Maria Grazia Clemente1, Elena Dore1, Lidia Abis1, Paola Molicotti2, Stefania Zanetti2, Paolina Olmeo1 and Roberto Antonucci1
1 Pediatric Clinic, Department of Surgical, Microsurgical and Medical Sciences, University of Sassari, Sassari, Italy.
2 Department of Biomedical Science, University of Sassari, Sassari, Italy.
Corresponding
author: Maria Grazia Clemente, Clinica
Pediatrica, Viale San Pietro 12, 07100 Sassari, Italy. Tel. (+39)
079-228457; fax (+39) 079-228459; cell phone (+39) 3336900504. E-mail:
mgclemente@uniss.it
Published: April 15, 2017
Received: October 26, 2016
Accepted: March 27, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017027 DOI
10.4084/MJHID.2017.027
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objectives:
Migration flux is an increasing phenomenon in Italy, and it raises
several public health issues and concerns in pediatric infectious
diseases. This study investigated the clinical characteristics and
outcomes of a pediatric population at high-risk for tuberculosis (TB)
and the potential role of immigration as a risk factor.
Design:
We performed an observational retrospective study of children referred
to the only Pediatric Infectious Diseases Unit for Northern Sardinia
over a 6-year-period (2009-2014). Main variables assessed included TB
skin test (TST), confirmed by quantiFERON Gold in Tube test, thorax
X-ray (TX), microbiological culture, direct microscopy for acid-fast
bacilli and molecular assays.
Results:
Of the 246 children (mean age = 5.8 ± 3.9 years) identified, 222
(90.2%) were native to Sardinia and 24 (9.8%) were immigrants. The
majority of children (n=205; 83%) were TB-exposed but not infected
based on a negative TST and TX. Among the TST positive group (n= 39;
16%), 19 (49%) had latent TB (TX negative), while 20 (51%) had active
TB (TX positive). The percent of TST positive children was
significantly higher in the immigrant than the native group (42.5%
versus 14%, p<0.001). Clinical presentations included pulmonary
involvement with hilar lymphadenopathy (72%), pleurisy (13,5%),
lateral-cervical lymphadenopathy (9%), pneumonia with calcifications
(4.5%) and disseminated TB (4.5%). One child had multidrug-resistant
tuberculosis.
Conclusions:
Pediatric TB represents a relevant and potentially worsening public
health problem in Northern Sardinia. A strict surveillance system and
appropriate treatment can prevent the most severe forms and reduce TB
transmission.
|
Introduction
Over
the past two decades, migration and the phenomenon of “boat migration”
to Italy and the island of Sardinia1 have progressively increased,
causing significant demographical changes. From 2009 to 2014, the
Italian National Centre for Statistics (ISTAT) recorded the arrival of
about 20.000 new immigrants to the island, of whom roughly one-third
reside in the Northern provinces. Upon arrival, migrants undergo
medical assistance and evaluation, especially for infectious
diseases.[1]
According to the Italian government guidelines,
migrants coming from high tuberculosis (TB) endemic countries receive
special care for potential Mycobacterium tuberculosis (MT)
infection.[1-2] About one-third of the population worldwide encounters
MT during their lifespan, remaining asymptomatic, a condition termed
latent tuberculosis infection (LTBI).[3,4] However, 5 to 10% of the
infected subjects are at risk of developing clinically evident TB, in
part depending on their immune system status. In fact, children aged
less than 5 years who are co-infected with HIV or treated with
immunosuppressants for other diseases are considered at higher risk for
