Emanuele Angelucci1,2, Silvana Anna Maria Urru3, Federica Pilo2 and Alberto Piperno4
1
Hematology, IRCCS Azienda Ospedaliera Universitaria San Martino – IST
Istituto Nazionale per la Ricerca sul Cancro, Genova. Italy.
2
Hematology and Bone Marrow Transplantation Unit, Ospedale Oncologico di
Riferimento Regionale “Armando Businco”, Cagliari, Italy.
3 CRS4, Biomedicine Sector, Scientific and Technology Park of Sardinia, Pula, Cagliari, Italy;
4
Internal Medicine 2, University of Milano-Bicocca, Centre for Disorders
of Iron Metabolism, ASST-Monza, S. Gerardo Hospital, Monza, Italy.
Corresponding
author: Emanuele Angelucci. IRCCS Azienda
Ospedaliera Universitaria San Martino - IST Istituto Nazionale per la
Ricerca sul Cancro. Largo Rosanna Benzi, 10 16132 Genova. Tel +39 010
555 3651. E-mail:
emnang@tin.it
Published: March 1, 2017
Received: December 12, 2016
Accepted: January 27, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017021 DOI
10.4084/MJHID.2017.021
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Over
recent decades we have been fortunate to witness the advent of new
technologies and of an expanded knowledge and application of chelation
therapies to the benefit of patients with iron overload. However,
extrapolation of learnings from thalassemia to the myelodysplastic
syndromes (MDS) has resulted in a fragmented and uncoordinated clinical
evidence base. We’re therefore forced to change our understanding of
MDS, looking with other eyes to observational studies that inform us
about the relationship between iron and tissue damage in these
subjects. The available evidence suggests that iron accumulation is
prognostically significant in MDS, but levels of accumulation
historically associated with organ damage (based on data generated in
the thalassemias) are infrequent. Emerging experimental data have
provided some insight into this paradox, as our understanding of
iron-induced tissue damage has evolved from a process of progressive
bulking of organs through high-volumes iron deposition, to one of
‘toxic’ damage inflicted through multiple cellular pathways. Damage
from iron may, therefore, occur prior to reaching reference thresholds,
and similarly, chelation may be of benefit before overt iron overload
is seen. In this review, we revisit the scientific and clinical
evidence for iron overload in MDS to better characterize the iron
overload phenotype in these patients, which differs from the classical
transfusional and non-transfusional iron overload syndrome. We hope
this will provide a conceptual framework to better understand the
complex associations between anemia, iron and clinical outcomes, to
accelerate progress in this area.
|
Introduction
Recent
retrospective studies suggest that MDS are more common than previously
recognized; these diseases affect predominantly older individuals, with
a median age at diagnosis of >70 years and with >10% of the
patients[1] in Europe and 6% in the United States being younger than 50 years of age.[2,3]
After
the development and introduction of oral iron chelators, the
possibility to chelate iron overload in MDS patients became a practical
option. This review will discuss theoretical basis and rationale for
iron chelation therapy in transfusion dependent patients affected by
myelodysplastic syndrome.
For a rational approach to this problem,
emphasis should be reserved for modern improvements in understanding
iron metabolism and iron toxicity.
Iron Balance and Overload
Iron
is essential for physiological health, playing an integral role in
oxygen transport/storage, DNA synthesis, translation, cellular
respiration, and a number of metabolic processes.[4] Excessive iron, however, is injurious to cells, tissues, and organs.
The liver peptide hepcidin (for review see reference)[5]
regulates intestinal iron absorption and iron release from storage
cells such as macrophages and hepatocytes. Hepcidin binds to
ferroportin causing its internalization and degradation, thus exerting
a general inhibitory effect on iron release within the body.[3,6] The hepcidin-ferroportin pathway is emerging as a therapeutic target for iron modulation,[7]
and a number of animal models have shown how hepcidin mimetics have the
capacity to reduce iron overload in response to hepcidin deficiency.[8]
Further, genetic deletion of the hepcidin inhibitor, Tmprss6, prevents
iron overload in animal models of hemochromatosis and β-thalassemia.[9,10]
Transferrin
is a blood protein that acts both as a chelator and transporter for
iron, taking it up into cells via the transferrin receptor 1 (TFR1).
Increased iron absorption due to inadequate suppression of hepcidin
(primary iron overload) that occurs as a consequence of HAMP
regulatory network alterations (as seen in hereditary haemochromatosis)
or ineffective erythropoiesis (as seen in non transfusion dependent
thalassemia = NTDT), causes oversaturation of transferrin, generation
of toxic non-transferrin bound iron (NTBI) and parenchymal iron
accumulation. In transfusion-dependent patients (as seen in thalassemia
major and myelodysplastic syndrome), iron accumulation occurs in the
reticuloendothelial system (RES), in the spleen and liver as a
consequence of parenteral input from blood transfusions (secondary iron
overload).[4] When the excess of iron overwhelms homeostatic mechanisms in RES cells, iron spills out into blood,[11] and transferrin becomes fully saturated leading to NTBI and parenchymal iron overload.[12]
NTBI
and its component labile plasma iron (LPI) are able to enter the cells
via an unregulated automatic way and disturb the delicate intracellular
balance between iron utilization, storage, and reactive oxygen species
(ROS) formation, finally leading to organelle damage and cell death.[13]
As a consequence of this new acquisition, it follows that iron toxicity
might develop long before the clear evidence of overload through the
production of tissue reactive iron and consequent reactive oxygen
species. Subsequently, iron overload constantly causes toxicity by
continuing to produce tissue reactive iron and ROS. In this setting,
the capacity to counteract these toxic effects might be relevant to the
development of cellular damage.
