Sumit Dahal1, Smrity Upadhyay2, Rashmi Banjade3, Prajwal Dhakal4, Nabin Khanal2 and Vijaya Raj Bhatt5
1 Interfaith Hospital, Department of Medicine, New York, USA
2 Creighton University Medical Center, Department of Internal Medicine, Omaha, Nebraska, USA
3 Montefiore New Rochelle Hospital, Department of Medicine, New York, USA
4 Michigan State University, Department of Medicine, East Lansing, Michigan, USA
5 University of Nebraska Medical Center, Department of Internal Medicine, Division of Hematology-Oncology, Omaha, Nebraska, USA
Corresponding
author: Prajwal Dhakal, MBBS. Department of Medicine, Michigan State
University. East Lansing, MI, 48824 Tel:. (W): 517-353-5100. E-mail:
prazwal@gmail.com
Published: March 1, 2017
Received: November 21, 2016
Accepted: February 7, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017019 DOI
10.4084/MJHID.2017.019
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Thrombocytopenia
in patients with chronic hepatitis C virus (HCV) infection is a major
problem. The pathophysiology is multifactorial, with
auto-immunogenicity, direct bone marrow suppression, hypersplenism,
decreased production of thrombopoietin and therapeutic adverse effect
all contributing to thrombocytopenia in different measures. The
greatest challenge in the care of chronic HCV patients with
thrombocytopenia is the difficulty in initiating or maintaining IFN
containing anti-viral therapy. Although at present, it is possible to
avoid this challenge with the use of the sole Direct Antiviral Agents
(DAAs) as the primary treatment modality, thrombocytopenia remains of
particular interest, especially in cases of advanced liver disease.
The
increased risk of bleeding with thrombocytopenia may also impede the
initiation and maintenance of different invasive diagnostic and
therapeutic procedures. While eradication of HCV infection itself is
the most practical strategy for the remission of thrombocytopenia,
various pharmacological and non-pharmacological therapeutic options,
which vary in their effectiveness and adverse effect profiles, are
available. Sustained increase in platelet count is seen with
splenectomy and splenic artery embolization, in contrast to only
transient rise with platelet transfusion. However, their routine use is
limited by complications. Different thrombopoietin analogues have been
tried. The use of synthetic thrombopoietins, such as recombinant human
TPO and pegylated recombinant human megakaryocyte growth and
development factor (PEG-rHuMDGF), has been hampered by the development
of neutralizing antibodies. Thrombopoietin-mimetic agents, in
particular, eltrombopag and romiplostim, have been shown to be safe and
effective for HCV-related thrombocytopenia in various studies, and they
increase platelet count without eliciting any immunogenicity Other
treatment modalities including newer TPO analogues- AMG-51, PEG-TPOmp
and AKR-501, recombinant human IL-11 (rhIL-11, Oprelvekin), recombinant
human erythropoietin (rhEPO), danazol and L-carnitine have shown
promising early result with improving thrombocytopenia.
Thrombocytopenia in chronic HCV infection remain a major problem,
however the recent change in DAAs without IFN, as the frontline
therapy for HCV, permit to avoid the dilemmas associated with
initiating or maintaining IFN based anti-viral therapy.
|
Introduction
Chronic hepatitis C virus (HCV) infection affects 3% of the world’s population and 1.3% of the United States’ population.[1,2]
It is a leading cause of chronic liver disease, cirrhosis, and
hepatocellular carcinoma, and is one of the most common causes of liver
transplants in the United States.[2] Besides hepatic
complications, chronic HCV infection is also associated with several
extra-hepatic manifestations including thrombocytopenia.
Thrombocytopenia in chronic HCV infection is a major problem,
particularly in patients with advanced liver disease. The risk of
serious bleeding with severe thrombocytopenia can prevent invasive
procedures including biopsies for staging.[3]
Thrombocytopenia can also complicate bleeding manifestations such as
variceal bleeding. It may impede the initiation and continuation of
antiviral therapy, potentially decreasing the probability of successful
HCV treatment.[4] Recent studies have evaluated the
underlying mechanism of thrombocytopenia in chronic HCV infection and
assessed the usefulness of several therapeutic options. Epidemiology
The
prevalence and degree of thrombocytopenia increase with the severity of
liver disease and correlates to hepatocellular damage and hepatic
fibrosis. [5] However, use of varying definition for
thrombocytopenia and insufficient data on study characteristics such as
age, gender, HCV treatment rates and disease severity preclude a more
accurate estimate of the overall prevalence.[6] A systematic review estimated the average prevalence of thrombocytopenia in chronic HCV infection to be nearly 24% (Table 1).[6]
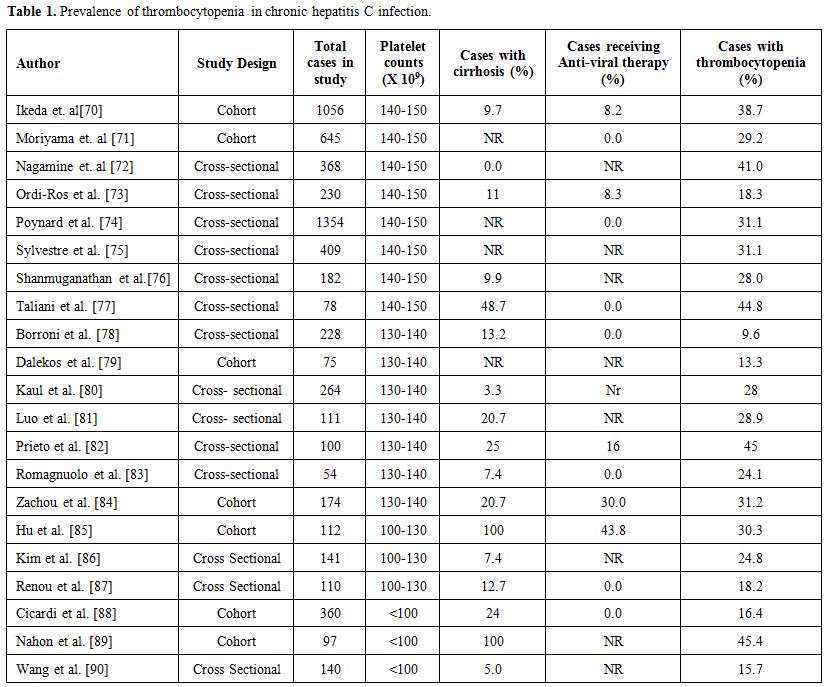 |
Table 1. Prevalence of thrombocytopenia in chronic hepatitis C infection. |
Mechanism
The
pathophysiology of thrombocytopenia in patients with HCV infection is
thought to be multifactorial. Besides inducing an autoimmune reaction
with production of anti-platelet antibodies, the virus also causes
direct bone marrow suppression with resulting thrombocytopenia.[7-10]
Chronic HCV infection induced liver fibrosis and cirrhosis leads to
portal hypertension with subsequent hypersplenism and sequestration of
platelets, decreased the production of thrombopoeitin, and endothelial
dysfunction, all of which can contribute to thrombocytopenia.[11-14]
Although uncommonly used in developed countries, interferon (IFN) and
ribavirin used as part of anti-HCV therapy can also contribute to low
platelet count.[15]
Impact on Clinical Management
Although
thrombocytopenia in chronic HCV infection is typically low grade and
not life-threatening, it represents an obstacle to different diagnostic
or therapeutic modalities and may preclude the use of anti-viral
treatment.
The greatest challenge in the care of chronic HCV
patients with thrombocytopenia is the difficulty in initiating or
maintaining IFN containing anti-viral therapy. Although this challenge
can be avoided with the use of sole DAAs as the primary treatment
modality, thrombocytopenia remains of particular interest, especially
in cases of advanced liver disease. In a study by Wang et al., baseline
thrombocytopenia increased the risk of drug cessation. Patients with
baseline thrombocytopenia actually exhibited compromised sustained
virologic response (SVR) rates while those with acquired
thrombocytopenia did not. Thus, use of growth factors to maintain SVR
rate would be beneficial in those with baseline thrombocytopenia rather
than in those who acquire it during therapy as dose reduction doesn’t
decrease SVR in such cases.[16]
Thrombocytopenia in HCV may also be a problem for patients with baseline platelet count of <50.000/mm3,
particularly in the presence of previous bleeding even when they are
treated with DAAs. However, patients with thrombocytopenia and fibrosis
have attained >90% SVR with DAAs even if in a proportion lower in
respect to patients with a normal platelet count. Thus, DAAs may be
continued most of the times without interruption and thrombopoietin
mimetics would be helpful only with severe thrombocytopenia (such as a
platelet count of <25,000/mm3).[17-19]
Directly-acting antivirals (DAAs):
Recently updated World Health Organization guidelines recommend that
DAA regimens (including simeprivir, grazoprevir, daclatasvir,
ledipasvir, and sofosbuvir) be used for the treatment of persons with
hepatitis C infection rather than regimens with pegylated interferon
and ribavirin.[16] Combinations of 2 or 3 DAAs have
been shown to be highly effective and safe in both cirrhotic and
non-cirrhotic patients in different phase III clinical trials and large
real life cohorts with providing SVR rates of >95%. While headache,
diarrhea, fatigue, and nausea have frequently been observed,
hematologic abnormalities including thrombocytopenia were reported in
no more than 1% of cases.[17,18] Lee et al. reported
