Andrea Gallamini1 and Anna Borra2
1 Research, innovation and statistics department - A Lacassagne Cancer Centre – Nice, France.
2 Haemato-oncology department- A Lacassagne Cancer Centre – Nice, France.
Corresponding
author: Prof. Andrea Gallamini. Research, Innovation and Statistics
Department. Lacassagne Cancer Center 33, Rue de Valombrose. 06129 -
Nice, France.
andreagallamini@gmail.com
Published: April 15, 2017
Received: September 11, 2016
Accepted: March 3, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017029 DOI
10.4084/MJHID.2017.029
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
In
the present review, the reader will be led to the most relevant
observations that prompted oncologists and haematologist to consider
FDG-PET/CT as a new paradigm for FL management in clinical practice.
The role of functional imaging in lymphoma staging, restaging,
prognostication, and metabolic tumour volume computing will be reviewed
in detail. Moreover, a special focus will be addressed to technical and
practical aspects of PET scan reporting, which have been set during the
last decade to ensure the reproducibility of the therapeutic results.
Finally, the predictive role of PET/CT on long-term treatment outcome
will be compared with another well-known prognosticator as minimal
residual disease (MRD) detection by Immunoglobulin gene rearrangement
assessment.
|
Introduction
Follicular
Lymphoma (FL) in the second most common lymphoma subtype accounting for
nearly one-quarter of all the non-Hodgkin lymphomas (NHL) in western
countries.[1-2] Despite the remarkable progress in
long-term disease control, nearly 20% of patients affected by this
lymphoma entity ultimately experience treatment failure and disease
progression within 2 years from diagnosis, with a 5-year overall
survival (OS) of only 50%.[3] Attempts to identify
these high-risk patients at diagnosis by the existing prognostic
indexes such as the Follicular Lymphoma International Prognostic Index
(FLIPI)[4] or FLIPI-2,[5] or
conventional radiological assessment of treatment outcome have
partially failed. Quite recently, a number of scientific reports
focusing on the role of 18F-Fluoro-deoxy-glucose positron emission
tomography (FDG-PET) combined with computed tomography (CT) in FL
staging, restaging and prognostication prompted clinicians and imaging
experts to reconsider the use of FDG-PET/CT (PET) in this disorder. In
lymphoma staging, PET provided information on the extra-nodal
involvement of the tumour at disease onset as well as on the
heterogeneity of clonal mechanisms underlying tumour spread and
aggressiveness. New insights on early detection of tumour
transformation to diffuse large B-cell lymphoma and practical
guidelines on how to detect it have also been achieved thanks to a
systematic use of PET in the for staging workup. The formal indication
on PET use in FL came from the recently published Lugano
recommendations for FDG-PET scan use for lymphoma staging and
restaging, clearly stating that functional imaging with FDG-PET is the
diagnostic standard tool for tumour burden assessment and treatment
response evaluation in several FDG-avid lymphomas, including FL.[6-7]
PET scan performed at the end of therapy resulted in the only factor
predictive of long-term disease control and overall survival in
prospective multicentre clinical trials, and proved able to identify a
fraction of patients (nearly 25%) with a particularly dismal prognosis.
Quite recently, end-of-treatment PET scan has been compared to minimal
residual disease detection by molecular biology in predicting long-term
treatment outcome in FL. These studies showed that PET is able to image
FL independently from the heterogeneity of the neoplastic clone, which
could be missed by molecular biology technique. The latter, in fact,
can precisely detect the single clonal disorder against which the
molecular probe has been constructed but not the entire tumour burden
when FL underwent a clonal evolution. The important information
generated by PET results could in future allow clinicians to
personalize treatment in FL maximizing the treatment efficacy and
reducing the cost of maintenance treatment for patients whose disease
could be controlled by standard immunochemotherapy
treatment.