As MDS is a disease characterized
by ineffective erythropoiesis, in which patients may eventually become
regularly transfused, both mechanisms are believed to be responsible
for the generation of free iron reactive species and iron overload,
although to varying degrees and at different stages of
transfusion-dependence. The kinetics of iron release from RES cells has
been partially studied in MDS, where there is a wide dispersion of
hepcidin levels.[6] Figure 1
illustrates iron homeostasis in pathologic conditions and the sequence
of events that leads to end-organ damage in response to iron overload.
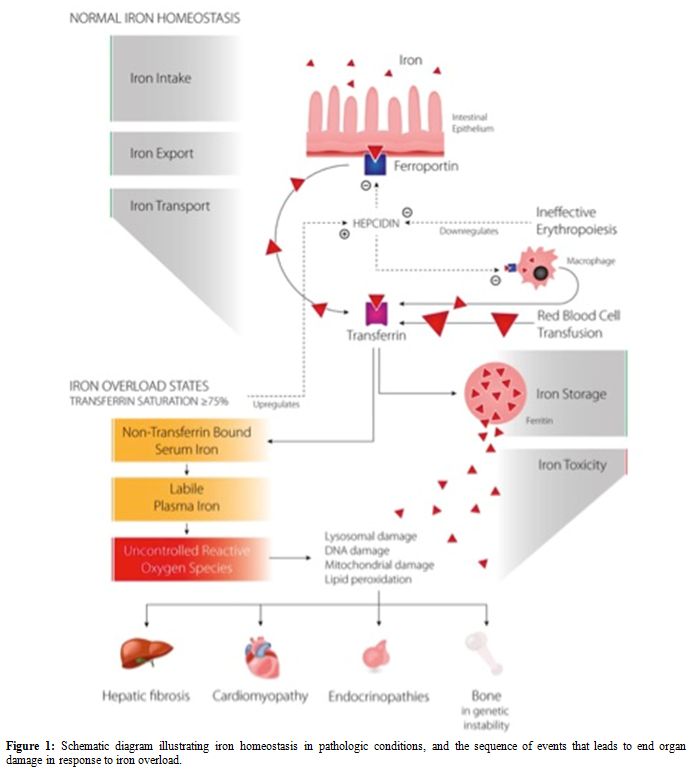 |
Figure 1. Schematic diagram illustrating
iron homeostasis in pathologic conditions, and the sequence of events
that leads to end organ damage in response to iron overload. |
Iron Overload in Myelodysplastic Syndromes
Myelodysplastic
syndromes represent a heterogeneous group of clonal stem cell disorders
associated with worsening cytopenias. In general, these patients
comprise frail and elderly individuals with multiple co-morbidities.
Indeed, the prognosis for these patients has traditionally been so poor
to negate consideration for novel therapeutic targets, as the
underlying disease process has historically determined outcomes.
Moreover, clinical researchers have reserved limited interest to what
they call supportive care. However supportive care can, in several
instances, substantially improves quality and duration of survival.
Obviously for what concern iron chelation therapy intervention with
daily subcutaneous chelating agents has not been an attractive option
for these patients.
Moreover, novel disease-modifying
agents and stem cell therapies have now extended the life
expectancy of these patients, allowing increased supportive care in the
form of blood transfusions.[14,15] In those patients with IPSS[16] low to intermediate risk MDS and probably even in those successfully receiving disease modifying agents,[17]
life expectancy is sufficiently long for chronic transfusion therapy to
generate iron free form and clinically relevant doses of iron.[18] With the emergence of acceptable oral chelating agents, examining the prognostic effect of iron toxicity in MDS is warranted.
Clinical Data
Leukemia-free
survival and overall survival (OS) have been shown to be lower in
transfusion-dependent patients with MDS (HR 1.91 and 1.84,
respectively) and these two parameters progressively decrease with each
subsequent transfusion.[19] Transfusion dependence has consequently been included in MDS risk calculations, with the introduction of the WPSS.[20] More recent longitudinal data on 2,994 MDS patients have provided further quantification of risk [21]
with those patients transfusion-dependent at baseline experiencing a
mean survival of 19 months, compared with 60 months in individuals
becoming transfusion-dependent during follow-up and 96 months in those
remaining transfusion-independent. Multivariate analysis of a subgroup
of these patients demonstrated high serum ferritin and transfusion
dependence to add significant prognostic value to overall survival in
IPSS and WPSS scores. Overall survival is reduced in MDS with
increasing ferritin levels, with a hazard ratio of 1.42 for every 500
ng/mL increase in ferritin over 1,000 ng/mL.[22]
The
baseline cardiovascular risk in these individuals is significant: of
1,000 newly diagnosed patients with low and intermediate-1 risk in the
European Leukemianet MDS (EUMDS) registry, 46% of patients had
hypertension, 18% diabetes mellitus, 12% arrhythmia, and 12% thyroid
disease.[23] Survival data were further confirmed by a recent update of the European registry.[24]
Malcovati et al. reported that 51% of non-leukemic causes of death were
due to cardiac failure in low-risk MDS, compared with 31% due to
infection and 8% due to hepatic cirrhosis.[20] In a
retrospective analysis of 840 MDS patients, 25% had cardiovascular
comorbidities, and 63% of deaths were due to cardiac failure.
Multivariate analyses showed that any cardiovascular comorbidity
increased non-leukemic deaths significantly, with an HR of 3.7.[25] This risk is even more pronounced in patients who are transfused.[26]
Indeed, the only study that do not report a correlation between
transfusion burden and survival was the retrospective study from the
Mayo Clinic, which examined a group of patients with RARS and limited
follow up.[27] Hepatic dysfunction also correlates, although to a lesser degree, with both transfusion history and ferritin levels.[28]
It
is tempting to link the cardiovascular events in MDS with data on
transfusion history and prognosis to speculate that iron loaded from
transfusions leads to cardiac siderosis, which then triggers
cardiovascular events.
A key confounding factor is, however, the
presence of anemia per se. In addition, aging and age-related disorders
may have a clinical impact on iron overload in MDS. It is well
established that chronic anemia is associated with adverse cardiac
outcomes.[29] Anemia triggers a compensatory process
of increased cardiac output to achieve sufficient oxygen delivery,
which overtime results in maladaptive cardiac morphology. Malign
remodeling has a higher metabolic demand, which is pro-ischemic and
overtimes leads to chamber dilatation and failure. Cardiac remodeling
is prevalent in individuals with transfusion dependence and reduced
mean haemoglobin levels.[30] Furthermore, in MDS,
anemia has been shown to be associated with left ventricular
hypertrophy, exacerbations of acute coronary syndromes, and coexistence
of renal disease, which in turn may result in decreased erythropoietin
(EPO) production and increasing severity of anemia.[29,30] Moreover, transfusion therapy causes abrupt changes in cardiac preload, which leads to altered haemodynamic.
Data from MRI studies of patients with MDS do not support a role for high-volume iron accumulation in the heart.[31] Our study,[32]
conducted on 27 chronic transfusion dependent patients with acquired
anemias revealed that only 3 patients with severe hepatic iron overload
(T2* <1.4 ms) showed cardiac T2* value indicative of dangerous
