 PMid:22307176

- Fognani
E, Giannini C, Piluso A, Gragnani L, Monti M, Caini P, Ranieri J,
Urraro T, Triboli E, Laffi G, Zignego AL. Role of microRNA profile
modifications in hepatitis C virus-related mixed cryoglobulinemia. PLoS
One. 2013;8(5):e62965. https://doi.org/10.1371/journal.pone.0062965 PMid:23650540 PMCid:PMC3641090

- Dispenzieri A, Gorevic PD. Cryoglobulinemia. Hematol Oncol Clin North Am 1999;13:1315–49. https://doi.org/10.1016/S0889-8588(05)70129-5

- Shozo Izui, Thierry Bemey, Takanori
Shibata, Thierry Fulpius. IgG3 cryoglobulins in autoimmune MRL-lpr/lpr
mice: immunopathogenesis, therapeutic approaches and relevance to
similar human diseases Annals of the Rheumatic Diseases. 1993; 52:
S48-S54. https://doi.org/10.1136/ard.52.Suppl_1.S48 PMid:8481059 PMCid:PMC1035026

- Otani M, Kuroki A, Kikuchi S, Kihara M,
Nakata J, Ito K, Furukawa J, Shinohara Y, Izui S. Sialylation
determines the nephritogenicity of IgG3 cryoglobulins. J Am Soc
Nephrol. 2012 Nov;23(11):1869-78. https://doi.org/10.1681/ASN.2012050477 PMid:23024299 PMCid:PMC3482736

- Gulli F, Basile U, Gragnani L, Fognani
E, Napodano C, Colacicco L, Miele L, De Matthaeis N, Cattani P, Zignego
AL, Rapaccini GL. Autoimmunity and lymphoproliferation markers in naïve
HCV-RNA positive patients without clinical evidences of
autoimmune/lymphoproliferative disorders. Dig Liver Dis. 2016
Aug;48(8):927-33. https://doi.org/10.1016/j.dld.2016.05.013 PMid:27289333

- Müller
RB, Vogt B, Winkler S, Mu-oz LE, Franz S, Kern P, Maihöfner C, Sheriff
A, von Kempis J, Schett G, Herrmann M. Detection of low level
cryoglobulins by flow cytometry. Cytometry A. 2012 Oct;81(10):883-7. https://doi.org/10.1002/cyto.a.22112 PMid:22961692

- Romitelli
F, Pucillo LP, Basile U and Di Stasio E. Comparison between the
Traditional and a Rapid Screening Test for Cryoimmunoglobulins
Detection. Biomed Res Int. 2015; 2015: 783063. https://doi.org/10.1155/2015/783063 PMid:25692146 PMCid:PMC4321088

- Shihabi ZK. Cryoglobulins: an
important but neglected clinical test. Ann Clin Lab Sci. 2006
Autumn;36(4):395-408. PMid:17127726

- Basile
U, Torti E, Dell'Abate MT, Colacicco L, Gulli F, Zuppi C, Rapaccini GL.
Pre-analytical phase in cryoglobulin (CRG) detection: an alternative
method for sample transport. Clin Chem Lab Med. 2016 Apr;54(4):e123-6. https://doi.org/10.1515/cclm-2015-0404 PMid:26587742

- Bakker
AJ, Slomp J, de Vries T, Boymans DA, Veldhuis B, Halma K, Joosten P.
Adequate sampling in cyioglobulinaemia: recommended warmly. Clin Chem
Lab Med 2003;41:85-9. https://doi.org/10.1515/CCLM.2003.015 PMid:12636055

- Kallemuchikkal U, Gorevic PD. Evaluation of cryoglobulins. Arch Pathol Lab Med 1999;123:119-25. PMid:10050784

- Dammacco F, Sansonno D, Piccoli C, Tucci FA, Racanelli V. The cryoglobulins: an overview. Eur J Clin Invest 2001;31:628-38. https://doi.org/10.1046/j.1365-2362.2001.00824.x PMid:11454019

- Fornasieri
A, Armelloni S, Bernasconi P, Li M, de Septis CP, Sinico RA, D'Amico G.
High binding of immunoglobulin M kappa rheumatoid factor from type II
cryoglobulins to cellular fibronectin: a mechanism for induction of in
situ immune complex glomerulonephritis?. Am J Kidney Dis
1996;27:476-83. https://doi.org/10.1016/S0272-6386(96)90156-0

- Andre M, Mahammedi H, Aumaitre O,
Tridon A, Tissot JD, Piette JC. A "missed" cryoglobulin: the importance
of in vitro calcium concentration. Ann Rheum Dis 2000;59:490. https://doi.org/10.1136/ard.59.6.490a PMid:10885976 PMCid:PMC1753150

- Tate J, Caldwell G, Daly J, Gillis D,
Jenkins M, Jovanovich S, Martin H, Steele R, Wienholt L, Mollee P;
Working Party on Standardised Reporting of Protein Electrophoresis.
Recommendations for standardized reporting of protein electrophoresis
in Australia and New Zealand. Ann Clin Biochem 2012;49:242–256. https://doi.org/10.1258/acb.2011.011158 PMid:22402916

- Shihabi
ZK. Analysis and general classification of serum cryoglobulins by
capillary zone electrophoresis. Electrophoresis 1996;17:1607-12. https://doi.org/10.1002/elps.1150171020 PMid:8957190

- Guerra
J, Garenne M, Mohamed MK, Fontanet A. HCV burden of infection in Egypt:
results from a nationwide survey. J Viral Hepat. 2012 Aug;19(8):560-7.
doi:10.1111/j.1365-2893.2011.01576.x. PubMed PMID: 22762140. https://doi.org/10.1111/j.1365-2893.2011.01576.x

- Daw
MA, El-Bouzedi A, Ahmed MO, Dau AA, Agnan MM; In association with the
Libyan Study Group of Hepatitis & HIV.. Epidemiology of hepatitis C
virus and genotype distribution in immigrants crossing to Europe from
North and sub-Saharan Africa. Travel Med Infect Dis.
2016;14(5):517-526. doi:10.1016/j.tmaid.2016.05.020. https://doi.org/10.1016/j.tmaid.2016.05.020

- AASLD/IDSA/IAS-USA.
Recommendations for testing, managing, and treating hepatitis C. 2014;
Retrieved April 24, 2014.

- Lagging M, Wejstål R, Norkrans G,
Karlström O, Aleman S, Weiland O, Castedal M, Josephson F; Swedish
Consensus Group.. Treatment of hepatitis C virus infection for adults
and children: Updated Swedish consensus recommendations. Infect Dis
(Lond) , 2016; 48, (4) 251–261. https://doi.org/10.3109/23744235.2015.1113438

- Basile U, Gragnani L, Piluso A, et al.
Assessment of free light chains in HCV-positive patients with mixed
cryoglobulinaemia vasculitis undergoing rituximab treatment. Liver
International 2015 https://doi.org/10.1111/liv.12829 PMid:25800731

- Eble
V, Legallicier B, Joly P, Vittecoq O, Caron F, Tamion F, Ducrotte P,
Levesque H, Menard JF, Jouen F, Guerrot D, Marie I. Long term outcome
of patients with low level of cryoglobulin (<0.05 g/L). Autoimmun
Rev 15 (2016) 440–446. https://doi.org/10.1016/j.autrev.2016.01.012 PMid:26827906

[TOP]










 .
. 




















