FDG-PET PET for FL Staging
FL is a lymphoma subset that proved FDG-avid in more than 95% of the cases.[8-10] Despite this high affinity, its use in FL for baseline staging in clinical practice became standard only in 2014.[6-7]
Several head-to-head studies reported a higher sensitivity of
FDG-PET/CT for FL staging compared to contrast-enhanced CT scan
(Ce/CT).[6-7] In a cohort of 45 FL patients, CeCT ad
PET/CT were performed in sequence for staging purpose: PET/CT detected
more nodal (+51%), and extranodal (+89%) lesions than Ce/CT; five
patients (11%) in early stage (I and II) by CeCT were upstaged to stage
III or IV by PET/CT.[11] The overall accuracy of
PET-CT and CeCT for tumour spread detection was 97% and 64%,
respectively. The most frequently detected extra-nodal sites by PET/CT
were bone marrow (13 Vs. 2) and spleen (11 Vs. 6). In a larger cohort
of 142 FL patients prospectively enrolled in the Italian Foll-5
clinical trial, Luminari et al retrospectively reassessed the role of
FDG-PET/CT and CeCT in the baseline staging.[12]
FDG-PET allowed the identification of more nodal areas than CeCT in 32%
of the patients and of 60 extranodal sites (ENS) in 47 patients. The
most frequently discovered new ENS was bone/bone marrow (34), spleen
(26), skin (12) and gastrointestinal tract (9). Interestingly, PET
staging modified also the FLIPI score, and the latter increased in 18%
of the patients and decreased in 6%. Finally, FDG-PET was able to
upstage as much as 62% of the patients in early stage (I and II) by
CeCT. As a matter of fact, bone marrow involvement (BMI) is the
most frequently detected ENS in baseline FL staging.[11-13]
Initial reports pointed towards a low sensitivity in detecting BMI by
PET/CT: in the study by Le Dorz comparing CeCT Vs. FDG-PET/CT for
initial staging in a cohort of 45 FL patients, PET detected 13 cases
(29%) of BMI, 11 of them not detected by CeCT: 5 with a diffuse and 8
with a focal pattern of FDG uptake. Bone marrow trephine biopsy (BMB)
was positive in all patients with diffuse uptake and in only 3 out of 8
with a focal uptake.[11] In another retrospective
study on a cohort of 64 FL patients, Wohrer et al. again found that the
most frequent ENS involved by lymphoma was the bone marrow, with a
pattern of FDG uptake suggestive of BMI in 13 out of 24 (56%) with BMI
by CeCT + BMB; nine had a diffuse uptake (all with a positive BMB) and
four a focal FDG uptake (all with a negative BMB). However, in the
remaining eleven patients with a positive BMB PET scan showed and
“indeterminate” pattern of FDG uptake. Overall, the sensitivity of
FDG-PET in detecting BMI was 54%.[13] In this pioneer
study, however, some degree of diffuse FDG uptake could be observed in
patients with a formally “negative” PET scan, prompting the Author’s
claim that a more sensitive threshold to detect an abnormal FDG uptake
in BM could be able to pick-up all the cases with a BMB-proven BMI and
a diffuse tracer uptake. This concept has been validated by a recently
published study by Perry et al.[14] In a
retrospective, single centre study of a series of 68 FL patients,
evidence of BMI by FDG-PET/CT imaging was recorded in 16 patients
(23.5%), 13 of them with a positive BMB. All the 8 patients with focal
and 5/8 with a diffuse FDG uptake had a positive BMB. Three patients
had a diffuse uptake, which disappeared with treatment and a positive
BMB. On the other hand, a diffuse “unspecific” FDG uptake was observed
in 17 patients (32.7%) with a negative BMB. As a consequence, BMI
detected by the visual assessment of FDG-PET had a very high Negative
Predictive Value (NPV): 100% and a disappointingly low Positive
Predictive Value (PPV) of 48.5%. By contrast, upon a quantitative
assessment of PET resulting by Standardized uptake value (SUV) a SUVmean
value < than 1.7 or higher of 2.7 as able to distinguish patients
with a non-invaded BM from those with a “true” BMI, showing a
sensitivity and specificity of 100% in both cases. Out of 20 cases
showing an “intermediate” SUVmean value between 1.7 and 2.7 only 5 had a biopsy-proven BMI.[14]
A particular interest of functional imaging in the baseline staging of
FL is the early detection of transformation into a large B-cell
lymphoma. This phenomenon, which occurs in 16% to 60% of the cases,
depending on the length of follow-up and re-biopsy policy, is a clonal