myocardial iron deposition as defined in young patients with
thalassemia.[33] It should be noted that these
studies are small, and the comparisons have been drawn against
functional thresholds, established in thalassemia, but not validated in
MDS. Similarly, thresholds for tissue toxicity and consequent fibrosis
and cirrhosis have not been established in MDS cohorts. It is
conceivable that iron may play a different role in these patients, and
perhaps not cause damage through the traditional paradigm of
transfusional siderosis. A more recent study reporting on a larger
patient series[34] did detect iron overload using T2*
values in 18.2% of regularly transfused MDS patients, with severe
overload in 4% (T2* ≤10 ms). They reported reduced T2* values
correlated with compromised left ventricular ejection fraction (LVEF)
using echocardiography.
These data, taken together, suggest that,
although iron infrequently accumulates to the degree seen with
iron-related target organ damage in thalassemia, its mild overload is
still associated with poor prognosis in patients with MDS. A
mechanistic illustration of disordered calcium handling and multiple
ion channel disruption as a result of iron influx into the cardiac
myocyte is shown in figure 2.
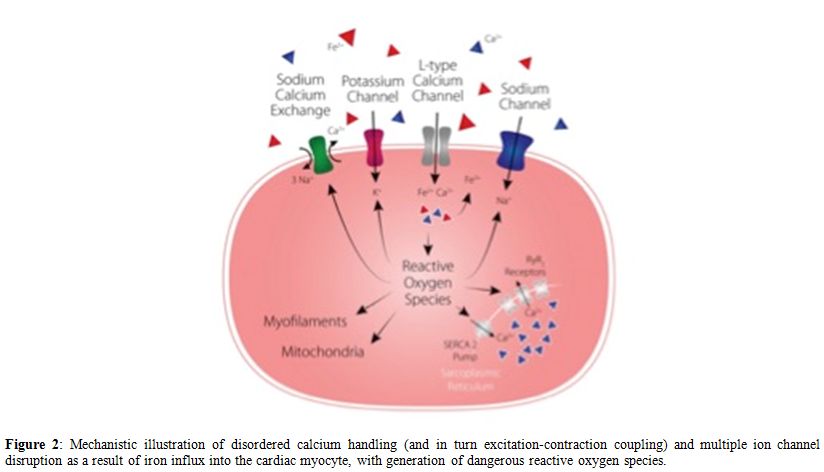 |
Figure 2. Mechanistic illustration of
disordered calcium handling (and in turn excitation-contraction
coupling) and multiple ion channel disruption as a result of iron
influx into the cardiac myocyte, with generation of dangerous reactive
oxygen species. |
There are two
possible explanation of this effect: 1) lower, not detectable, levels
of iron accumulation can have dangerous clinical negative effect
2) circulating “reactive iron species - free iron forms” in myocyte cells can damage without clear evidence of overload.
MDS and other Diseases
MDS
has been the last “iron overload” disease to be included in iron
chelation therapy program. Several of the evidence on the clinical
impact of iron toxicity and iron overload come from other diseases like
thalassemia major and hereditary hemochromatosis. However, these other
diseases are characterized by deep clinical differences (age,
comorbidity, functionality of stem cell, anemia, non proliferative
diseases, gastro intestinal iron absorption, life expectancy, etc.).
Consequently MDS patient is a completely different clinical scenario
whose characteristics in term of tissue and organ morbidity, quality of
life, therapeutic options and finally survival is completely to be “de
novo“ designed. Table 1 describes few of the various differences between MDS and the other iron overload disease.
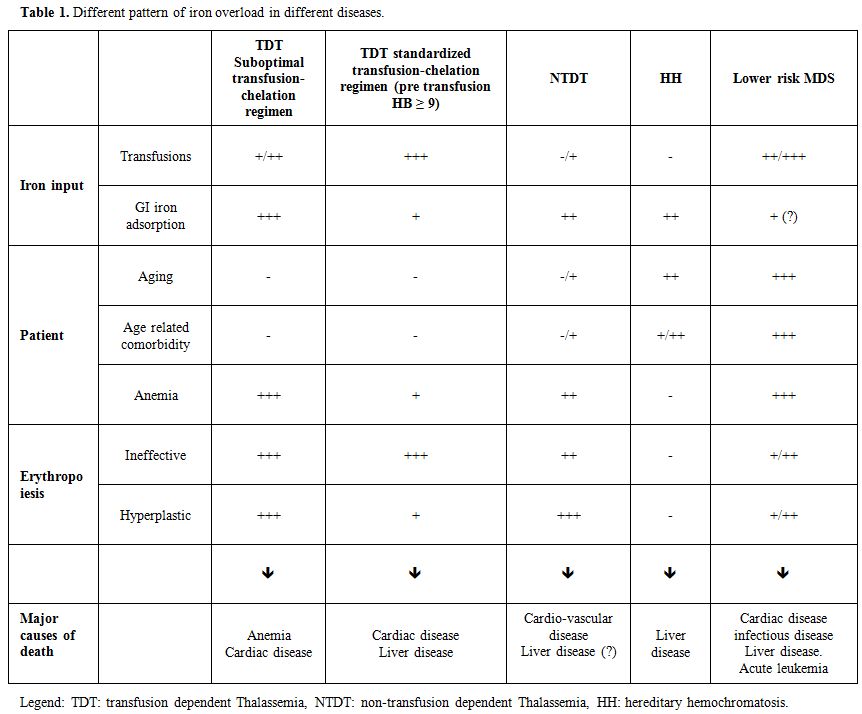 |
Table 1. Different pattern of iron overload in different diseases. |
Therapeutic Potential in MDS Patients
A
number of medium-sized retrospective studies and single-armed
prospective trials have tested the role of chelation therapy in MDS.
These studies have shown that deferasirox is capable of lowering serum
ferritin and liver iron concentration in MDS.[35-39] The US03[40] and EPIC[41] trials, demonstrated sustained reductions in labile plasma iron (LPI). Post-hoc analyses from both studies[40,42]
showed improvements in erythroid, platelet, and neutrophil counts in
subgroups of patients (in the range of 13-22%). In the US experience,
median ferritin reductions were greater in patients with hematological
improvements compared to patients without, but with no statistical
differences detected in terms of LPI levels. There have also been some
reports of transfusion independence in patients with MDS treated with
iron chelation,[43-50] and sub-analysis of 116
patients from the EPIC cohort reports hematological improvements with
deferasirox treatment in patients with aplastic anemia.[51]
Our findings from the GIMEMA MDS0306 trial provide the first
prospective evidence for positive hematological responses with
deferasirox chelation therapy in MDS.[52]
Importantly, a subset of patients in this multicenter study also
achieved transfusion-independence, which may be related to
iron-dependent or independent pathways.[52] Iron
depletion and scavenging reactive oxygen species from the bone marrow
and other organs involved in erythropoiesis is one potential mechanism.[53,54]
Experimental data support the idea that removal of excess iron from the
iron- and oxygen-dependent propyl hydroxylase in the renal oxygen
sensing system may benefit erythropoietin production.[55,56]
Iron accumulation is known to have a suppressive effect on erythroid
production (in vitro), elevated ferritin levels are associated with
suppression of erythroid progenitor cells in non transfusion dependent
thalassemia (NTDT).[57] Hartmann et al. recently
demonstrated that iron overload causes suppression of erythroid
progenitor cells (BFU-E) in MDS and that patients with even slight
elevations in serum ferritin have impaired proliferation capacity
compared to those with normal ferritin levels. Interestingly, iron
chelation can restore this deficit.[54]
Iron Chelation and Survival
While
chronic transfusion therapy is associated with reduced overall and
leukemia-free survival, a clear confounder to any causative conclusions
lie in the fact that transfusion-dependency represents more progressive
bone marrow disease.
Iron overload does, however, remain prognostically important in multivariate analyses,[20,21] suggesting a contributing role of iron on survival. More debate is on the role of iron overload in leukemia transformation.
Recent data from a prospective US registry of 600 lower-risk MDS patients with transfusional iron overload over 5 years[58]
report improved median overall survival in those patients chelated for
a minimum of 6 months, as compared with non-chelated individuals – in
both low-risk and intermediate-1 patients (median survival 98.7 vs.
53.6 months and 70 vs. 44.7 months, respectively). However, there were
no statistically significant differences in the causes of deaths
between groups, although there was a signal towards shorter AML-free
survival in non-chelated patients. A matched-pair analysis of 188
patients with iron overload or a history of chronic iron transfusion in
