Elisabetta Metafuni1, Rosaria Santangelo2, Patrizia Chiusolo1, Luca Laurenti1, Federica Sorà1, Sabrina Giammarco1 and Simona Sica1.
1 Hematology Department, Fondazione Policlinico Agostino Gemelli, Università Cattolica del Sacro Cuore, Rome, Italy.
2 Microbiology Department, Fondazione Policlinico Agostino Gemelli, Università Cattolica del Sacro Cuore, Rome, Italy.
Corresponding
author: Simona Sica, Hematology Department, Fondazione Policlinico
Agostino Gemelli, Università Cattolica del Sacro Cuore, Largo Agostino
Gemelli, 8 00168 Rome, Italy, Tel: +39 0630155300, Fax +39 063017319.
E-mail:
simona.sica@unicatt.it
Published: March 1, 2017
Received: December 19, 2016
Accepted: January 23, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017024 DOI
10.4084/MJHID.2017.024
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
Dear Editor,
We read with interest the paper by La Torre et al.,[1]
which conducted two systematic reviews and a meta-analysis to summarize
the results of scientific works about influenza and pneumococcal
vaccines in onco-hematological patients. We paid specific attention to
influenza vaccine results. The protection rate of H1N1 vaccine resulted
in 31% and 30% after the first dose and booster dose, respectively.
Protection rate of H3N2 and influenza B first dose was 42.6% and 39.6%,
respectively. Considering the response rate, the pooled prevalence for
H1N1 was 30% and 35% after the first dose and booster dose,
respectively. The response rate for H3N2 was 21.7% after first dose and
24% after booster dose. Finally, the response rate for influenza B was
23.6% after the first dose and 29% after booster dose. Only a minority
of patients experienced adverse events, most of them were mild and did
not require treatment. Even though influenza vaccine elicits a low
response rate among onco-hematological patients, particularly in those
who received transplantation, splenectomy or rituximab, it is an
inexpensive intervention with few side effects. However, the first
vaccine dose induces a small response, and the booster dose induces
additional antibodies. Therefore, it is recommended to vaccinate these
patients twice,[2] and the higher immunogenicity of adjuvated vaccines may be the way to improve response rate.[3] To further decrease influenza risk, also household members and care providers should receive vaccination.[4]
Patients
with hematological malignancies presented an increased susceptibility
to influenza because of their underlying disease or treatment
associated immunodeficiency.[5] Recently, we evaluated the trend of influenza cases in our center from 2011 to 2016 (Figure 1),
according to patients adherence to vaccine campaign. Our hematology
unit is composed of a section for non-transplant hematological patients
(22 beds), another section for autologous and allogeneic stem cell
transplantation (SCT) (8 beds) and an outpatient clinic. Visitors can
access to the unit only during visiting hours, one visitor at the time
for patients by wearing the mask. Moreover, in the transplant unit
visitor must wear mask, gown, headdress and gloves. Patients were
advised to receive influenza vaccine, in the influenza season,
available for risk categories by our Minister of Heath. In addition,
care workers and patients’ family members were strictly encouraged to
perform seasonal influenza vaccine to reduce possible sources of
infection both at home and in the hospital. Throughout the influenza
season, all patients were tested for influenza viruses at the admission
to the ward and in the case of symptoms development for hospitalized
patients and outpatients. We documented the first outbreak of H1N1
influenza in 2011, when, despite recommendations, the percentage of
general population submitted to vaccination in our region Lazio was of
18.9%.[6] Since that, fragile patients were strongly
advised to adhere to vaccination campaign that was entrusted to family
doctors and was focused on elderly, immunocompromised and chronic
diseases affected people. Because of a major percentage of vaccine
coverage, in the following years, few cases of influenza infections
were registered. Unfortunately, in the 2014-2015 season, we fell in a
broader A flu outbreak, most likely due to the lack of adherence of
patients, care workers and family members to influenza vaccination
program, with a fall in vaccine administration down to 14% in region
Lazio.[6] The incidence of influenza cases in Italy in
this season was the highest registered since 2009 pandemia, with upon
to 10.6 cases every 1000 observed in the central weeks of the season.[7]
In Italy, at least two episodes determined a drastic reduction of
adherence to vaccination campaign in the last years. In 2013 several
batches of influenza vaccines were temporarily taken off the market and