that DAA therapy in one patient precipitated ITP refractory to various
treatment modalities and it required several weeks of therapy with
multiple platelet transfusions, intravenous immunoglobulin, steroids
and romiplostim to achieve a stable platelet count of 40,000/mm3 with no signs of bleeding.[19]
However, this is only one case describing any relation of DAA with
thrombocytopenia. A study by Forns et al. showed that HCV genotype
1a-infected patients with surrogate markers of portal hypertension or
impaired liver function such as thrombocytopenia and hypoalbuminemia at
baseline achieved high SVR rates with ombitasvir/paritaprevir/ritonavir
and dasabuvir with ribavirin and treatment was well tolerated.[20]
Additionally, reduction in liver fibrosis markers such as fibrosis-4
score and aspartate transaminase platelet ratio along with regression
of transient elastography have been reported with use of DAAs in
chronic hepatitis C.[21] In any case, by the time,
thrombocytopenia improves following SVR obtained with any antiviral
therapy among chronic HCV infected patients with
advanced hepatic fibrosis.[21,22]
INF based antiviral therapy:
Although IFN based antiviral therapy is uncommonly used in developed
countries nowadays, the prohibitive cost of DAA may require the use of
INF based therapy along with the addition of thrombopoietin mimetics,
if required, in economically disadvantaged areas. Additionally,
in chronic hepatitis C cases treated with pegylated INF plus ribavirin,
single nucleotide polymorphisms at or near the IL-28B gene have been
shown to be a predictor of SVR.[23,24] The American
Gastroenterological Association recommends dose reduction of INF with a
platelet count between 25,000-50,000 and withdrawal of INF-based
treatment with a count below 25,000.[25] This is important because the antiviral therapy itself may cause a further drop in platelet count.[26]
Studies have shown IFN-based therapy to cause severe thrombocytopenia
in up to 13% of patients, with the incidence higher in patients with
lower baseline platelet count.[27,28] The
modifications in IFN-based therapy have potential to lower the chances
of attaining SVR. The increased risk of bleeding may also impede the
initiation and maintenance of different invasive diagnostic and
therapeutic procedures such as liver biopsy, variceal banding,
paracentesis and thoracentesis, central line insertion, endoscopy and
elective surgery.
Management
Various
pharmacological and non-pharmacological therapeutic options are
available for the management of thrombocytopenia in chronic HCV
infection (Table 2). These
treatment modalities vary in their effectiveness and adverse effect
profiles. The most practical strategy in treating HCV-related
thrombocytopenia is based on the principle that eradication of HCV
infection may result in remission of thrombocytopenia. By
eradicating HC virus, DAAs are supposed to improve
thrombocytopenia related to hepatitis C infection but may not
ameliorate thrombocytopenia related to cirrhosis or portal
hypertension. In cases of IFN based antiviral therapy, the usual
approach is to continue with the therapy, reducing the dose if platelet
count drops below 50,000 cells/μL or discontinuing it for a platelet
count of below 25,000 cells/μL.[25] The measures
described below are mostly supportive. As expected, there is a lot of
published data on how these measures might be necessary to IFN-based
therapy but not to them with DAAs.
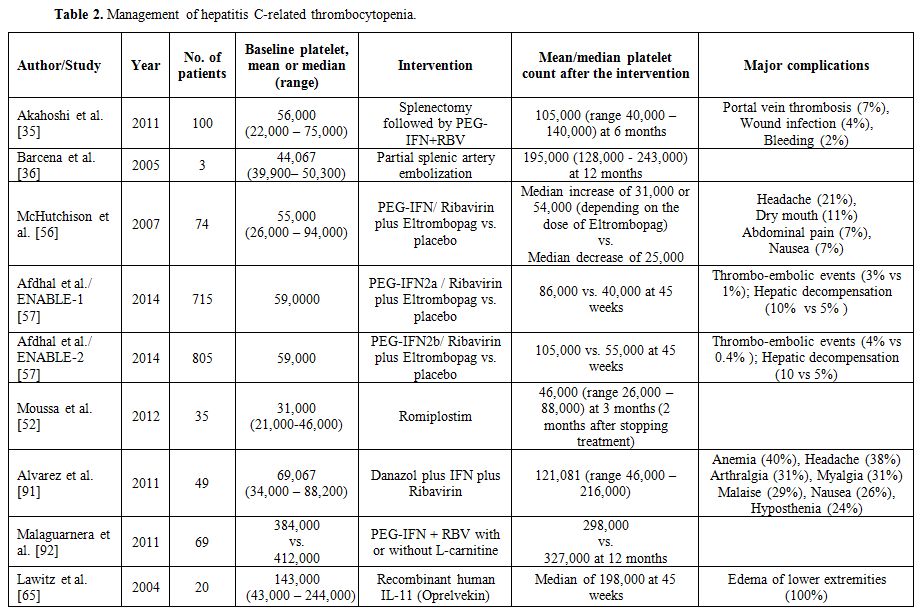 |
Table 2. Management of hepatitis C-related thrombocytopenia |
Platelet transfusion:
Though widely used for the management of thrombocytopenia, platelet
transfusion has several limitations, especially in patients with
chronic liver disease. The increase in platelet count is transient, and
hence useful only for procedures or during bleeding. Patients are also
at risk for transfusion-related complications, which can occur in up to
30% of the recipients and include viral or bacterial infection, febrile
non-hemolytic reactions, and iron overload.[29]
Nearly half of all patients undergoing multiple platelet transfusions
can develop platelet refractoriness secondary to human leukocyte
antigen (HLA) alloimmunization.[30,31] It may not always ensure maintenance of homeostatic platelet levels.[32] Besides, the requirement of hospitalization and high cost may be prohibitive in a resource-poor setting.
Splenectomy and splenic artery embolization:
Splenectomy and splenic artery embolization have been used to correct
thrombocytopenia in patients with hypersplenism, producing significant
and persistent increases in platelet count.[33,34]
Akahoshi et al. studied the effect of splenectomy in patients with
HCV-associated thrombocytopenia and found above 200% rise in mean
platelet count at 1 month after splenectomy.[35] In
cases of IFN-based antiviral therapy, the positive effect is known to
persist even after the initiation of antiviral therapy, with the mean
platelet count nearly 80% above baseline after 12 months of the
therapy. Splenectomy, however, is an invasive procedure with high risk
of bleeding, sepsis and portal vein thrombosis. Asplenic patients are
susceptible to overwhelming post-splenectomy infection. Splenic artery
embolization may be an alternative option. In a study by Barcena et
al., the mean platelet count increased by 342% from the baseline after
12 weeks of partial splenic artery embolization.[36]
Splenic artery embolization, though associated with lower morbidity and
mortality than splenectomy, is not free of complications.
Pharmacotherapy: Steroids:
With HCV reported to play a pathogenic role in some cases of immune
thrombocytopenic purpura, there have been case reports of significant
improvement in HCV-related thrombocytopenia with the use of
corticosteroid.[37] As described earlier, Lee et al.
described a case of resistant ITP which developed after DAA therapy and
did not respond to high dose prednisone.[20] Lebano
et al. reported a case where the platelet count increased by 175% from
baseline six months after steroid therapy and improved further (360%
above baseline) after another six months of IFN and ribavirin.[37]
Despite similar reports of steroids causing a variable rise in platelet
counts, they are not routinely considered in the management of
thrombocytopenia in HCV infection because of the possible risk of
worsening viral loads and liver damage.[38,39]
Thrombopoietin analogue:
Thrombopoietin (TPO) is a cytokine predominantly synthesized by the
hepatocytes in the liver and plays a central role in thrombopoiesis. It
binds to TPO receptors (mpl) expressed on the surface of megakaryocyte
precursor cells and megakaryocytes, activating signal transduction
cascades that result in proliferation and maturation of megakaryocytes.[40]
A better understanding of TPO and its role in platelet production and
function has led to newer treatment modalities. Synthetic
thrombopoietins such as recombinant human TPO and pegylated recombinant
human megakaryocyte growth and development factor (PEG-rHuMDGF) cause
an increase in platelet count.[41,42] However, their
use has been hampered by the appearance of neutralizing antibodies that
cross-reacts with both recombinant and endogenous TPO.[43]
In a study using PEG-rHuMDGF injection by Li et al., an initial rise in
platelet count was followed by the development of an antibody against
TPO, detected as early as 56 days after the initial injection.[44]
This was associated with corresponding fall in platelet count and a
marked decrease in bone marrow megakaryocytes, with an average nadir
platelet count of 6% to 8% of baseline.
Thrombopoietin-mimetic
agents, in particular, eltrombopag and romiplostim, have been shown to
increase platelet count without eliciting any immunogenicity.[45-47]
Romiplostin is a peptibody composed of four TPO mimetic peptides
attached by glycine bridges to the heavy chain portion of
immunoglobulin G. It acts by dimerizing the TPO receptor via its paired
peptides, which stimulates platelet production.[48]
It is given by weekly subcutaneous injections. Various clinical trials
in patients with chronic immune thrombocytopenic purpura have shown