TB.[3-5] Among children less than 5 years, those less than 2 years are
at even greater risk of severe disseminated TB, including pulmonary
miliary TB and meningitis. The diagnosis of LTBI is made when the
tuberculosis skin test (TST) is positive in subjects who are not
vaccinated and in the absence of any other clinical and/or radiological
evidence for active TB. Subjects with LTBI harbor an inactive form of
MT. Adequate prophylaxis with isoniazid in LTBI subjects is able to
reduce if not eliminate the risk of active disease.[3,4] However, the
distinction between latent and active TB may be at times particularly
challenging, leading to the concept of a “pediatric TB spectrum”
ranging from asymptomatic to lethal TB.[5-7] Children with positive TST
but negative chest X-ray (TX) have been recently found to have active
TB by microbiological analysis that was confirmed by chest CT scan.[6]
As a consequence of this complexity, the “Paediatric Tuberculosis
Network European Trials group” has recently proposed to replace LTBI
with the new definition of “TB with a positive immunological test
result [TST and/or interferon-gamma release assay (IGRA)] in the
absence of active disease”, in which the word “latent” is omitted. [5]
Data
recently published by the TB surveillance and monitoring in 29 European
States for the year 2012, have shown that 2,845 (4%) out of the total
68,423 cases of TB were found in children.[7] These data show a
decrease of 2% compared to the year 2011.[8]
According to the
World Health Organization, Italy is one of the countries with a low
incidence of TB.[8] However, the progressive reduction of surveillance
measures along with the increasing number of immigrants coming from
countries at high TB incidence are reasons for the recent alarming
reports of a reemerging TB in developed countries.[8]
In Italy,
inter-regional differences exist. A recent study on the prevalence of
LTBI among 733 healthcare students in Genoa reported a prevalence as
low as 1.4%, with migrants coming from a geographical area at high
incidence of TB being those most commonly affected.[9] Differently, in
Tuscany, the study of a large series of children with TB (n=484),
almost half of whom were immigrants, has found no difference between
native and immigrant youth.[10] This study has also reported an
increase in pediatric TB incidence in Tuscany, where children less than
5 years were at high risk of severe TB.[10]
The proportion of TB
affected children in Sardinia, and the impact of the migration flux
from TB endemic countries is unknown. Our study is the first report on
the clinical characteristics and outcomes of pediatric TB in Northern
Sardinia, assessed by TST, IGRA, TX, microbiological culture, direct
microscopy for acid-fast bacilli and molecular assays.
Study Population and Methods
We
performed an observational retrospective study relative to the
6-year-period 2009-2014 by reviewing clinical records of a TB high-risk
pediatric population at the Pediatric Infectious Diseases Unit of the
Pediatric Clinic, University of Sassari, Italy. This is the only
referral Center for pediatric TB in the Northern Sardinia and is
responsible for the evaluation of children referred from the Public
Health Service because of household or occasional contacts of TB index
cases. Less commonly, symptomatic children come to the Center for a
general clinical evaluation; in these cases, it is not always possible
to identify the index case. Both de novo active TB children and
children identified because of close or occasional contacts with TB