in 2014 an alert on sudden deaths after influenza vaccines had an
impressive echo on social media. Despite the prompt response of the
European Medicines Agency’s Pharmacovigilance Risk Assessment Committee
concluding that there was no evidence of an association between the
reported deaths and the vaccine, flu vaccination has decreased by 80%,
resulting in an estimated decrease of 25-30% on the overall 2014
national immunization campaign.[8]
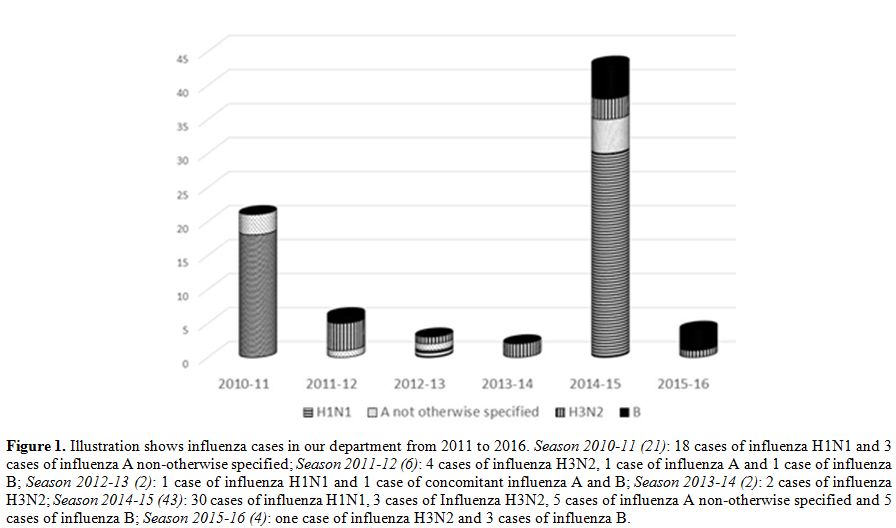 |
Figure 1. Illustration shows influenza cases in our department from 2011 to 2016. Season 2010-11 (21): 18 cases of influenza H1N1 and 3 cases of influenza A non-otherwise specified; Season2011-12 (6): 4 cases of influenza H3N2, 1 case of influenza A and 1 case of influenza B; Season 2012-13 (2): 1 case of influenza H1N1 and 1 case of concomitant influenza A and B; Season 2013-14 (2): 2 cases of influenza H3N2; Season2014-15 (43):
30 cases of influenza H1N1, 3 cases of Influenza H3N2, 5 cases of
influenza A non-otherwise specified and 5 cases of influenza B; Season2015-16 (4): one case of influenza H3N2 and 3 cases of influenza B. |
Between
December 2014 to March 2015 we diagnosed fourty-three cases of
influenza in 35 hospitalized patients and 8 outpatients: H1N1 in 30
cases (69.8%), influenza A not-otherwise specified in 5 cases (11.6%),
H3N2 in 3 cases (7%) and influenza B in 5 cases (11.6%). Only three
patients had received influenza vaccination, two of them developed
influenza B infection and another one H1N1 influenza with mild
symptoms. Patients’ characteristics as age, underlying disease and
status are reported in Table 1.
All outpatients were screened for influenza infection because of
respiratory symptoms development, while only 20 (57.1%) of the
hospitalized patients presented already symptoms at admission: asthenia
in one case, only fever in 5 cases, fever and diarrhea in one case,
cough in 6 cases, and both fever and cough in 7 cases. The others 15
patients developed respiratory symptoms during the hospital stay. The
overall median duration of hospitalization was 13 days (range, 2 to
90), and median time to swab positivization was 4 days (range, 1 to 27)
after admission. At the date of swab positivity, patients showed median
neutrophils and lymphocytes count of 1.89x109/L (range, 0.01 to 17.96) and 0.45x109/L
(range, 0.01 to 23.77), respectively. All of them received oseltamivir
administration within 24-48 hours from symptoms development.
Twenty-five patients (58.1%) presented abnormal radiological study
ranging from isolated small opacity to extensive and bilateral
pulmonary involvement with pleural effusion.
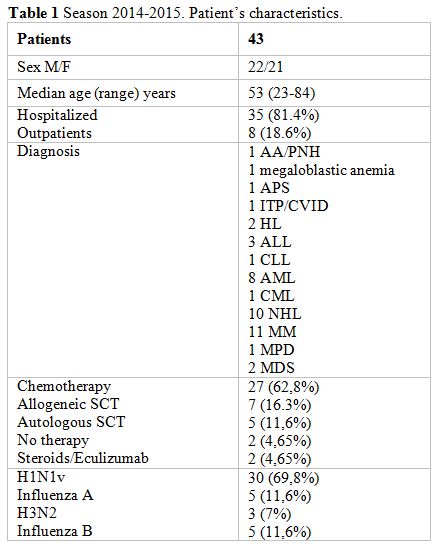 |
Table 1. Season 2014-2015. Patient’s characteristics. |
Seventeen
patients (39.5%) developed hypoxia requiring oxygen therapy. Seven
patients (41.2%) were supported with Venturi mask while three patients
(17.6%) required C-PAP mask support. The other seven patients (41.2%)
presented a severe respiratory impairment requiring orotracheal
intubation and were transferred in Intensive Care Unit. Two of them
also required extracorporeal membrane oxygenation (ECMO) support to
manage the severe acute respiratory distress, unfortunately
unsuccessfully. Cumulative mortality incidence in the study’s group was
23.3% (10/43), while it was 58.8% among the hypoxemic patients (10/17).
Death cause was attributable to a refractory and progressive
respiratory distress leading to pulmonary failure in a half of the
cases, while a multi organ failure (MOF) sepsis-driven was responsible
for death in the remaining cases.
The vast majority of influenza
cases among our patients during the 2014-2015 season were due to H1N1v
rather than H3N2, opposite to that reported by several authors for the
same season. Even if we thought that lack of vaccination was the main
responsible for this new influenza outbreak, we could not exclude a
virus neuraminidase inhibitor resistance conferred by amino acid
substitution.[9]
In order to limit the influenza
infection and nosocomial spread, our hospital organized a vaccination
planning for care workers, in ward patients and outpatients increasing
awareness and vaccination rate. Considering the results observed in the
last season, it seems that our effort was able to avoid a new outbreak
of influenza in our department.
Ackowledgements
This
study was supported by Centro di Ricerca sulle Cellule Staminali
Emopoietiche e le Terapie Cellulari, Università Cattolica del Sacro
Cuore in Rome.
References
- La Torre G, Mannocci A, Colamesta V, D'Egidio V,
Sestili C, Spadea A. Influenza and Pneumococcal Vaccination in
Hematological Malignancies: a Systematic Review of Efficacy,
Effectiveness, and Safety. Mediterr J Hematol Infect Dis. 2016 Sep
1;8(1):e2016044. https://doi.org/10.4084/MJHID.2016.044