romiplostin to cause a dose dependent increase in platelet count,
resulting in lower rates of treatment failure, decreased the need for
splenectomy and improved quality of life.[49-51] Lee et al. described romiplostim use in a case of resistant ITP after DAA therapy.[20]
A study by Moussa et al. in 35 patients with chronic liver disease and
thrombocytopenia secondary to HCV infection showed more than three-fold
increase in mean platelet count from the baseline after 3 weeks of
therapy.[52] And the mean platelet count remained 1.5
times above the baseline even after 2 months of stopping the drug.
Similarly, Voican et al. reported two cases where romiplostin was used
to control severe thrombocytopenia; this allowed anti-HCV treatment
with pegylated-IFN and ribavirin to be completed successfully without
any dose reduction or discontinuation.[53]
Eltrombopag,
an orally active TPO agonist, interacts with the trans-membrane domain
of the thrombopoietin receptor, activating JAK2/STAT signaling pathways
and increasing proliferation and differentiation of human bone marrow
progenitor cells into megakaryocytes.[54] Preclinical
studies have shown the binding site on the receptor and the signal
transduction mechanism to be different for eltrombopag as compared to
thrombopoeitin, causing the two to have an additive effect on platelet
production.[55] Eltrombopag has been found to be safe and effective in the management of HCV-related thrombocytopenia.[56,57] In a phase II trial,[56]
71-91% of the patients receiving eltrombopag had a dose dependent
increase in their platelet counts to levels which allowed initiation of
antiviral therapy. 36-65% of patients in the eltrombopag group
completed first 12 weeks of antiviral therapy compared to 6% in the
placebo group. Though platelet counts decreased during the antiviral
treatment phase despite the use of eltrombopag, the count consistently
remained above baseline as well as above the level at which a reduction
in the pegylated-IFN dose is recommended (<50,000 per cubic
millimeter). Another phase III trial, Eltrombopag to Initiate and
Maintain Interferon Antiviral Treatment to Benefit Subjects with
Hepatitis C-Related Liver Disease (ENABLE-1 and ENABLE-2), showed a
higher rate of sustained virological response with the use of
eltrombopag than placebo (23% vs. 14%, p=0.0064 in ENABLE-1 and 19% vs.
13%, p=0.0202 in ENABLE-2).[57] Pegylated-IFN was
administered at higher doses, with fewer dose reductions in the
eltrombopag group. Throughout the antiviral treatment, a platelet
count of 50,0000 per cubic millimeter or higher was maintained in more
patients receiving eltrombopag than placebo (69% vs. 15% in ENABLE-1
and 81% vs. 23% in ENABLE-2).
The most common side effect with
these thrombopoietin-mimetic agents is a headache, with the reported
incidence in clinical trials ranging from 7% to 21%.[49-51,56,57]
Eltrombopag also commonly causes dry mouth, abdominal pain, and nausea,
and may be associated with hepatic decompensation like ascites and
hepatic encephalopathy.[56,57] Romiplostin may be associated with increased deposition of reticulin in the bone marrow, and possibly marrow fibrosis.[58] The risk of thromboembolic events like portal vein thrombosis is seen with all these agents.[57,58]
Other
newer drugs currently under investigation include the peptidic
compounds like AMG-531 and PEG-TPOmp, non-peptidic compound like
AKR-501, and monoclonal antibodies. AMG-531, a TPO agonist, has been
designed with no sequence homology to human TPO to reduce the
likelihood of an anti-TPO immune response. Phase II and III studies in
ITP patients have shown promising early results with a dose-dependent
increase in platelet count with no serious adverse events.[59,60]
PEG-TPOmp is a pegylated TPO peptide agonist and has shown to be
effective in animal studies. Similarly, AKR-501 is an orally active TPO
agonist and has been shown to be effective in clinical studies
involving healthy volunteers.[60] In vitro studies have shown engineered monoclonal antibodies to bind mpl and activate TPO-expressing cell lines.[61]
However, all these compounds and drugs need further clinical studies,
including in patients with HCV and chronic liver disease before they
can be considered for routine use.
Cytokines with thrombopoietic potential:
Cytokine such as interleukin-11 (IL-11) has thrombopoietic activity.
Recombinant human IL-11 (rhIL-11, Oprelvekin), approved for the
management of chemotherapy-related thrombocytopenia, has also been
shown to increase platelet count in chronic HCV infection.[62-64]
In a study by Lawitz et al., use of rhIL-11 (Oprelvekin) in patients
with advanced liver disease associated with chronic HCV infection
caused a 38% increase in mean platelet count from baseline after 12
weeks of therapy, along with an improvement in the mean Knodell
Histology Activity Index from 7.3 to 5.9 (p= 0.006).[65] However, the platelet level tends to fall back on discontinuing the drug.[62]
It also causes fluid retention in most patients, and this can be a
significant management problem in patients with decompensated
cirrhosis.[64]
Erythropoietin:
The amino-terminal domain on TPO, which binds to thrombopoietin
receptor shares significant homology with erythropoietin. Recombinant
human erythropoietin (rhEPO) has shown promising results in improving
thrombocytopenia in cirrhotic patients.[66,67] Pirisi
et al. studied the effect of rhEPO on the platelet count in 19 patients
with thrombocytopenia related to chronic liver disease, and found an
increase in mean platelet count by 45% from the baseline in the
treatment group as compared to 0% in the placebo group (p < 0.02).[67]
As rhEPO has also been suggested for the treatment of ribavirin-induced
anemia in patients with HCV, this provides the possibility of using a
single drug for the treatment of both thrombocytopenia and anemia
related to the INF-based antiviral therapy. However, further studies
are needed to confirm this.
Danazol:
Danazole used in immune thrombocytopenic purpura may have a role in
HCV-related thrombocytopenia. In a study by Alvarez et al., the use of
danazol along with the anti-HCV treatment resulted in a 75% increase in
the mean platelet count from the baseline and allowed 90% of the
patients to complete their antiviral treatment.[68]
Anemia, headache, arthralgia and myalgia were some of the common
adverse effects of the combination therapy reported in the study.
L-carnitine:
L-carnitine is a nutrient synthesized from amino acids lysine and
methionine. In a study, the addition of L-carnitine to
pegylated-IFN-α plus ribavirin resulted in a decrease in the incidence
of thrombocytopenia during antiviral therapy.[69]
Conclusions
Thrombocytopenia
in chronic HCV infection has a multifactorial pathophysiology and
remains a major problem. The recent change in DAAs without IFN,
as the frontline therapy for HCV, permit to avoid the dilemmas
associated with initiating or maintaining IFN based anti-viral therapy.
DAAs,
with high SVR and less than 1% of hematological adverse effects, have
been shown to improve thrombocytopenia associated with HCV infection as
well as advanced hepatic disease. While eradication of HCV infection
itself is the most practical strategy for the remission of
thrombocytopenia, various pharmacological and non-pharmacological
therapeutic options, which vary in their effectiveness and adverse
effect profiles, are available. Thrombopoietin-mimetic agents like
eltrombopag and romiplostim have been shown to be safe and effective
for HCV-related thrombocytopenia in various studies.
Studies of the long-term effects of DAA on extrahepatic consequences of HCV infection are in progress..
Acknowledgement
Vijaya
Bhatt is supported by the 2016-2017 Physician-Scientist Training
Program Grant from the College of Medicine, University of Nebraska
Medical Center.
References
- Armstrong GL, Wasley A, Simard EP, McQuillan GM,
Kuhnert WL, Alter MJ: The prevalence of hepatitis C virus infection in
the United States, 1999 through 2002. Ann Intern Med 2006;144(10):
705-714. https://doi.org/10.7326/0003-4819-144-10-200605160-00004 PMid:16702586