evolution from a classical FL, and it is characterized by an increased
number of large B cell centroblasts.[15-16] The
definition of FL transformation has in the past included progression
from grade 1-2 to 3 or development of a diffuse pattern “d’emblée” with
persistence of follicular morphology. The clinical behaviour of a
transformed FL (tFL) is very aggressive, and treatment outcome is
usually poor, with a median survival from the transformation of 1.2
years. A prompt identification of patients with tFL is therefore
needed, as these patients could be treated from the beginning with
intensive chemotherapy followed by autologous stem cell transplantation
and, once in CR, could experience prolonged survival.[17]
Several attempts have been made to correlate the histologic FL grade
and FL transformation with the intensity of FDG uptake in PET/CT. In
general non-tFL show a moderate FDG avidity, with a SUVmax values never exceeding 11.[18] On the other hand, the majority of tFLs have SUVmax values comparable to that of Diffuse Large B-cell Lymphoma (DLBCL).[19]
In a series of 17 FL and 2 tFL staged at baseline, Kharam et al. were
not able to show a significant differences in the entity of FDG uptake
across the three histologic grade of FL; the mean SUVmax
being 5.8 ± 2.6 for grade 1, 8.1 ± 4.8 for grade 2, 7.9 ± 1.3 for grade
3 (p= 0.1). By contrast, a significant difference was recorded between
non-tFL and tFL: 7.66 ± 4.59 Vs. 13.9 ± 10.2 (p<0.01).[20]
Quite recently, Novelli et al. in a longitudinal observational study
performed on 16 FL and 5 DLBCL patients during 3.5 years, undergoing a
PET-guided biopsy in the hottest FDG uptake site, were able to
demonstrate a close correlation between histologic grade and the SUVmax detected on the biopsied node. The SUVmax
was 6.7 (3.0-146) for grade 1, 9.3 for grade 2 (4.3-13-3), 12.7
(5.0-24.0) for grade 3a and 13.5 (3.0-40.0) for grade 3b and DLBCL. The
Ki-67 (r=0.73) and FL grade (r=0.75) at the biopsy showed significant
correlation with the SUVmax at diagnosis (p<0.01).[21]
Wondergem et al. compared 18F-FDG and 3’-Deoxy-3’-18F-Fluorothymidine
(FLT) PET scan to detect FL transformation. In this study, 18F-FDG and
18F-FLT PET scan were performed in 17 non-tFL and tFL, and the highest
SUVmax was measured in both scans in every patient. SUVrange was also measured, defined as the difference between the SUVmax of the lymph node with the highest and lowest uptake per patient. The highest SUVmax was significantly higher in tFL than in non-tFL, both in FDG and FLT-PET (p<0.001). The SUVrange
was significantly higher for tFL than FL with FDG-PET (p=0.029) but not
with FLT-PET (p=0.075). The ability of FDG-PET to discriminate between
FL and tFL was superior to that of FLT-PET for both the highest SUVmax (p= 0.039) and the SUVrange (p=0.012). The cutoff value of SUVmax to differentiate FL and tFL with FDG-PET with the highest sensitivity (100%) and specificity (82%) at a ROC analysis was 14.5.[22]
In
conclusion, FDG PET proved more accurate compared to standard
radiological means in FL staging, being able to upstage as much as 62%
of the patients in early stage (I and II) by CeCT, with the highest
sensitivity for ENS and, first among them, bone marrow. SUVmax values > 10 are usually found in grade 3 or transformed FL. DG-PET for FL Prognostication at Baseline
Quantitative
metrics for PET scan assessment have been used to assess the prognostic
role of baseline imaging with FDG-PET. In a retrospective study
including 45 histologically proven FL patients treated with Rituximab
and Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone
(R-CHOP), Le Dorz et al. showed a strong predictive value on treatment
outcome of baseline FDG-PET score. The latter assigned 1 point for each
of the following: (a) 1 point for osteo-medullar FDG uptake; (b) 1
point for SUVmax ≥ 15, (c) 1 point for extra-nodal involvement other
than BMI on PET; (d) 1 point for the largest diameter of lesion ≥ 7
cm., (e) 1 point for number of nodal sites involved by lymphoma of PET
≥ 6. Its ability to predict an incomplete response or an early relapse
was compared with follicular lymphoma prognostic index (FLIPI) using a
Receiver Operation Curve (ROC) analysis for sensitivity, specificity
and overall accuracy.[11] The ROC values for sensitivity of PET score ≥
2 and FLIPI in predicting treatment failure were 0.856 (95% C.I.