the Düsseldorf registry[59] showed no association
between chelation therapy and the risk of leukemic transformation,
although there was improved mean survival in chelated versus
non-chelated patients (74 vs. 49 months, respectively). Inconsistencies
in these data may reflect both limitations in registry data, such as
selection bias (those patients with better overall performance status
are chosen for treatment with chelation), as well as inherent
challenges in MDS cohorts, including very high-dropout rates.[52]
Recent
meta-analyses further support this statement: of 8 studies, comparing
chelation versus not chelation in MDS, 7 showed a significant
statistical benefit on survival, while the other one showed a not
statistically significant advantage for chelation.[60]
However, it should be underlined that evidence of these study is
limited being or retrospective or match paired or prospective but not a
randomized study. Existing data, taken collectively, indicate a role
for iron chelation therapy in MDS, and this is reflected by its
inclusion in a number of societies and national guidelines.[17,61,62] We are currently coordinating the phase II randomized TELESTO trial (URL: http://clinicalTrials.gov/ct2/show/NCT00940602),
which has completed the recruitment of patients with low and
intermediate-1 risk MDS to receive either deferasirox monotherapy or
placebo. The trial will include a composite primary endpoint of death
and non-fatal cardiac and hepatic events in lower risk MDS patients
(with secondary outcomes including metabolic effects and disease
progression). This will hopefully provide definitive evidence for the
efficacy of iron chelation therapy in MDS. When considering the
heterogeneity of MDS, the complexity of the patient cohort, with an
elderly population and multiple comorbidities, a “blanket” approach to
treatment is unlikely to be the best by utilizing chelating agents. A
more sophisticated approach will require a better understanding of
pathophysiology and toxicity of iron in specific subgroups of MDS.
Perspectives
Removal
of iron is a slow and progressive process. Compliance with chelation
therapy is often challenging in elderly patients with multiple
comorbidities and polypharmacy, as demonstrated by the high drop-out
rate in all existing MDS chelation studies.[36,40]
Against this background, and when considering that reparative
mechanisms are likely compromised or even absent in this frail cohort,
“debulking” of iron from organs is unlikely to be the relevant
mechanism. Given that the reduced overall survival in MDS occurs prior
to a transfusion history expected to cause cardiac siderosis, it seems
likely that alterative mechanisms of tissue damage are taking place.
Recent studies have seen a transition in our understanding of
transfusional iron overload, from a disease of “bulking” of organs
through progressive accumulation, to one of “toxic” damage. Indeed,
increased LPI and NTBI levels may cause injury in the absence of
evidence of iron loading on MRI, although this is yet to be determined
in clinical studies. Importantly, these toxic iron species are
chelatable targets as also evidenced in studies using deferasirox in
MDS patients.[40] Any protective benefits conferred
by chelators in MDS may, therefore, occur through mechanisms at least
partially independent of “debulking” of iron stores in the liver and
heart. Instead, a more accurate picture may be one of “detoxifying”
against deleterious derivates of iron, such as reductions in LPI and
NTBI levels. The dynamic regulation of iron loading between the heart
and liver (relative delay in heart iron loading and unloading) is yet
to be determined but it may be that initial exposure to NTBI even in
iron-free hearts is sufficient to establish cardiovascular disease in
these elderly patients.
Conclusions
In
patients with thalassemia, iron-induced tissue damage occurs through
high-volume deposition of iron in target organs, with progressive
bulking and eventual mechanical failure. In MDS the prognostic
consequences of iron appear to occur prior to perceived thresholds of
significant iron burden. Advances in our understanding of the
mechanisms of iron-induced tissue damage have shown direct toxic
cellular damage through multiple molecular pathways. In frail elderly
cohorts with MDS, on a background of poor physiological reserve and
anemia, iron may provide the final insult through toxic ROS,
endothelial injury, and erythroid suppression, causing tissue death and
triggering a cycle of progressive anemia and organ damage. Toxic
thresholds in this setting may, therefore, be dramatically lower than
those used to assess cardiac siderosis in thalassemia, as iron is
working through fundamentally different pathways to confer organ
damage. Worryingly, current MDS protocols have adopted a paradigm
identical to iron in thalassemia, which involves waiting for iron
levels to reach levels representative of target organ injury in
disorders of secondary iron overload. These reference thresholds are
invalid in MDS, and during this observation period clinically relevant
injury may be occurring at the cellular level, and the window of
opportunity to halt organ damage may be lost.
Ultimately,
if the basis in which we treat patients and design trials is incorrect,
advancing the management and therapeutic framework will be severely
hindered. As the iron overload phenotype in MDS remains
uncharacterized, we urgently require further experimental studies in
models of MDS, in tandem with dedicated clinical trials, to build a
legitimate evidence base for iron toxicity and the role of chelation
therapy in MDS.
Contributions
All
Authors equally contributed the conception and design of the
study, analysis, and interpretation of the results and writing of
the paper.
All Authors and has approved the final submitted version of the manuscript.
Acknowledgements
Editorial assistance was provided by Kaivan Khavandi, MD.References
- Neukirchen
J, Schoonen WM, Strupp C, Gattermann N, Aul C, Haas R, Germing U.
Incidence and prevalence of myelodysplastic syndromes: Data from the
Dusseldorf MDS-registry. Leuk Res. 2011; 35: 1591-6. https://doi.org/10.1016/j.leukres.2011.06.001 PMid:21708407