- Verna
EC, Brown Jr RS: Hepatitis C and liver transplantation: enhancing
outcomes and should patients be retransplanted. Clinics in liver
disease 2008;12(3): 637-659. https://doi.org/10.1016/j.cld.2008.03.010 PMid:18625432

- Seeff
LB, Everson GT, Morgan TR, Curto TM, Lee WM, Ghany MG, Shiffman ML,
Fontana RJ, Di Bisceglie AM, Bonkovsky HL: Complication rate of
percutaneous liver biopsies among persons with advanced chronic liver
disease in the HALT-C trial. Clinical Gastroenterology and Hepatology
2010;8(10): 877-883. https://doi.org/10.1016/j.cgh.2010.03.025 PMid:20362695 PMCid:PMC3771318

- Karasu
Z, Tekin F, Ersoz G, Gunsar F, Batur Y, Ilter T, Akarca US: Liver
fibrosis is associated with decreased peripheral platelet count in
patients with chronic hepatitis B and C. Digestive diseases and
sciences 2007;52(6): 1535-1539. https://doi.org/10.1007/s10620-006-9144-y PMid:17464564

- Wang
C-S, Yao W-J, Wang S-T, Chang T-T, Chou P: Strong association of
hepatitis C virus (HCV) infection and thrombocytopenia: implications
from a survey of a community with hyperendemic HCV infection. Clinical
infectious diseases 2004;39(6): 790-796. https://doi.org/10.1086/423384 PMid:15472809

- Louie
KS, Micallef JM, Pimenta JM, Forssen UM: Prevalence of thrombocytopenia
among patients with chronic hepatitis C: a systematic review. J Viral
Hepat 2011;18(1): 1-7. https://doi.org/10.1111/j.1365-2893.2010.01366.x PMid:20796208

- Aref
S, Sleem T, El Menshawy N, Ebrahiem L, Abdella D, Fouda M, Abou Samara
N, Menessy A, Abdel-Ghaffar H, Bassam A: Antiplatelet antibodies
contribute to thrombocytopenia associated with chronic hepatitis C
virus infection. Hematology 2009;14(5): 277-281. https://doi.org/10.1179/102453309X439818 PMid:19843383

- Linares
M, Pastor E, Hernandez F, Montagud M, Blanquer A: Autoimmune
thrombocytopenia and hepatitis C virus infection. American journal of
hematology 1996;53(4): 284-284. https://doi.org/10.1002/(SICI)1096-8652(199612)53:4<284::AID-AJH20>3.0.CO;2-B

- Bordin
G, Ballare M, Zigrossi P, Bertoncelli MC, Paccagnino L, Baroli A,
Brambilla M, Monteverde A, Inglese E: A laboratory and thrombokinetic
study of HCV-associated thrombocytopenia: a direct role of HCV in bone
marrow exhaustion? Clin Exp Rheumatol 1995;13 Suppl 13: S39-43.
PMid:8730475

- Weksler
BB: Review article: the pathophysiology of thrombocytopenia in
hepatitis C virus infection and chronic liver disease. Aliment
Pharmacol Ther 2007;26 Suppl 1: 13-19. https://doi.org/10.1111/j.1365-2036.2007.03512.x PMid:17958515

- Kedia
S, Goyal R, Mangla V, Kumar A, S S, Das P, Pal S, Sahni P, Acharya SK:
Splenectomy in cirrhosis with hypersplenism: improvement in cytopenias,
Child's status and institution of specific treatment for hepatitis C
with success. Ann Hepatol 2012;11(6): 921-929. PMid:23109457