index cases were included in the present study. We classified as native
children those born from both native parents, while as immigrant
children those with at least one foreign-born parent.
For the
purpose of this study, any child referred because of
household/occasional contact or affected by active TB underwent a
personal and family history collection, complete physical examination,
TB skin test (TST), interferon-gamma release assay (IGRA) and a thorax
X-ray (TX).[10-11] The TST (Biocine Test PPD; Chiron, Siena, Italy) was
considered positive when producing a diameter ≥ 5 mm at 48 to 72 hours.
Interferon-gamma release assay (IGRA).
The IGRA assay was the quantiFERON Gold in Tube test (QFT). One mL of
blood sample was added into three QFT tubes containing either TB
antigens ESAT-6, CFP-10 and TB7.7, a positive control (mitogen) or a
negative control (Nil). After 16-24 hours incubation at 37°C, plasma IFN-γ
concentration was measured by ELISA. QFT results were scored as
indicated by the manufacturer (cut-off value for a positive test was
≥0.35 IU/ml).[11-12]
Microbiological diagnosis of tuberculosis.
To confirm active TB, standard microbiological culture and molecular
genetic assays were performed on patients’ biological samples as
previously described.[11-12] Gastric aspirates were collected from
children who were TST and/or IGRA positive. Staining and microscopy,
nested polymerase chain reaction (PCR) and culture tests were performed
as previously described.[11-12] All clinical samples were stained with
Zielh-Neelsen and inoculated into liquid and solid medium (MGIT 960 and
Lowenstein-Jensen respectively). Molecular tests were carried out using
the Dx MTB Assay (Bio-Rad), a Real-time PCR that targets the IS6110
element and the RD9 specific region. DNA was extracted using a
chelating ion-exchange resin (InstaGene matrix; Bio-Rad, Hercules, CA)
and amplified according to the manufacturer's instructions.
Patient management.
Depending on the absence/presence of clinical symptoms, TST/IGRA and TX
results, patients were classified into three standard categories of (1)
exposed but not infected (asymptomatic, negative to both TST and TX),
(2) LTBI (asymptomatic, TST positive but TX negative) and (3) active TB
(symptomatic, TX positive or negative, TST positive or negative).
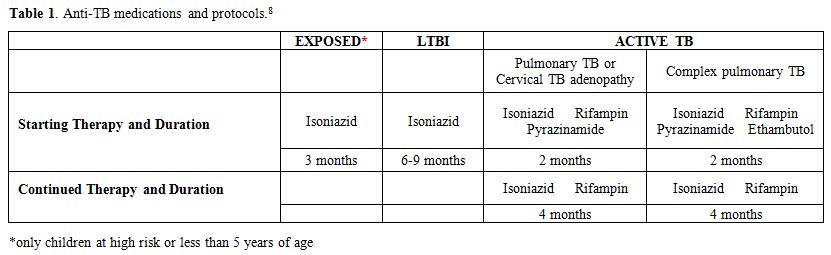
The exposed but not infected children did not receive any treatment but were re-tested for TST and IGRA three months later (Table 1).
In accordance with WHO global guidelines,[4] children in this category
who were less than 5 years or at risk for developing active TB (e.g,,
children in close contact with an index case of TB) received isoniazid
at the dosage of 10 mg/kg/day for 3 months before being re-tested (Table 1). Isoniazid was discontinued if both tests were still negative at the second evaluation.
 |
Table
1. Anti-TB medications and protocols.[8] |
All children who were LTBI received isoniazid prophylaxis for 9 months (Table 1),
while all children with active TB underwent a further investigation to
confirm the diagnosis. In children with active TB, a DNA fingerprinting
assay was performed according to standard protocols.[11-12] Children
affected by active TB received the conventional anti-tubercular
therapy[13] (Table 1).
Treatment for TB lateral-cervical adenopathy was both medical and
surgical when indicated. The surgical excision of the affected nodes
was used for both diagnostic (microbiological analysis) and therapeutic
purposes.