- Ma
X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: Incidence and
survival in the United States. Cancer. 2007; 109:1536-42. https://doi.org/10.1002/cncr.22570 PMid:17345612

- Piperno
A, Mariani R, Trombini P, Girelli D. Hepcidin modulation in human
diseases: From research to clinic. World Journal of Gastroenterology.
2009; 538-51. https://doi.org/10.3748/wjg.15.538 PMid:19195055 PMCid:PMC2653344

- Brittenham GM. Iron-chelating therapy for transfusional iron overload. N Engl J Med 2011; 364: 146-56. https://doi.org/10.1056/NEJMct1004810 PMid:21226580 PMCid:PMC3078566

- Hentze
MW, Muckenthaler MU, Galy B, Camaschella C. Two to Tango: Regulation of
Mammalian Iron Metabolism. Cell. 2010; 142: 24-38. https://doi.org/10.1016/j.cell.2010.06.028 PMid:20603012

- Santini
V, Girelli D, Sanna A, Martinelli N, Duca L, Campostrini N, Cortelezzi
A, Corbella M, Bosi A, Reda G, Olivieri O, Cappellini MD. Hepcidin
levels and their determinants in different types of myelodysplastic
syndromes. PLoS One. 2011;6(8). https://doi.org/10.1371/journal.pone.0023109

- Camaschella C. Treating Iron Overload. N Engl J Med. 2013; 368:2325-7. https://doi.org/10.1056/NEJMcibr1304338 PMid:23758239

- Ramos
E, Ruchala P, Goodnough JB, Kautz L, Preza GC, Nemeth E,Ganz T.
Minihepcidins prevent iron overload in a hepcidin-deficient mouse model
of severe hemochromatosis. Blood. 2012; 120: 3829-36. https://doi.org/10.1182/blood-2012-07-440743 PMid:22990014 PMCid:PMC3488893

- Finberg
KE, Whittlesey RL, Andrews NC. Tmprss6 is a genetic modifier of the
Hfe-hemochromatosis phenotype in mice. Blood. 2011; 117: 4590-9. https://doi.org/10.1182/blood-2010-10-315507 PMid:21355094 PMCid:PMC3099575

- Nai
A, Pagani A, Mandelli G, Lidonnici MR, Silvestri L, Ferrari G,
Camaschella C. Deletion of TMPRSS6 attenuates the phenotype in a mouse
model of?beta-thalassemia. Blood. 2012; 119: 5021-9. https://doi.org/10.1182/blood-2012-01-401885 PMid:22490684 PMCid:PMC3426375

- Ghugre
NR, Gonzalez-Gomez I, Butensky E, Noetzli L, Fischer R, Williams R,
Harmatz P,Coates TD, Wood JC. Patterns of hepatic iron distribution in
patients with chronically transfused thalassemia and sickle cell
disease. Am J Hematol. 2009; 84: 480-3. https://doi.org/10.1002/ajh.21456 PMid:19536851 PMCid:PMC2884400

- Wood JC. Cardiac iron across different transfusion-dependent diseases. Blood Rev. 2016; 22: S14-21. https://doi.org/10.1016/S0268-960X(08)70004-3

- Coates
TD. Physiology and pathophysiology of iron in hemoglobin-associated
diseases. Free Radical Biology and Medicine. 2014:23-40. https://doi.org/10.1016/j.freeradbiomed.2014.03.039 PMid:24726864 PMCid:PMC4940047

- Wood
JC. History and Current Impact of Cardiac Magnetic Resonance Imaging on
the Management of Iron Overload. Circulation 2009; 120: 1937-9. https://doi.org/10.1161/CIRCULATIONAHA.109.907196 PMid:19884464 PMCid:PMC2884388

- Gujja
P, Rosing DR, Tripodi DJ, Shizukuda Y. Iron overload cardiomyopathy:
Better understanding of an increasing disorder. J Am Coll Cardiol.
2010: 56: 1001-12. https://doi.org/10.1016/j.jacc.2010.03.083 PMid:20846597 PMCid:PMC2947953