- Adinolfi
LE, Giordano MG, Andreana A, Tripodi MF, Utili R, Cesaro G, Ragone E,
Mangoni ED, Ruggiero G: Hepatic fibrosis plays a central role in the
pathogenesis of thrombocytopenia in patients with chronic viral
hepatitis. British journal of haematology 2001;113(3): 590-595. https://doi.org/10.1046/j.1365-2141.2001.02824.x

- Giannini
E, Borro P, Botta F, Fumagalli A, Malfatti F, Podestà E, Romagnoli P,
Testa E, Chiarbonello B, Polegato S: Serum thrombopoietin levels are
linked to liver function in untreated patients with hepatitis C
virus-related chronic hepatitis. Journal of hepatology 2002;37(5):
572-577. https://doi.org/10.1016/S0168-8278(02)00274-X

- Osada
M, Kaneko M, Sakamoto M, Endoh M, Takigawa K, Suzuki-Inoue K, Inoue O,
Satoh K, Enomoto N, Yatomi Y: Causes of thrombocytopenia in chronic
hepatitis C viral infection. Clinical and Applied Thrombosis/Hemostasis
2012;18(3): 272-280. https://doi.org/10.1177/1076029611429124 PMid:22327815

- Sulkowski MS: Management of the hematologic complications of hepatitis C therapy. Clinics in liver disease 2005;9(4): 601-616. https://doi.org/10.1016/j.cld.2005.07.007 PMid:16207566

- Wang
H, Innes H, Hutchinson SJ, Goldberg DJ, Allen S, Barclay ST, Bramley P,
Fox R, Fraser A, Hayes PC, Kennedy N, Mills PR, Dillon JF: The
prevalence and impact of thrombocytopenia, anaemia and leucopenia on
sustained virological response in patients receiving hepatitis C
therapy: evidence from a large 'real world' cohort. Eur J Gastroenterol
Hepatol 2016;28(4): 398-404. PMid:26695428

- Feld
JJ, Jacobson IM, Hezode C, Asselah T, Ruane PJ, Gruener N, Abergel A,
Mangia A, Lai CL, Chan HL, Mazzotta F, Moreno C, Yoshida E, Shafran SD,
Towner WJ, Tran TT, McNally J, Osinusi A, Svarovskaia E, Zhu Y,
Brainard DM, McHutchison JG, Agarwal K, Zeuzem S: Sofosbuvir and
Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med
2015;373(27): 2599-2607. https://doi.org/10.1056/NEJMoa1512610 PMid:26571066

- Foster
GR, Afdhal N, Roberts SK, Brau N, Gane EJ, Pianko S, Lawitz E, Thompson
A, Shiffman ML, Cooper C, Towner WJ, Conway B, Ruane P, Bourliere M,
Asselah T, Berg T, Zeuzem S, Rosenberg W, Agarwal K, Stedman CA, Mo H,
Dvory-Sobol H, Han L, Wang J, McNally J, Osinusi A, Brainard DM,
McHutchison JG, Mazzotta F, Tran TT, Gordon SC, Patel K, Reau N, Mangia
A, Sulkowski M: Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3
Infection. N Engl J Med 2015;373(27): 2608-2617. https://doi.org/10.1056/NEJMoa1512612 PMid:26575258

- Lee
LM, Johansen ME, Jy W, Horstman LL, Ahn Y-S: Second Generation
Direct-Acting Antiviral Agents Eradicate Hepatitis C Virus (HCV) but
Exacerbate Thrombocytopenia in a Patient with HCV-Associated Immune
Thrombocytopenic Purpura (ITP): Case Report. Blood 2014;124(21): 5022.

- Forns
X, Poordad F, Pedrosa M, Berenguer M, Wedemeyer H, Ferenci P, Shiffman
ML, Fried MW, Lovell S, Trinh R, Lopez-Talavera JC, Everson G:
Ombitasvir/paritaprevir/r, dasabuvir and ribavirin for cirrhotic HCV
patients with thrombocytopenia and hypoalbuminaemia. Liver
International 2015;35(11): 2358-2362. https://doi.org/10.1111/liv.12931

- Bachofner
JA, Valli PV, Kröger A, Bergamin I, Künzler P, Baserga A, Braun D,
Seifert B, Moncsek A, Fehr J, Semela D, Magenta L, Müllhaupt B,
Terziroli Beretta-Piccoli B, Mertens JC: Direct antiviral agent
treatment of chronic hepatitis C results in rapid regression of
transient elastography and fibrosis markers fibrosis-4 score and
aspartate aminotransferase-platelet ratio index. Liver International:
2016 Sep 28. doi: 10.1111/liv.13256. [Epub ahead of print] https://doi.org/10.1111/liv.13256

- van
der Meer AJ, Maan R, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert
F, Duarte-Rojo A, Manns MP, Zeuzem S, Hofmann WP, de Knegt RJ, Hansen
BE, Janssen HL: Improvement of platelets after SVR among patients with
chronic HCV infection and advanced hepatic fibrosis. J Gastroenterol
Hepatol 2016;31(6): 1168-1176. https://doi.org/10.1111/jgh.13252 PMid:26647353

- Afzal
MS: Predictive potential of IL-28B genetic testing for interferon based
hepatitis C virus therapy in Pakistan: Current scenario and future
perspective. World J Hepatol 2016;8(26): 1116-1118. https://doi.org/10.4254/wjh.v8.i26.1116 PMid:27660680 PMCid:PMC5026995

- Gonzalez
SA, Keeffe EB: IL-28B As a Predictor of Sustained Virologic Response in
Patients with Chronic Hepatitis C Virus Infection. Gastroenterology
& Hepatology 2011;7(6): 366-373
 .
.
- Dienstag
JL, McHutchison JG: American Gastroenterological Association technical
review on the management of hepatitis C. Gastroenterology 2006;130(1):
231-264. https://doi.org/10.1053/j.gastro.2005.11.010 PMid:16401486

- McHutchison
JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, Dienstag J, Lee
WM, Mak C, Garaud JJ: Adherence to combination therapy enhances
sustained response in genotype-1–infected patients with chronic
hepatitis C. Gastroenterology 2002;123(4): 1061-1069. https://doi.org/10.1053/gast.2002.35950 PMid:12360468

- Hermos
JA, Quach L, Gagnon DR, Weber HC, Altincatal A, Cho K, Lawler EV,
Grotzinger KM: Incident severe thrombocytopenia in veterans treated
with pegylated interferon plus ribavirin for chronic hepatitis C
infection. Pharmacoepidemiol Drug Saf 2014;23(5): 480-488. https://doi.org/10.1002/pds.3585 PMid:24677630

- Lin
KH, Hsu PI, Yu HC, Lin CK, Tsai WL, Chen WC, Chan HH, Lai KH: Factors
linked to severe thrombocytopenia during antiviral therapy in patients
with chronic hepatitis c and pretreatment low platelet counts. BMC
Gastroenterol 2012;12: 7. https://doi.org/10.1186/1471-230X-12-7 PMid:22257364 PMCid:PMC3275508

- Kaushansky K: Thrombopoietin. The New England journal of medicine 1998;339(11): 746-754. https://doi.org/10.1056/NEJM199809103391107 PMid:9731092