Statistical analysis. Statistical comparisons between groups were performed with the χ2 test or the Fisher’s exact test when indicated. A p-value < 0.05 was considered to be statistically significant.
Results
Over
the 6 years period of study, a total of 246 children (0-14 years; mean
age = 5.8 ± 3.9 years; M: F=1:1) were observed at our unit, among whom
222 (90.2%) were native Sardinians, and 24 (9.8%) were immigrants.
As shown in Figure 1,
about 2/3 (67%) of the immigrants arrived in Sardinia more recently,
during the years 2013 and 2014, confirming the reported trend towards a
progressive increase in the number of immigrants from countries where
TB is endemic, including East Europe and Africa.
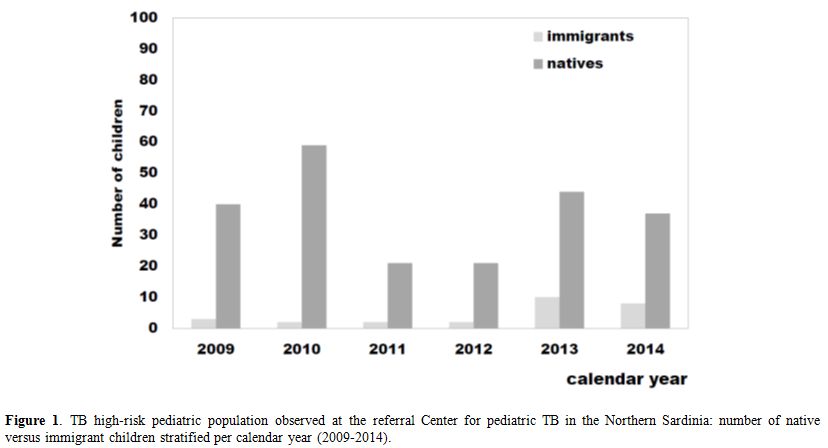 |
Figure 1. TB high-risk pediatric
population observed at the referral Center for pediatric TB in the
Northern Sardinia: number of native versus immigrant children
stratified per calendar year (2009-2014). |
The
study population included 111 (45.1%) children who were less than 5
years old, 117 (47.5%) 5-12 years old, and 18 (7.3%) over 12 years old.
In our cohort, the majority of children (n=205; 83.3%) were found to be
exposed to TB but not infected, 19 (7.7%) had evidence for LTBI, and 22
children (8.9%) had active TB (Table 2 and Figure 2).
As our study involved a high-risk population within institutional
settings, a higher prevalence of active TB was found compared to LTBI.
The
exposed but not infected group consisted of 145 children (71%) who were
not treated and 60 (29%) children who were at risk for developing TB
and received isoniazid prophylaxis (Table 2 and Figure 2). Of those children, 53 (25.8%) were native Sardinians, and 7 (3.4%) were immigrants.
Among
the 39 children who were TST-positive, 19 (49%) were diagnosed with
LTBI because of negative TX and asymptomatic, while 20 (51%) showed
active TB (Table 2 and Figure 2).
Interestingly, of children who were TST positive, significantly higher
proportions were immigrants compared to native Sardinians (42.5% versus
14%, p < 0.001), and there was a trend for immigrant children to
have active TB (12.5% vs. 9%, p = 0.050).
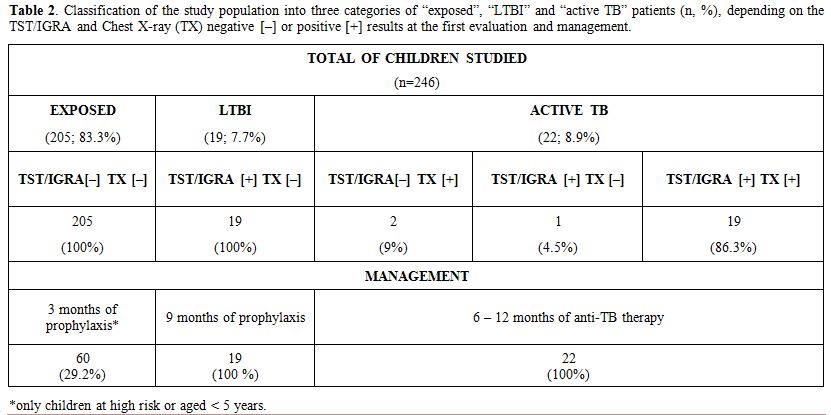 |
Table 2.
Classification of the study population into three categories of
“exposed”, “LTBI” and “active TB” patients (n, %), depending on the
TST/IGRA and Chest X-ray (TX) negative [–] or positive [+] results at
the first evaluation and management. |
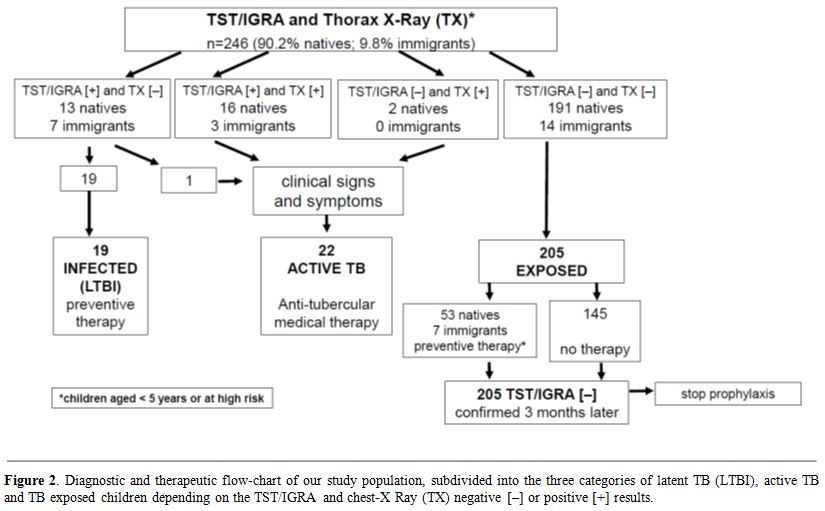 |
Figure 2. Diagnostic and therapeutic
flow-chart of our study population, subdivided into the three
categories of latent TB (LTBI), active TB and TB exposed children
depending on the TST/IGRA and chest-X Ray (TX) negative [–] or positive
[+] results. |
In the LTBI group of
19 children, all but four were TST and IGRA positive. Of these, three
(16%; 5, 9 and 11 years old) were TST and IGRA negative, and one (5%;
12 years old) was TST negative but IGRA positive.
Among the 22
children with active TB, 10 (45.4%) children were less than 5 years
old, 10 (45.4%) were 5-12 years old, and two (9%) were 14 years old.
Half of all TB diagnoses (11/22; 50%) were made in 2009, 5/22 (22%) in
2013, 3/22 (13.6%) in 2010 and 2014 and none in 2011 and 2012. All but
three children were TST and IGRA positive. Two of them (9%) tested
negative at the first TST and IGRA evaluation (Table 3).
One of these was a 9-year old boy under immunosuppressive treatment for
juvenile idiopathic arthritis, who underwent TB investigation because
of fever and cough. At the initial evaluation, TST and IGRA were
negative, but the TX and the culture of gastric aspirate were