0.745-0.967) and 0,594 (95% C.I. 0.387-0.801), with a significant
advantage for PET score (p<0.0001). High tumour burden has been
considered in the past an important prognostic factor in FL, and a
number of parameters surrogate for high tumour burden have been
proposed to identify poor-prognosis patients. Such are any nodal or
extranodal tumour mass with a diameter larger than 7 cm., involvement
of at least three nodal areas, each of which with a diameter ≥ 3 cm.,
systemic symptoms, substantial splenic enlargement, pleural effusion,
orbital or epidural involvement and leukemic presentation.[23]
More recently, more sophisticated tools such as functional imaging with
FDG-PET/CT allowed displaying the morphology and the functional
activity of tumour burden. Moving from quantitative metrics of
FDG-avidity such as SUVmax, dedicated software permitted to quantify the metabolically active tumour volume (MTV).[24] The latter, calculated on baseline FDG-PET scan proved a very strong predictor of treatment outcome in Hodgkin Lymphoma,[25] in diffuse large B-cell lymphoma,[26] Primary Mediastinal B-cell lymphoma[27] and peripheral T-cell lymphoma.[28]
In a pooled retrospective analysis on 185 FL patients enrolled in
different prospective clinical trial and treated with
immunochemotherapy, Meignan et al., upon visual assessment and manual
contouring of all the lesions visible in baseline PET, using a fixed
threshold method of 41% of the SUVmax value to measure MTV, demonstrated that MTV, computed at baseline FGD-PET scan and a cutoff value of 510 cm3,
was able to identify patients with a poor response to therapy, with a
5-Y PFS of 33% Vs. 65% for low and high MTV, respectively (p<.001) (Figure 1).
In multivariate analysis only MTV and FLIPI-2 retained their
independent prognostic value but, when both were used in a combined
prognostic model, they were able to single out three classes with
significant different 5-y PFS: (I) MTV ≤510 and FLIPI-2 0-2, median 5-Y
PFS 69%; (II) MTV > 510 or FLIPI-2 3-5, 5-Y PFS 46% and (III) both
MTV > 510 and FLIPI-2 3-5, 5-Y PFS 20%; group 1 Vs. 2: p=.007; Group
1 Vs. group 3: p<.001; group 2 Vs. group 3: p=.004.[29]
In conclusion, semi-quantitative parameters on baseline PET (Q-PET) such as SUVmax and MTV proved informative of long-term FL treatment outcome.
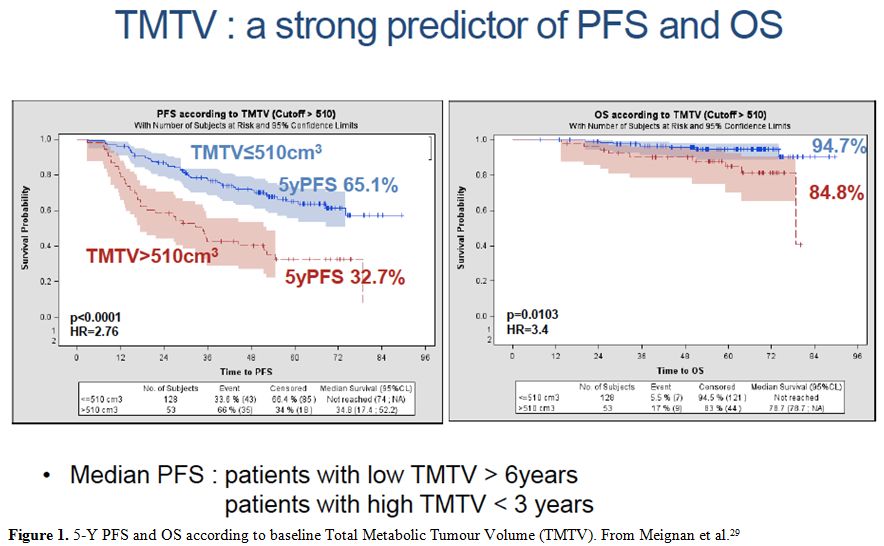 |
Figure 1. 5-Y PFS and OS according to baseline Total Metabolic Tumour Volume (TMTV). From Meignan et al.[29] |
FDG-PET for Treatment Response Evaluation in FL
FDG-PET showed a higher performance in tumor restaging, compared to traditional radiological means in solid cancers,[30] HL and NHL.[31]
In a retrospective study comparing FDG-PET/CT with contrast-enhanced CT