- Murphy M, Waters A: Clinical aspects of platelet transfusions. Blood Coagulation & Fibrinolysis 1991;2(2): 389-396. https://doi.org/10.1097/00001721-199104000-00026 PMid:1893071

- Afdhal
N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B,
Esteban R: Thrombocytopenia associated with chronic liver disease. J
Hepatol 2008;48(6): 1000-1007. https://doi.org/10.1016/j.jhep.2008.03.009 PMid:18433919

- Schiffer
CA, Anderson KC, Bennett CL, Bernstein S, Elting LS, Goldsmith M,
Goldstein M, Hume H, McCullough JJ, McIntyre RE: Platelet transfusion
for patients with cancer: clinical practice guidelines of the American
Society of Clinical Oncology. Journal of Clinical Oncology 2001;19(5):
1519-1538. PMid:11230498

- McCormick
PA, Murphy KM: Splenomegaly, hypersplenism and coagulation
abnormalities in liver disease. Best Practice & Research Clinical
Gastroenterology 2000;14(6): 1009-1031. https://doi.org/10.1053/bega.2000.0144 PMid:11139352

- Shah
R, Mahour GH, Ford E, Stanley P: Partial splenic embolization. An
effective alternative to splenectomy for hypersplenism. The American
surgeon 1990;56(12): 774-777. PMid:2268105

- Akahoshi
T, Tomikawa M, Kawanaka H, Furusyo N, Kinjo N, Tsutsumi N, Nagao Y,
Hayashi J, Hashizume M, Maehara Y: Laparoscopic splenectomy with
interferon therapy in 100 hepatitis-C-virus-cirrhotic patients with
hypersplenism and thrombocytopenia. J Gastroenterol Hepatol 2012;27(2):
286-290. https://doi.org/10.1111/j.1440-1746.2011.06870.x PMid:21793908

- Barcena
R, Gil-Grande L, Moreno J, Foruny JR, Oton E, Garcia M, Blazquez J,
Sanchez J, Moreno A, Moreno A: Partial splenic embolization for the
treatment of hypersplenism in liver transplanted patients with
hepatitis C virus recurrence before peg-interferon plus ribavirin.
Transplantation 2005;79(11): 1634-1635. https://doi.org/10.1097/01.TP.0000155424.52939.3D PMid:15940057

- Lebano
R, Rosato V, Masarone M, Romano M, Persico M: The effect of antiviral
therapy on hepatitis C virus-related thrombocytopenia: a case report.
BMC Res Notes 2014;7: 59. https://doi.org/10.1186/1756-0500-7-59 PMid:24457056 PMCid:PMC3915622

- Hernandez
F, Blanquer A, Linares M, Lopez A, Tarin F, Cervero A: Autoimmune
thrombocytopenia associated with hepatitis C virus infection. Acta
haematologica 1998;99(4): 217-220. https://doi.org/10.1159/000040842 PMid:9644300

- Rajan
S, Liebman HA: Treatment of hepatitis C related thrombocytopenia with
interferon alpha. American journal of hematology 2001;68(3): 202-209. https://doi.org/10.1002/ajh.1180 PMid:11754404

- Afdhal
NH, McHutchison JG: Review article: pharmacological approaches for the
treatment of thrombocytopenia in patients with chronic liver disease
and hepatitis C infection. Aliment Pharmacol Ther 2007;26 Suppl 1:
29-39. https://doi.org/10.1111/j.1365-2036.2007.03511.x PMid:17958517

- Vadhan-Raj
S, Murray LJ, Bueso-Ramos C, Patel S, Reddy SP, Hoots WK, Johnston T,
Papadopolous NE, Hittelman WN, Johnston DA, Yang TA, Paton VE, Cohen
RL, Hellmann SD, Benjamin RS, Broxmeyer HE: Stimulation of
megakaryocyte and platelet production by a single dose of recombinant
human thrombopoietin in patients with cancer. Ann Intern Med
1997;126(9): 673-681. https://doi.org/10.7326/0003-4819-126-9-199705010-00001 PMid:9139552

- Harker
LA, Roskos LK, Marzec UM, Carter RA, Cherry JK, Sundell B, Cheung EN,
Terry D, Sheridan W: Effects of megakaryocyte growth and development
factor on platelet production, platelet life span, and platelet
function in healthy human volunteers. Blood 2000;95(8): 2514-2522.
PMid:10753829

- Basser R. The impact of thrombopoietin on clinical practice. Curr Pharm Des. 2002;8(5):369-77. Review. PubMed PMID: 12069375
 .
.
- Li
J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, Kuter DJ:
Thrombocytopenia caused by the development of antibodies to
thrombopoietin. Blood 2001;98(12): 3241-3248. https://doi.org/10.1182/blood.V98.12.3241 PMid:11719360

- De
Serres M, Ellis B, Dillberger JE, Rudolph SK, Hutchins JT, Boytos CM,
Weigl DL, DePrince RB: Immunogenicity of thrombopoietin mimetic peptide
GW395058 in BALB/c mice and New Zealand white rabbits: evaluation of
the potential for thrombopoietin neutralizing antibody production in
man. Stem Cells 1999;17(4): 203-209. https://doi.org/10.1002/stem.170203 PMid:10437983

- Dower
WJ, Cwirla SE, Balasubramanian P, Schatz PJ, Barrett RW, Baccanari DP:
Peptide agonists of the thrombopoietin receptor. Stem Cells
1998;16(S1): 21-29. https://doi.org/10.1002/stem.5530160705 PMid:11012174

- Kaushansky K: Hematopoietic growth factor mimetics. Annals of the New York Academy of Sciences 2001;938(1): 131-138. https://doi.org/10.1111/j.1749-6632.2001.tb03582.x PMid:11458500

- Broudy VC, Lin NL: AMG531 stimulates megakaryopoiesis in vitro by binding to Mpl. Cytokine 2004;25(2): 52-60. https://doi.org/10.1016/j.cyto.2003.05.001 PMid:14693160

- Bussel
JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, Lichtin
AE, Lyons RM, Nieva J, Wasser JS: AMG 531, a thrombopoiesis-stimulating
protein, for chronic ITP. New England Journal of Medicine 2006;355(16):
1672-1681. https://doi.org/10.1056/NEJMoa054626 PMid:17050891

- Kuter
DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM,
Aledort LM, George JN, Kessler CM, Sanz MA: Efficacy of romiplostim in
patients with chronic immune thrombocytopenic purpura: a double-blind
randomised controlled trial. The Lancet 2008;371(9610): 395-403. https://doi.org/10.1016/S0140-6736(08)60203-2

- Kuter
DJ, Rummel M, Boccia R, Macik BG, Pabinger I, Selleslag D, Rodeghiero
F, Chong BH, Wang X, Berger DP: Romiplostim or standard of care in
patients with immune thrombocytopenia. New England Journal of Medicine
2010;363(20): 1889-1899. https://doi.org/10.1056/NEJMoa1002625 PMid:21067381

- Moussa
MM, Mowafy N: Preoperative use of romiplostim in thrombocytopenic
patients with chronic hepatitis C and liver cirrhosis. J Gastroenterol
Hepatol 2013;28(2): 335-341. https://doi.org/10.1111/j.1440-1746.2012.07246.x PMid:22849409

- Voican
CS, Naveau S, Perlemuter G: Successful antiviral therapy for hepatitis
C virus-induced cirrhosis after an increase in the platelet count with
romiplostim: two case reports. Eur J Gastroenterol Hepatol 2012;24(12):
1455-1458. https://doi.org/10.1097/MEG.0b013e328357d5f2 PMid:22890208