positives, allowing the diagnosis of active TB (Table 3).
Three months later, at the second evaluation, TST and IGRA turned
positive. The second patient was a 2-year old girl with a few months
history of fever of unknown origin, lack of appetite and irritability.
Because of the initial negative laboratory tests, the diagnosis of TB
went unrecognized until she presented with the severe clinical picture
of disseminated TB, including pulmonary miliary, meningitis, bone and
renal involvement (Table 3, Figure 3).
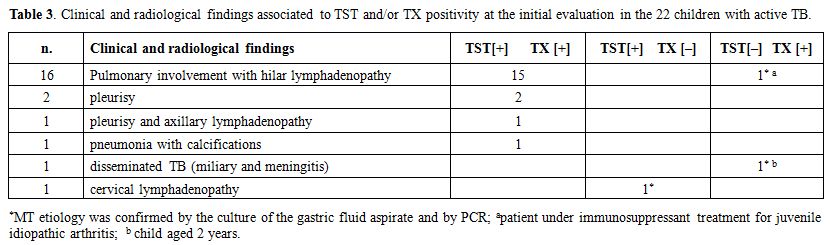 |
Table 3.
Clinical and radiological findings associated to TST and/or TX
positivity at the initial evaluation in the 22 children with active TB. |
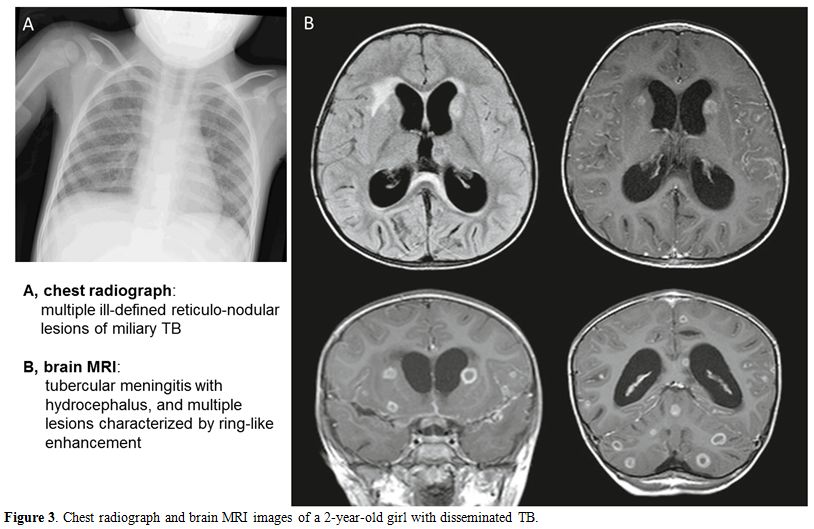 |
Figure 3. Chest radiograph and brain MRI images of a 2-year-old girl with disseminated TB. |
In
the 22 children with active TB, pulmonary involvement with hilar
lymphadenopathy was the most common clinical manifestation (n=16
patients, 72%), followed by pleurisy in two patients (13,6%), and
one patient each (4.5%) had pleurisy and axillary lymphadenopathy,
pneumonia with calcifications, cervical lymphadenopathy, or
disseminated TB (miliary and meningitis) (Table 3, Figures 3-5).
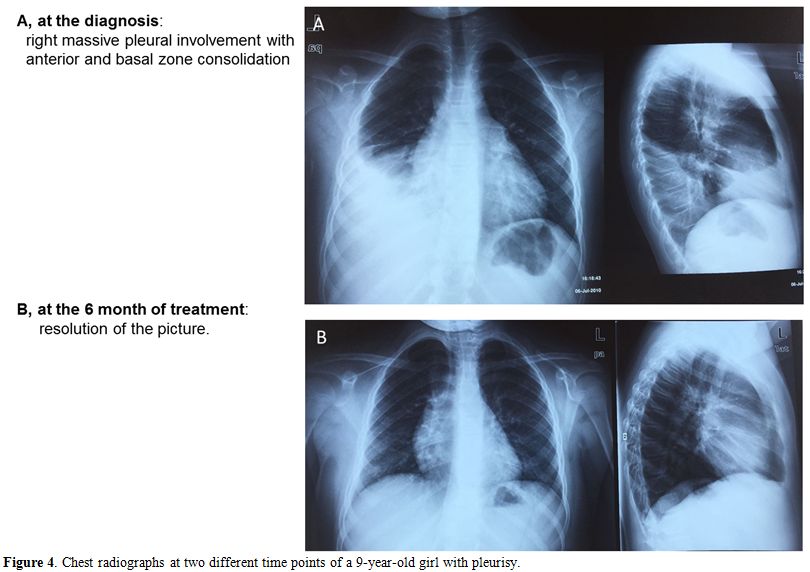 |
Figure 4.
Chest radiographs at two different time points of a 9-year-old girl with pleurisy. |
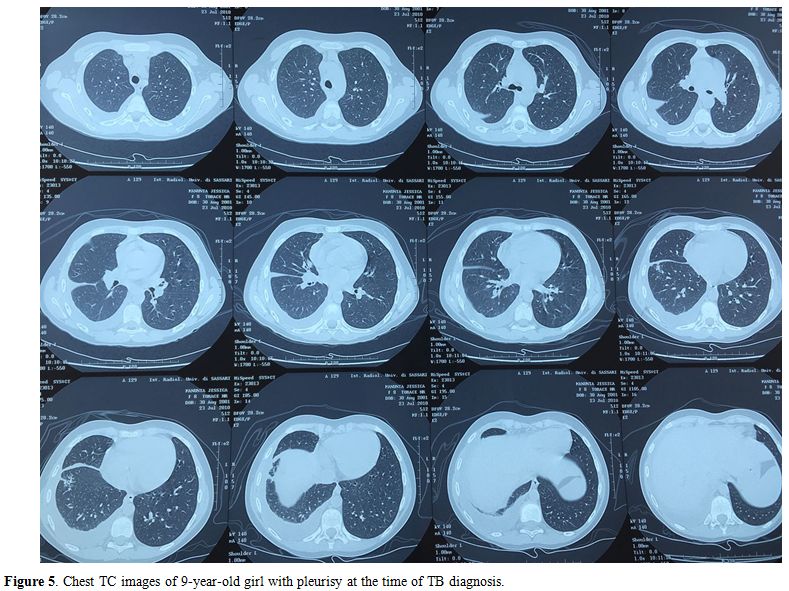 |
Figure 5. Chest TC images of 9-year-old girl with pleurisy at the time of TB diagnosis. |
In one of two
patients who presented with adenopathy as extra-pulmonary TB, the
initial ultrasound imaging revealed cervical lymphoadenopathy with a
concomitant involvement of the parotid gland, which is a known
contraindication for surgical treatment (potential risk of causing VII
cranial nerve lesion). This patient was a 3-year-old girl in whom the
MT etiology was confirmed by culture of the gastric fluid aspirate and
by the PCR of the caseous material collected from an external skin
fistula that was drained from the colliquative node. The second patient
who developed axillary adenopathy as secondary MDR-TB was an 8-year-old
boy with Down syndrome, who was initially affected with tubercular
pleurisy. According to the conventional therapy protocol, this child
received treatment with rifampin, isoniazid, ethambutol and
pyrazinamide for 18 months. At the fifth month of follow-up, he
presented with axillary lymphoadenopathy. The culture of surgically
excised lymphonodal tissue revealed multi-drug resistant MT.[13,14]
Anti-TB treatment with amikacin, ethionamide, levofloxacin, and
pyrimethamine was successful, with complete recovery after one year of
therapy.
Among the children who were symptomatic when they came to
the Center, it was possible to identify the index case only in 6
children (50%), while the identification of the TB contact preceded the
diagnosis of TB in all the 10 children referred to the Center by the
Public Health Service. When known, the index case was an adult in all
but one case (a school companion). In the majority (9/16 children), it
was one of the parents, mostly the father, followed by an uncle in four
cases, a grandparent in one, and a neighbor in another one. Discussion
Recent
data on pediatric TB indicate that immigration is an important risk
factor for TB in countries where the incidence of TB is low.[15-17]
Our study is the first report on pediatric TB in Northern Sardinia,
where the native children represented the large majority (9:1 ratio).
However, immigrant children showed a trend toward a higher proportion