- Greenberg
P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T,
Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J.
International scoring system for evaluating prognosis in
myelodysplastic syndromes. Blood. 1997; 89: 2079-88.
PMid:9058730

- Santini
V, Alessandrino PE, Angelucci E, Barosi G, Billio A, Di Maio M, Finelli
C, Locatelli F, Marchetti M, Morra E, Musto P, Visani G, Tura S.
Clinical management of myelodysplastic syndromes: Update of SIE, SIES,
GITMO practice guidelines. Leuk Res. 2010; 34: 1576-88. https://doi.org/10.1016/j.leukres.2010.01.018 PMid:20149927

- Leitch HA. Controversies surrounding iron chelation therapy for MDS. Blood Rev. 2011; 25: 17-31. https://doi.org/10.1016/j.blre.2010.09.003 PMid:21030120

- Malcovati
L. Impact of transfusion dependency and secondary iron overload on the
survival of patients with myelodysplastic syndromes. Leuk Res 2016; 31:
S2-6. https://doi.org/10.1016/S0145-2126(07)70459-9

- Malcovati
L, Della Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E,
Passamonti F, Arcaini L, Maffioli M, Bernasconi P, Lazzarino M, Cazzola
M. Prognostic factors and life expectancy in myelodysplastic syndromes
classified according to WHO criteria: A basis for clinical decision
making. J Clin Oncol. 2005; 23: 7594-603. https://doi.org/10.1200/JCO.2005.01.7038 PMid:16186598

- Remacha
ÁF, Arrizabalaga B, Villegas A, Durán MS, Hermosín L, de Paz R, Garcia
M, Diez Campelo M, Sanz G; IRON-2 Study Group. Evolution of iron
overload in patients with low-risk myelodysplastic syndrome: iron
chelation therapy and organ complications. Ann Hematol. 2015; 94:
779-87. https://doi.org/10.1007/s00277-014-2274-y PMid:25516455

- Gattermann N, Rachmilewitz EA. Iron overload in MDS-pathophysiology, diagnosis, and complications. Ann Hematol. 2011: 90: 1-10. https://doi.org/10.1007/s00277-010-1091-1 PMid:20938663

- de
Swart L, Smith A, Fenaux P, Sanz G, Hellstrom-Lindberg E, Symeonidis A,
CermakJ, Germing U, Stauder R, Georgescu O, MacKenzie M, Malcovati L,
Holm MS, Madry K, Park S, Beyne-Rauzy O, Droste J, Bowen D, de Witte T.
Early Mortality in 1000 Newly Diagnosed MDS Patients with Low- and
Intermediate-1 Risk MDS in the European Leukemianet MDS (EUMDS)
Registry. Blood. 2012; 120: 3830

- Langemeijer
S, De Swart L, Yu G, Smith A, Crouch S, Johnston T, Fenaux P,
Symeonidis A, Cermak J, Hellstrom-Lindberg E, Sanz G, Stauder R,
Malcovati L, Germing U, MS Holm, Mittelman M, Madry K, Tatic A, Almeida
A, Savic A, Park S, Beyne-Rauzy O, Itzykson R, van Marrewijk C, Bowen
D, de Witte T. Impact of Treatment with Iron Chelators in Lower-Risk
MDS Patients Participating in the European Leukemianet MDS (EUMDS)
Registry. Blood. 2016;128:3186

- della
Porta MG, Malcovati L, Strupp C, Ambaglio I, Kuendgen A, Zipperer E,
Travaglino E, Invernizzi R, Pascutto C, Lazzarino M, Germing U, Cazzola
M Risk stratification based on both disease status and
extra-hematologic comorbidities in patients with myelodysplastic
syndrome. Haematologica. 2011;96: 441-9. https://doi.org/10.3324/haematol.2010.033506 PMid:21134982 PMCid:PMC3046276

- Goldberg
SL, Chen E, Corral M, Guo A, Mody-Patel N, Pecora AL, Laouri M.
Incidence and clinical complications of myelodysplastic syndromes among
United States Medicare beneficiaries. J Clin Oncol. 2010; 28: 2847-52. https://doi.org/10.1200/JCO.2009.25.2395 PMid:20421543

- Chee
CE, Steensma DP, Wu W, Hanson CA, Tefferi A. Neither serum ferritin nor
the number of red blood cell transfusions affect overall survival in
refractory anemia with ringed sideroblasts. Am J Hematol. 2008; 83:
611-3. https://doi.org/10.1002/ajh.21192 PMid:18442062

- Takatoku
M, Uchiyama T, Okamoto S, Kanakura Y, Sawada K, Tomonaga M, Nakao S,
Nakahata T, Harada M, Murate T, Ozawa K. Retrospective nationwide
survey of Japanese patients with transfusion-dependent MDS and aplastic
anemia highlights the negative impact of iron overload on
morbidity/mortality. Eur J Haematol. 2007; 78: 487-94. https://doi.org/10.1111/j.1600-0609.2007.00842.x PMid:17391310

- Platzbecker
U, Hofbauer LC, Ehninger G, Hölig K. The clinical, quality of life, and
economic consequences of chronic anemia and transfusion support in
patients with myelodysplastic syndromes. Leuk Res. 2012; 36: 525-36. https://doi.org/10.1016/j.leukres.2012.01.006 PMid:22300879

- Cazzola
M, Della Porta MG, Malcovati L. Clinical relevance of anemia and
transfusion iron overload in myelodysplastic syndromes. Hematology Am
Soc Hematol Educ Program. 2008;166-75. https://doi.org/10.1182/asheducation-2008.1.166 PMid:19074076

- Konen
E, Ghoti H, Goitein O, Winder A, Kushnir T, Eshet Y, Rachmilewitz E. No
evidence for myocardial iron overload in multitransfused patients with
myelodysplastic syndrome using cardiac magnetic resonance T2*
technique. Am J Hematol. 2007; 82: 1013-6. https://doi.org/10.1002/ajh.20980 PMid:17654681