- Erickson-Miller
C, Delorme E, Giampa L, Hopson C, Valoret E, Tian S-S, Miller SG,
Keenan R, Rosen J, Dillon S: Biological activity and selectivity for
Tpo receptor of the orally bioavailable, small molecule Tpo receptor
agonist, SB-497115. Blood 2004;104(11): 2912-2912
 .
.
- Erickson-Miller
CL, Delorme E, Tian SS, Hopson CB, Landis AJ, Valoret EI, Sellers TS,
Rosen J, Miller SG, Luengo JI: Preclinical Activity of Eltrombopag
(SB-497115), an Oral, Nonpeptide Thrombopoietin Receptor Agonist. Stem
Cells 2009;27(2): 424-430. https://doi.org/10.1634/stemcells.2008-0366 PMid:19038790 PMCid:PMC2729672

- McHutchison
JG, Dusheiko G, Shiffman ML, Rodriguez-Torres M, Sigal S, Bourliere M,
Berg T, Gordon SC, Campbell FM, Theodore D: Eltrombopag for
thrombocytopenia in patients with cirrhosis associated with hepatitis
C. New England Journal of Medicine 2007;357(22): 2227-2236. https://doi.org/10.1056/NEJMoa073255 PMid:18046027

- Afdhal
NH, Dusheiko GM, Giannini EG, Chen PJ, Han KH, Mohsin A,
Rodriguez-Torres M, Rugina S, Bakulin I, Lawitz E, Shiffman ML, Tayyab
GU, Poordad F, Kamel YM, Brainsky A, Geib J, Vasey SY, Patwardhan R,
Campbell FM, Theodore D: Eltrombopag increases platelet numbers in
thrombocytopenic patients with HCV infection and cirrhosis, allowing
for effective antiviral therapy. Gastroenterology 2014;146(2): 442-452
e441.

- Bussel
JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL: Safety and
efficacy of long-term treatment with romiplostim in thrombocytopenic
patients with chronic ITP. Blood 2009;113(10): 2161-2171. https://doi.org/10.1182/blood-2008-04-150078 PMid:18981291

- Kuter
D, Bussel J, George J, Aledort L, Lichtin A, Lyons R, Nieva J, Wasser
J, Bourgeois E, Kappers-Klunne M. Long-Term Dosing of AMG 531 in
Thrombocytopenic Patients with Immune Thrombocytopenic Purpura: 48-Week
Update. ASH Annual Meeting Abstracts. 2006: 476.

- Desjardins
RE, Tempel DL, Lucek R, Kuter DJ: Single and multiple oral doses of
AKR-501 (YM477) increase the platelet count in healthy volunteers.
Blood 2006;108: 477a.

- Orita
T, Tsunoda H, Yabuta N, Nakano K, Yoshino T, Hirata Y, Ohtomo T, Nezu
J, Sakumoto H, Ono K, Saito M, Kumagai E, Nanami M, Kaneko A, Yoshikubo
T, Tsuchiya M: A novel therapeutic approach for thrombocytopenia by
minibody agonist of the thrombopoietin receptor. Blood 2005;105(2):
562-566. https://doi.org/10.1182/blood-2004-04-1482 PMid:15374889

- Lawitz
EJ, Hepburn MJ, Casey TJ: A pilot study of interleukin-11 in subjects
with chronic hepatitis C and advanced liver disease nonresponsive to
antiviral therapy. The American journal of gastroenterology
2004;99(12): 2359-2364. https://doi.org/10.1111/j.1572-0241.2004.40047.x PMid:15571583

- Ong
JP, Younossi ZM: Managing the hematologic side effects of antiviral
therapy for chronic hepatitis C: anemia, neutropenia, and
thrombocytopenia. Cleveland Clinic journal of medicine 2004;71(Suppl
3): S17. https://doi.org/10.3949/ccjm.71.Suppl_3.S17 PMid:15468613

- Mendenhall
CL, Shakir AR, Zoiss EA, Reese C, Bui H, Goldberg S, Roselle GA:
Thrombocytopenia (T) in patients with chronic hepatitis C: Management
with interleukin 11. Gastroenterology 2003;124(4): A770. https://doi.org/10.1016/S0016-5085(03)83891-X

- Lawitz
EJ, Hepburn MJ, Casey TJ: A pilot study of interleukin-11 in subjects
with chronic hepatitis C and advanced liver disease nonresponsive to
antiviral therapy. Am J Gastroenterol 2004;99(12): 2359-2364. https://doi.org/10.1111/j.1572-0241.2004.40047.x PMid:15571583

- Homoncik
M, Jilma-Stohlawetz P, Schmid M, Ferlitsch A, Peck-Radosavljevic M:
Erythropoietin increases platelet reactivity and platelet counts in
patients with alcoholic liver cirrhosis: a randomized, double-blind,
placebo-controlled study. Alimentary pharmacology & therapeutics
2004;20(4): 437-443. https://doi.org/10.1111/j.1365-2036.2004.02088.x PMid:15298638

- Pirisi
M, Fabris C, Soardo G, Cecchin E, Toniutto P, Bartoli E:
Thrombocytopenia of chronic liver disease corrected by erythropoietin
treatment. Journal of hepatology 1994;21(3): 376-380. https://doi.org/10.1016/S0168-8278(05)80316-2

- Álvarez
GC, Gómez-Galicia D, Rodríguez-Fragoso L, Marina VM, Dorantes LC,
Sánchez-Alemán M, Méndez-Sánchez N, Esparza JR: Danazol improves
thrombocytopenia in HCV patients treated with peginterferon and
ribavirin. Ann Hepatol 2011;10(4): 458-468. PMid:21911886

- Malaguarnera
M, Vacante M, Giordano M, Motta M, Bertino G, Pennisi M, Neri S,
Malaguarnera M, Volti GL, Galvano F: L-carnitine supplementation
improves hematological pattern in patients affected by HCV treated with
Peg interferon-a 2b plus ribavirin. World journal of gastroenterology:
WJG 2011;17(39): 4414. https://doi.org/10.3748/wjg.v17.i39.4414 PMid:22110268 PMCid:PMC3218156

- Ikeda
M, Fujiyama S, Tanaka M, Sata M, Ide T, Yatsuhashi H, Watanabe H: Risk
factors for development of hepatocellular carcinoma in patients with
chronic hepatitis C after sustained response to interferon. J
Gastroenterol 2005;40(2): 148-156. https://doi.org/10.1007/s00535-004-1519-2 PMid:15770398

- Moriyama
M, Matsumura H, Aoki H, Shimizu T, Nakai K, Saito T, Yamagami H, Shioda
A, Kaneko M, Goto I, Tanaka N, Arakawa Y: Long-term outcome, with
monitoring of platelet counts, in patients with chronic hepatitis C and
liver cirrhosis after interferon therapy. Intervirology 2003;46(5):
296-307. https://doi.org/10.1159/000073209 PMid:14555850

- Nagamine
T, Ohtuka T, Takehara K, Arai T, Takagi H, Mori M: Thrombocytopenia
associated with hepatitis C viral infection. J Hepatol 1996;24(2):
135-140. https://doi.org/10.1016/S0168-8278(96)80021-3