of both TST positivity and active TB, even though the latter did not
reach statistical significance likely due to the small sample size.
Interestingly, the rate of immigrant children undergoing isoniazid
prophylaxis was three-fold higher than that of native children. This is
perhaps not surprising as these immigrant children were from Eastern
Europe (i.e. Romania) and Africa (i.e. Morocco), areas where TB is
endemic. For this reason, we argue that increased TB surveillance is
necessary as the “boat migration” phenomenon is progressively
increasing in Sardinia as well as in other European nations.
The
results of our study showed a strong concordance between TST and IGRA
tests, which appears to be not affected by a younger age. The higher
percentage of discordant tests was noted among LTBI cases that
presented with negative IGRA in spite of a positive TST (3 patients).
All this suggests the utility of performing both the tests in all
children, as it has been previously reported.[18]
The
clinical study revealed that hilar lymphoadenopathy with pneumonia is
the most common manifestation of TB in children. These were mostly the
children referred because of household or occasional contacts with
index cases. On the other hand, children in our study with the most
advanced and severe TB manifestations were exclusively those with de
novo diagnosis, confirming that contact tracing plays a key role in
limiting the consequence of TB in children.
In addition, it
important to note that of the children who received isoniazid
prophylaxis, none developed active TB. At least one-third of these
children were immigrants. Thus, pediatric TB prevention assumes a
priority in public health.
Conclusions
In conclusion, our data show that:
• pediatric TB is a public health issue in Northern Sardinia;
• immigrant children have a high rate of LTBI;
• the
progressively increasing “boat migration” phenomenon requires immediate
action on the TB surveillance level in current low incidence countries;
• TB
diagnosis cannot be excluded in children with initial negative TST and
IGRA and in whom other diseases have been ruled out by appropriate
investigations; a delay in TB diagnosis and treatment is potentially
fatal;
• the presence of MDR-TB should always be expected, especially in children living in high-risk families;
•
the household or occasional contacts of index cases referred by
the local Public Health System significantly benefit from earlier
diagnosis and treatment compared to the ex-novo active TB children.
Author Contributions
The
first two authors (Maria Grazia Clemente and Elena Dore) contributed
equally and wrote the first draft of the paper. Lidia Abis and Paolina
Olmeo performed the clinical study. Paola Molicotti and Stefania
Zanetti were responsible for the microbiological culturing and
molecular genetic assays for MT detection. Paolina Olmeo and Roberto
Antonucci were co-senior authors.
Acknowledgements
We are grateful to
Professor Giovanni Fadda for advice and helpful discussion and to Dr.
Mark Soloski and Mary Blue for assistance with writing in the English
language. This work was partially supported by the Autonomous Region of
Sardinia.References
- Italian Ministry of Health. Guidelines for the
control of tuberculosis on the proposal of the Minister of Health under
Article 115, paragraph 1, letter b of Legislative Decree 31, March
1998, no. 112.
- Bua
A, Cubeddu M, Piras D, Delogu R, Zanetti S, Molicotti P. Tuberculosis
screening among asylum seekers in Sardinia. J Public Health (Oxf). 2016
Jan 24. pii: fdv215. [Epub ahead of print]