- Di
Tucci AA, Matta G, Deplano S, Gabbas A, Depau C, Derudas D, Caocci G,
Agus A, Angelucci E. Myocardial iron overload assessment by T2*
magnetic resonance imaging in adult transfusion dependent patients with
acquired anemias. Haematologica. 2008; 93: 1385-8. https://doi.org/10.3324/haematol.12759 PMid:18603557

- Anderson
LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, Firmin DN,
Wonke B, Porter J, Walker JM, Pennell DJ. Cardiovascular T2-star (T2*)
magnetic resonance for the early diagnosis of myocardial iron overload.
Eur Heart J 2001; 22: 2171-79 https://doi.org/10.1053/euhj.2001.2822 PMid:11913479

- Pascal
L, Beyne-Rauzy O, Brechignac S, Marechaux S, Vassilieff D, Ernst O,
Berthon C, Gyan E,Dreyfus F, Fenaux P,Rose C. Cardiac iron overload
assessed by T2* magnetic resonance imaging and cardiac function in
regularly transfused myelodysplastic syndrome patients. Br J Haematol
2013;162: 413-5 https://doi.org/10.1111/bjh.12368 PMid:23656172

- Nolte
F, Höchsmann B, Giagounidis A, Lübbert M, Platzbecker U, Haase D, Lück
A, Gattermann N, Taupitz M, Baier M, Leismann O, Junkes A, Schumann C,
Hofmann WK, Schrezenmeier H. Results from a 1-year, open-label, single
arm, multi-center trial evaluating the efficacy and safety of oral
Deferasirox in patients diagnosed with low and int-1 risk
myelodysplastic syndrome (MDS) and transfusion-dependent iron overload.
Ann Hematol. 2013; 92: 191-8. https://doi.org/10.1007/s00277-012-1594-z PMid:23073603

- Gattermann
N, Jarisch A, Schlag R, Blumenstengel K, Goebeler M, Groschek M, Losem
C, Procaccianti M, Junkes A, Leismann O, Germing U. Deferasirox
treatment of iron-overloaded chelation-naïve and prechelated patients
with myelodysplastic syndromes in medical practice: Results from the
observational studies eXtend and eXjange. Eur J Haematol. 2012; 88:
260-8. https://doi.org/10.1111/j.1600-0609.2011.01726.x PMid:22023452 PMCid:PMC3505370

- Greenberg
PL, Koller CA, Cabantchik ZI, Warsi G, Glynos T, Paley C, Schiffer C.
Prospective assessment of effects on iron-overload parameters of
deferasirox therapy in patients with myelodysplastic syndromes. Leuk
Res. 2010; 34: 1560-5. https://doi.org/10.1016/j.leukres.2010.06.013 PMid:20615548

- Metzgeroth
G, Dinter D, Schultheis B, Dorn-Beineke A, Lutz K, Leismann O, Hehlmann
R, Hastka J. Deferasirox in MDS patients with transfusion-caused iron
overload - A phase-II study. Ann Hematol. 2009; 88: 301-10. https://doi.org/10.1007/s00277-008-0588-3 PMid:18758781

- Porter
J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Cappellini MD,
Olivieri N, Piga A, Cunningham MJ, Soulières D, Gattermann N, Tchernia
G, Maertens J, Giardina P, Kwiatkowski J, Quarta G, Jeng M, Forni GL,
Stadler M, Cario H, Debusscher L, Della Porta M, Cazzola M, Greenberg
P, Alimena G, Rabault B, Gathmann I, Ford JM, Alberti D, Rose C.
Relative response of patients with myelodysplastic syndromes and other
transfusion-dependent anaemias to deferasirox (ICL670): A 1-yr
prospective study. Eur J Haematol. 2008; 80: 168-76. PMid:18028431
PMCid:PMC2268958

- List
AF, Baer MR, Steensma DP, Raza A, Esposito J, Martinez-Lopez N, Paley
C, Feigert J, Besa E. Deferasirox reduces serum ferritin and labile
plasma iron in RBC transfusion-dependent patients with myelodysplastic
syndrome. J Clin Oncol. 2012; 30: 2134-9. https://doi.org/10.1200/JCO.2010.34.1222 PMid:22547607

- Gattermann
N, Finelli C, Porta MD, Fenaux P, Ganser A, Guerci-Bresler A, Schmid M,
Taylor K, Vassilieff D, Habr D, Domokos G, Roubert B, Rose C, EPIC
study investigators. Deferasirox in iron-overloaded patients with
transfusion-dependent myelodysplastic syndromes: Results from the large
1-year EPIC study. Leuk Res. 2010; 34:1143-50. https://doi.org/10.1016/j.leukres.2010.03.009 PMid:20451251

- Gattermann
N, Finelli C, Porta M Della, Fenaux P, Stadler M, Guerci-Bresler A,
Schmid M, Taylor K, Vassilieff D, Habr D, Marcellari A, Roubert B, Rose
C. Hematologic responses to deferasirox therapy in
transfusion-dependent patients with myelodysplastic syndromes.
Haematologica. 2012; 97: 1364-71. https://doi.org/10.3324/haematol.2011.048546 PMid:22419577 PMCid:PMC3436237

- Badawi
MA, Vickars LM, Chase JM, Leitch HA. Red blood cell transfusion
independence following the initiation of iron chelation therapy in
myelodysplastic syndrome. Adv Hematol. 2010;2010:164045. https://doi.org/10.1155/2010/164045 PMid:20368773 PMCid:PMC2846339

- Breccia
M, Finsinger P, Loglisci G, Federico V, Santopietro M, Colafigli G,
Petrucci L, Salaroli A, Serrao A, Latagliata R, Alimena G. Deferasirox
treatment for myelodysplastic syndromes: Real-life efficacy and
safety in a single-institution patient population. Ann Hematol. 2012;
91: 1345-9. https://doi.org/10.1007/s00277-012-1481-7 PMid:22569854

- Capalbo
S, Spinosa G, Franzese MG, Palumbo G. Early Deferasirox Treatment in a
Patient with Myelodysplastic Syndrome Results in a Long-Term Reduction
in Transfusion Requirements. Acta Haematol 2009; 121: 19-20. https://doi.org/10.1159/000209206 PMid:19287132