- Ordi-Ros
J, Villarreal J, Monegal F, Sauleda S, Esteban I, Vilardell M:
Anticardiolipin antibodies in patients with chronic hepatitis C virus
infection: characterization in relation to antiphospholipid syndrome.
Clin Diagn Lab Immunol 2000;7(2): 241-244. https://doi.org/10.1128/cdli.7.2.241-244.2000

- Poynard
T, Schiff E, Terg R, Moreno-Otero R, Flamm SL, Schmidt WN, Berg T,
Goncales Jr FL, Heathcote J, Diago M: S1000 Results from the Epic3
Program: Platelet Counts Are Strong Predictors of Sustained Viral
Response (SVR) in the RE-Treatment of Previous Interferon/Ribavirin
Non-Responders (Nr). Gastroenterology 2008;134(4): A-772. https://doi.org/10.1016/S0016-5085(08)63605-7

- Sylvestre DL, Clements BJ: The utility of indirect predictors of hepatitis C viremia. Drug Alcohol Depend 2004;74(1): 15-19. https://doi.org/10.1016/j.drugalcdep.2003.11.006 PMid:15072803

- Shanmuganathan
G, Palaniappan S, Khor B, Radhakrishnan A, Raj M: The
clinico-epidemiological pattern of hepatitis C in a tertiary care
hospital in Malaysia: the Kuala Lumpur Hospital experience. 6th Asian
Pacific Digestive Week (ADPW 2006). Cebu, Philippines 2006.

- Taliani
G, Duca F, Clementi C, De Bac C: Platelet-associated immunoglobulin G,
thrombocytopenia and response to interferon treatment in chronic
hepatitis C. J Hepatol 1996;25(6): 999. https://doi.org/10.1016/S0168-8278(96)80309-6

- Borroni
G, Ceriani R, Cazzaniga M, Tommasini M, Roncalli M, Maltempo C, Felline
C, Salerno F: Comparison of simple tests for the non-invasive diagnosis
of clinically silent cirrhosis in chronic hepatitis C. Aliment
Pharmacol Ther 2006;24(5): 797-804. https://doi.org/10.1111/j.1365-2036.2006.03034.x PMid:16918883

- Dalekos
GN, Kistis KG, Boumba DS, Voulgari P, Zervou EK, Drosos AA, Tsianos EV:
Increased incidence of anti-cardiolipin antibodies in patients with
hepatitis C is not associated with aetiopathogenetic link to
anti-phospholipid syndrome. Eur J Gastroenterol Hepatol 2000;12(1):
67-74. https://doi.org/10.1097/00042737-200012010-00013 PMid:10656213

- Kaul
V, Friedenberg FK, Braitman LE, Anis U, Zaeri N, Fazili J, Herrine SK,
Rothstein KD: Development and validation of a model to diagnose
cirrhosis in patients with hepatitis C. Am J Gastroenterol 2002;97(10):
2623-2628. https://doi.org/10.1111/j.1572-0241.2002.06040.x PMid:12385450

- Luo
JC, Hwang SJ, Chang FY, Chu CW, Lai CR, Wang YJ, Lee PC, Tsay SH, Lee
SD: Simple blood tests can predict compensated liver cirrhosis in
patients with chronic hepatitis C. Hepatogastroenterology 2002;49(44):
478-481. PMid:11995477

- Prieto
J, Yuste JR, Beloqui O, Civeira MP, Riezu JI, Aguirre B, Sangro B:
Anticardiolipin antibodies in chronic hepatitis C: implication of
hepatitis C virus as the cause of the antiphospholipid syndrome.
Hepatology 1996;23(2): 199-204. https://doi.org/10.1002/hep.510230201 PMid:8591841

- Romagnuolo
J, Jhangri GS, Jewell LD, Bain VG: Predicting the liver histology in
chronic hepatitis C: how good is the clinician? Am J Gastroenterol
2001;96(11): 3165-3174. https://doi.org/10.1111/j.1572-0241.2001.05275.x PMid:11721766

- Zachou
K, Liaskos C, Christodoulou DK, Kardasi M, Papadamou G, Gatselis N,
Georgiadou SP, Tsianos EV, Dalekos GN: Anti-cardiolipin antibodies in
patients with chronic viral hepatitis are independent of
beta2-glycoprotein I cofactor or features of antiphospholipid syndrome.
Eur J Clin Invest 2003;33(2): 161-168. https://doi.org/10.1046/j.1365-2362.2003.01110.x PMid:12588291

- Hu
KQ, Tong MJ: The long-term outcomes of patients with compensated
hepatitis C virus-related cirrhosis and history of parenteral exposure
in the United States. Hepatology 1999;29(4): 1311-1316. https://doi.org/10.1002/hep.510290424 PMid:10094980

- Kim
YS, Lee HS, Ahn YO: Factors associated with positive predictability of
the anti-HCV ELISA method with confirmatory RT-PCR. J Korean Med Sci
1999;14(6): 629-634. https://doi.org/10.3346/jkms.1999.14.6.629 PMid:10642940 PMCid:PMC3054447

- Renou
C, Muller P, Jouve E, Bertrand JJ, Raoult A, Benderriter T, Halfon P:
Revelance of moderate isolated thrombopenia as a strong predictive
marker of cirrhosis in patients with chronic hepatitis C virus. Am J
Gastroenterol 2001;96(5): 1657-1659. https://doi.org/10.1111/j.1572-0241.2001.03830.x PMid:11374731

- Cicardi
M, Cesana B, Del Ninno E, Pappalardo E, Silini E, Agostoni A, Colombo
M: Prevalence and risk factors for the presence of serum cryoglobulins
in patients with chronic hepatitis C. J Viral Hepat 2000;7(2): 138-143.
https://doi.org/10.1046/j.1365-2893.2000.00204.x PMid:10760044

- Nahon
P, Ganne-Carrie N, Degos F, Nahon K, Paries J, Grando V, Chaffaut C,
Njapoum C, Christidis C, Trinchet JC, Chevret S, Beaugrand M: Serum
albumin and platelet count but not portal pressure are predictive of
death in patients with Child-Pugh A hepatitis C virus-related
cirrhosis. Gastroenterol Clin Biol 2005;29(4): 347-352. https://doi.org/10.1016/S0399-8320(05)80779-1

- Wang
CS, Yao WJ, Wang ST, Chang TT, Chou P: Strong association of hepatitis
C virus (HCV) infection and thrombocytopenia: implications from a
survey of a community with hyperendemic HCV infection. Clin Infect Dis
2004;39(6): 790-796. https://doi.org/10.1086/423384 PMid:15472809

- Alvarez
GC, Gomez-Galicia D, Rodriguez-Fragoso L, Marina VM, Dorantes LC,
Sanchez-Aleman M, Mendez-Sanchez N, Esparza JR: Danazol improves
thrombocytopenia in HCV patients treated with peginterferon and
ribavirin. Ann Hepatol 2011;10(4): 458-468. PMid:21911886

- Malaguarnera
M, Vacante M, Giordano M, Motta M, Bertino G, Pennisi M, Neri S,
Malaguarnera M, Li Volti G, Galvano F: L-carnitine supplementation
improves hematological pattern in patients affected by HCV treated with
Peg interferon-alpha 2b plus ribavirin. World J Gastroenterol
2011;17(39): 4414-4420. https://doi.org/10.3748/wjg.v17.i39.4414 PMid:22110268 PMCid:PMC3218156

[TOP]



























 .
.

















 .
.









 .
.




