- Abubakar
I, Griffiths C, Ormerod P. Diagnosis of active and latent tuberculosis:
summary of NICE guidance. BMJ 2012; 345: e6828.

- WHO. Global tuberculosis report 2016. World Health Organization, Geneva; 2016 http://www.who.int/tb/publications/global_report/en/

- Tebruegge
M, Salo E, Ritz N, Kampmann B. On Behalf Of The Paediatric Tuberculosis
Network European Trialsgroup Ptbnet (2013) Inclusion of latent
tuberculosis infection as a separate entity into the international
classification of diseases. Thorax 68: 588. thoraxjnl-2012-202824. https://doi.org/10.1136/thoraxjnl-2012-202824

- Buonsenso
D, Sali M, Focarelli B, Onesimo R, Palucci I, Delogu G, et al. The
tuberculosis spectrum: Translating basic research into pediatric
clinical practice. Med Hypotheses. 2015 Oct 28. pii:
S0306-9877(15)00410-7.

- Sali
M, Buonsenso D, Goletti D, D'Alfonso P, Zumbo A, Fadda G, et al.
Accuracy of QuantiFERON-TB Gold Test for Tuberculosis Diagnosis in
Children. PLoS One. 2015 Oct 6;10(10):e0138952. doi:
10.1371/journal.pone.0138952. https://doi.org/10.1371/journal.pone.0138952