- Nishiuchi
T, Okutani Y, Fujita T, Yoshida K, Ohnishi H, Haba R. Effect of iron
chelator deferasirox on chronic anemia and thrombocytopenia in a
transfusion-dependent patient with myelodysplastic syndrome. Int J
Hematol 2010; 91: 333-5. https://doi.org/10.1007/s12185-010-0500-5 PMid:20127527

- Oliva
EN, Ronco F, Marino A, Alati C, Praticò G, Nobile F. Iron chelation
therapy associated with improvement of hematopoiesis in
transfusion-dependent patients. Transfusion. 2010; 50: 1568-70. https://doi.org/10.1111/j.1537-2995.2010.02617.x PMid:20230535

- Maurillo
L, Breccia M, Buccisano F, Voso MT, Niscola P, Trapè G, Tatarelli C,
D'Addosio A, Latagliata R, Fenu S, Piccioni AL, Fragasso A, Aloe
Spiriti MA, Refrigeri M, Criscuolo M, Musto P, Venditti A. Deferasirox
chelation therapy in patients with transfusion-dependent MDS: a
real-worl d report from two regional Italian registries: Gruppo
Romano Mielodisplasie and Registro Basilicata. Eur J Haematol. 2015; 95
:52-6. https://doi.org/10.1111/ejh.12476 PMid:25764148

- Improta
S, Villa MR, Volpe A, Lombardi A, Stiuso P, Cantore N, Mastrullo L.
Transfusion-dependent low-risk myelodysplastic patients receiving
deferasirox: Long-term follow-up. Oncol Lett 2013; 6:1 774-8.

- Rose
C, Fitoussi O, Gyan E, Hacini M, Amé S, Corront B, Beyne-Rauzy O, Adiko
DI, Loppinet EA, Ali-Ammar N, Wattel E, Dreyfus F, Cheze S. Prospective
Evaluation of the Effect of Deferasirox on Hematologic Response in
Transfusion-Dependent Patients with Low-Risk MDS and Iron Overload: The
Rythmex Study. Blood.2016;128:2008

- Lee
JW, Yoon S-S, Shen ZX, Ganser A, Hsu H-C, El-Ali A, Habr D, Martin N,
Porter JB. Hematologic responses in patients with aplastic anemia
treated with deferasirox: a post hoc analysis from the EPIC study.
Haematologica. 2013; 98: 1045-1048. https://doi.org/10.3324/haematol.2012.077669 PMid:23585526 PMCid:PMC3696607

- Angelucci
E, Santini V, Di Tucci AA, Quaresmini G, Finelli C, Volpe A, Quarta G,
Rivellini F, Sanpaolo G, Cilloni D, Salvi F, Caocci G, Molteni A,
Vallisa D, Voso MT, Fenu S, Borin L, Latte G, Alimena G, Storti S,
Piciocchi A, Fazi P, Vignetti M, Tura S. Deferasirox for
transfusion-dependent patients with myelodysplastic syndromes: safety,
efficacy, and beyond (GIMEMA MDS0306 Trial). Eur J Haematol 2014; 92:
527-36. https://doi.org/10.1111/ejh.12300 PMid:24580147

- Ghoti
H, Fibach E, Merkel D, Perez-Avraham G, Grisariu S, Rachmilewitz EA.
Changes in parameters of oxidative stress and free iron biomarkers
during treatment with deferasirox in iron-overloaded patients with
myelodysplastic syndromes. Haematologica. 2010: 1433-4. https://doi.org/10.3324/haematol.2010.024992 PMid:20421274 PMCid:PMC2913096

- Hartmann
J, Braulke F, Sinzig U, Wulf G, Maas JH, Konietschke F, Haase D. Iron
overload impairs proliferation of erythroid progenitors cells (BFU-E)
from patients with myelodysplastic syndromes. Leuk Res. 2013; 37:
327-32. https://doi.org/10.1016/j.leukres.2012.11.005 PMid:23259989

- Ren
X, Dorrington KL, Maxwell PH, Robbins P. Effects of desferrioxamine on
serum erythropoietin and ventilatory sensitivity to hypoxia in humans.
J Appl Physiol 2000; 89: 680-6. PMid:10926654

- Jaakkola
P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegshei
A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW,
Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau
ubiquitylation complex by O2-regulated prolyl hydroxylation. Science
2001;292: 468-72. https://doi.org/10.1126/science.1059796 PMid:11292861

- Musallam
KM, Cappellini MD, Wood JC, Taher AT. Iron overload in
non-transfusion-dependent thalassemia: A clinical perspective. Blood
Rev. 2012;26(SUPPL.1) S 16-9.

- Lyons
RM, Marek BJ, Paley C, Esposito J, Garbo L, DiBella N, Garcia-Manero G.
Comparison of 24-month outcomes in chelated and non-chelated lower-risk
patients with myelodysplastic syndromes in a prospective registry. Leuk
Res. 2014; 38: 149-54. https://doi.org/10.1016/j.leukres.2013.11.004 PMid:24314590

- Neukirchen
J, Fox F, Kündgen A, Nachtkamp K, Strupp C, Haas R, Germing U,
Gattermann N. Improved survival in MDS patients receiving iron
chelation therapy - A matched pair analysis of 188 patients from the
Düsseldorf MDS registry. Leuk Res. 2012;36:1067-70. https://doi.org/10.1016/j.leukres.2012.04.006 PMid:22564985

- Mainous
AG, Tanner RJ, Hulihan MM, Amaya M, Coates TD. The impact of chelation
therapy on survival in transfusional iron overload: a meta-analysis of
myelodysplastic syndrome. Br J Haematol 2014; 167: 720-3. https://doi.org/10.1111/bjh.13053 PMid:25048454 PMCid:PMC4991026

- Gattermann
N. Overview of guidelines on iron chelation therapy in patients with
myelodysplastic syndromes and transfusional iron overload. Int J
Hematol. 2008; 88: 24-9. https://doi.org/10.1007/s12185-008-0118-z PMid:18581200 PMCid:PMC2516534

- Bennett JM. Consensus statement on iron overload in myelodysplastic syndromes. Am J Hematol. 2008; 83: 858-61. https://doi.org/10.1002/ajh.21269 PMid:18767130

[TOP]

































