scan in different NHL subtypes, PET showed higher sensitivity (86.1%
Vs. 59.4%) and specificity (99.4% Vs. 96.1%) than CeCT alone.[32]
Due to these and several other observations pointing toward its
superiority in lymphoma restaging in different histotype, FDG-PET has
been included among the mandatory investigation tests to assess
treatment response in HL and NHL.[6] However, only
three years earlier, after the publication of the results of three
important trials, the LYSA trial PRIMA, the GOELAM trial and the FIL
Foll-05 trial, aimed at assessing the efficacy of immunochemotherapy in
FL, the interest of clinicians focused on the role of PET scan at the
end-of-treatment for assessing the response.[33-35]
Impressive similitudes in trial results have been reported across these
studies, regarding: (a) the percentage of patients showing a positive
end-of-treatment PET scan: 26%, 22%, and 24%, respectively; (b)
treatment outcome of PET-positive Vs. PET-negative patients, with a 3-Y
PFS of 33% Vs. 71%, a 2-Y PFS of 51% Vs. 87% and a 3-Y PFS of 35% and
66% respectively; (c) the prognostic role of end-of-therapy PET scan,
which turned out as the only factor associated with overall survival in
multivariate analysis in an independent way from other prognostic
factors such as FLIPI. Interestingly, data were fully reproducible
using a common readout for PET scan interpretation, the five-point
Deauville scale (5P-S).[36] In a retrospective pooled
analysis on 439 patients enrolled in the three trials whose PET scan
images were centrally reviewed adopting 5P-S with a cut-off value of a
positive scan ≥ 4, Trotman et al. confirmed the independent prognostic
value of end-of-therapy PET scan. Patients with a score 4 or more had a
4-years PFS of 22.3% compared with 63.4% for patients showing a score 3
or less (p<0.0001).[37] It is also noteworthy that
long-term survival was also significantly correlated with PET results,
irrespective of second and further-line treatment: patients with a
positive or negative PET after first-line treatment showed an OS of
87.1% and 97.2%, respectively (p<0.00001) (Figure 2).
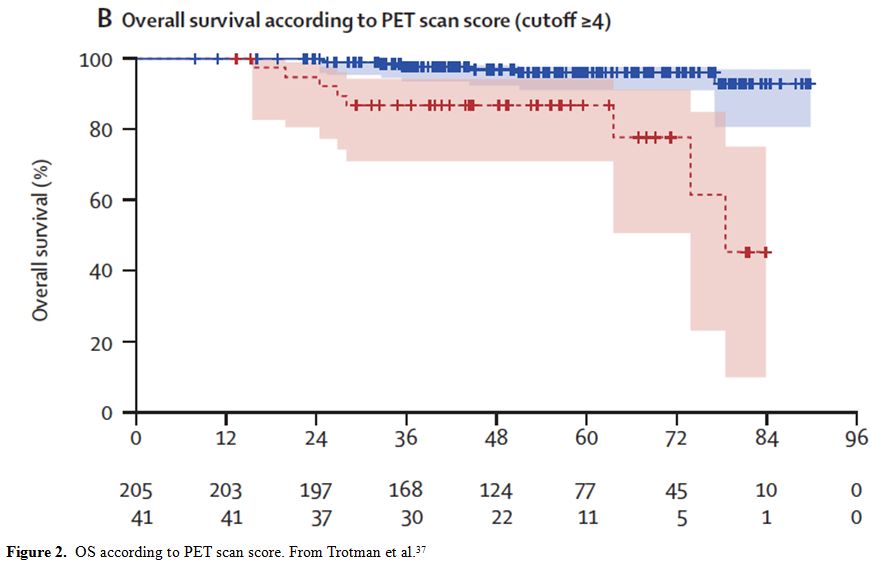 |
Figure 2. OS according to PET scan score. From Trotman et al.[37] |
In
a recent, systematic review on the prognostic value of end-of-treatment
FDG-PET in FL, Adams et al. reported the results of a pooled analysis
of 748 patients from eight different studies.[37] In
six of them the association of a scan results with PFS was sought, and
in five of them, a significant correlation between FDG positive
patients and worse PFS was reported. Only three out four studies
comparing OS and PET results showed a strict association, with a
significantly lower survival in patients with a positive scan.