- Eurosurveillance
Editorial Team. ECDC and WHO/Europe joint report on tuberculosis
surveillance and monitoring in Europe. Euro Surveill. 20140;19(11).
pii: 20741.

- Durando
P, Alicino C, Orsi A, Barberis I, Paganino C, Dini G, et al. Latent
tuberculosis infection among a large cohort of medical students at a
teaching hospital in Italy. Biomed Res Int. 2015;2015:746895. https://doi.org/10.1155/2015/746895 PMid:25705685 PMCid:PMC4331323

- Chiappini
E, Bonsignori F, Orlandini E, Sollai S, Venturini E, Galli L, de
Martino M. Increasing incidence of tuberculosis in Tuscan youth, 1997
to 2011. Pediatr Infect Dis J. 2013; 32(11):1289-91 https://doi.org/10.1097/INF.0b013e31829e7d81 PMid:24141802

- Bua
A, Molicotti P, Cannas S, Ruggeri M, Olmeo P, Zanetti S. Tuberculin
skin test and QuantiFERON in children. New Microbiol. 2013;36
(2):153-6. PMid:23686121

- Molicotti
P., Bua A., Mela G., Olmeo P., Ortu S., Sechi L.A., et al. Performance
of Quantiferon TB testing in a outbrak at a primary school J. Pediatr.
2008; 152 (4): 585-86 https://doi.org/10.1016/j.jpeds.2007.12.014 PMid:18346520

- Graham SM. Treatment of paediatric TB: revised WHO guidelines. Paediatr Respir Rev. 2011;12 (1):22-6. https://doi.org/10.1016/j.prrv.2010.09.005 PMid:21172671

- Santiago
B, Baquero-Artigao F, Mejías A, Blázquez D, Jiménez MS, Mellado-Pe-a
MJ; EREMITA Study Group. Pediatric drug-resistant tuberculosis in
Madrid: family matters. Pediatr Infect Dis J. 2014;33 (4):345-50. https://doi.org/10.1097/INF.0000000000000111 PMid:24622395

- Pang
J, Teeter LD, Katz DJ, Davidow AL, Miranda W, Wall K, et al.
Tuberculosis Epidemiologic Studies Consortium. Epidemiology of
tuberculosis in young children in the United States. Pediatrics.
2014;133(3):e494-504. https://doi.org/10.1542/peds.2013-2570 PMid:24515517 PMCid:PMC5135007

- Durando
P, Sotgiu G, Spigno F, Piccinini M, Mazzarello G, Viscoli C, et al.
Latent tuberculosis infection and associated risk factors among
undergraduate healthcare students in Italy: a cross-sectional study.
BMC Infect Dis. 2013;13:443 https://doi.org/10.1186/1471-2334-13-443 PMid:24059355 PMCid:PMC3848912

- Galli
L, Lancella L, Tersigni C, Venturini E, Chiappini E, Bergamini BM, et
al. Pediatric Tuberculosis in Italian Children: Epidemiological and
Clinical Data from the Italian Register of Pediatric Tuberculosis. Int
J Mol Sci. 2016 Jun 17;17(6). pii: E960. doi: 10.3390/ijms17060960. https://doi.org/10.3390/ijms17060960

- Garazzino
S, Galli L, Chiappini E, Pinon M, Bergamini BM, Cazzato S, et al.;
SITIP IGRA Study Group. Performance of interferon-? release assay for
the diagnosis of active or latent tuberculosis in children in the first
2 years of age: a multicenter study of the Italian Society of Pediatric
Infectious Diseases. Pediatr Infect Dis J. 2014 Sep;33(9):e226-31. https://doi.org/10.1097/INF.0000000000000353 PMid:25361032

[TOP]