- Ide
Y, Imamura Y, Ohfuji S, Fukushima W, Ide S, Tsutsumi C, Koga M, Maeda
K, Hirota Y. Immunogenicity of a monovalent influenza A(H1N1)pdm09
vaccine in patients with hematological malignancies. Hum Vaccin
Immunother. 2014;10(8):2387-94. https://doi.org/10.4161/hv.29094 PMid:25424946 PMCid:PMC4896784

- Cherif
H, Höglund M, Pauksens K. Adjuvanted influenza a (H1N1) 2009 vaccine in
patients with hematological diseases: good safety and immunogenicity
even in chemotherapy-treated patients. Eur J Haematol. 2013
May;90(5):413-9. Epub 2013 Mar 18. https://doi.org/10.1111/ejh.12094

- Engelhard
D, Zakay-Rones Z, Shapira MY, Resnick I, Averbuch D, Grisariu S, Dray
L, Djian E, Strauss-Liviatan N, Grotto I, Wolf DG, Or R. The humoral
immune response of hematopoietic stem cell transplantation recipients
to AS03-adjuvanted A/California/7/2009 (H1N1)v-like virus vaccine
during the 2009 pandemic. Vaccine. 2011 Feb 17;29(9):1777-82 https://doi.org/10.1016/j.vaccine.2010.12.113 PMid:21216315

- Lalayanni
C, Sirigou A, Iskas M, Smias C, Sakellari I, Anagnostopoulos A.
Outbreak of novel influenza A (H1N1) in an adult haematology department
and haematopoietic cell transplantation unit: clinical presentation and
outcome. J Infect. 2010;61(3):270-2. https://doi.org/10.1016/j.jinf.2010.06.013 PMid:20600296

- Vaccinazione antinfluenzale in Italia. Stagioni 2000-2001/2015-2016. Ministero della Salute. http://www.salute.gov.it/imgs/C_17_tavole_19_allegati_iitemAllegati_0_fileAllegati_itemFile_3_file.pdf
- Epicentro. Istituto Superiore di Sanità. Bollettino epidemiologico settimanale delle sindromi influenzali. 1/2015. http://www.epicentro.iss.it/problemi/influenza/FluNews/FluNews_2015-4.pdf
- Signorelli
C, Odone A, Conversano M, Bonanni P. Deaths after Fluad flu vaccine and
the epidemic of panic in Italy. BMJ. 2015;350:h116. https://doi.org/10.1136/bmj.h116 PMid:25589037

- Hurt
AC, Besselaar TG, Daniels RS, Ermetal B, Fry A, Gubareva L, Huang W,
Lackenby A, Lee RT, Lo J, Maurer-Stroh S, Nguyen HT, Pereyaslov D,
Rebelo-de-Andrade H, Siqueira MM, Takashita E, Tashiro M, Tilmanis D,
Wang D, Zhang W, Meijer A. Global update on the susceptibility of human
influenza viruses to neuraminidase inhibitors, 2014-2015. Antiviral
Res. 2016 Aug;132:178-85. https://doi.org/10.1016/j.antiviral.2016.06.001 PMid:27265623

[TOP]