Curiously in one single study[38] only a trend toward
significance was found between 3-Y PFS and scan results, with a PFS of
74.4% and 38.2% for PET-negative and positive patients, respectively
(p=0.083). In the same study FDG-PET was predictive of OS, showing mean
OS of 95.2 and 45 months in PET-negative and positive patients,
respectively (p<0.001). Overall, the above results seem to confirm
the concept that relying on morphologic parameters alone such as tumour
shrinkage after treatment is not adequate to predict long-term survival
in FL. As a matter of fact, the association of radiological and
functional imaging in the so-called IHP criteria for the first time
showed that a tumour size reduction associated with a persistent FDG
avidity had a much worst prognostic meaning than it would have been
predicted by the classical radiologic IWC criteria alone.[39]
Minimal residual disease (MRD), assessed on tumour DNA by molecular
biology has been advocated as a predictive parameter on long-term
treatment outcome in FL.[40] Upon systematic use of
end-of-treatment PET scan to assess the response of first line
treatment in FL, data on MRD and PET after therapy were concurrently
available. In an interesting preliminary report, Luminari et al. tried
to correlate the predictive role of both methods in the same patients
enrolled in the Italian FIL study Foll-05.[41] FDG-PET scan was centrally reviewed and scored according to 5-PS,[36]
and MRD was assessed on bone marrow aspirate with a qualitative and
quantitative assessment of the BCL2/IGH fusion gene, after nested
qualitative PCR.[42] A total of 41 subjects had
available data on both PET and BCL2/IGH at the end of treatment.
PET/MRD concordance was only 76% with a kappa value of 0.249,
suggesting the both parameters were not strongly correlated. In
univariate analysis, EOT PET was associated with poorer PFS (HR 3.61 p=
0.028) while MRD showed only a trend toward a shorter PFS (HR 2.54; P=
0.06).
In conclusion, there is largely documented evidence that
achieving a complete metabolic response (CMR) at the end of treatment
is the single most powerful predictor of long-term disease control and
survival in FL.
FDG-PET for Radioimmunotherapy (RIT)
In 2003 the Food and Drug Administration (FDA) approved the use of 131I-tositumomab (Bexxar® Glaxo Smith Kline), and later of 90Y-Ibritumomab
(Zevalin® Biogen-IDEC) and for the treatment of relapsed, refractory
FL. Ibritumomab is the murine parent of the anti-CD20 antibody (IDEC)
from which the human chimeric antibody rituximab was engineered. Both
agents target the CD 20 antigen expressed on the surface of B-cell
lymphoproliferative disorders. Upon binding of these MoAbs on their
ligand on the cell surface, a cell apoptosis or a cell lysis does
occur, mediated by the complement or by the Fc part of the antibody
binding to the Fc receptor on the cytotoxic T-cells. A synergistic
action of the radio-conjugate consists in the cytolytic action of the
neoplastic cells by the β-particles emitted by the radiotracer.[43] The efficacy of both radio-conjugates in relapsed or refractory FL is similar, with an overall response rate of the 60%-83%.[44-46] Front-line treatment of high-risk FL with 131I-tositumomab induce even higher overall response rate (95%), with as much as 75% of the patients attaining CR.[47]
However, advantages of RIT over standard immunochemotherapy with R-CHOP
in untreated FL were less evident in prospective, randomized studies
and contradictory results have been published. In a well-designed
prospective randomized trial, 532 patients with stage II-IV and grade
1-3 FL were randomly assigned to CHOP-R or CHOP-21 followed by 4-8
weeks after the 6th CHOP cycle by 131I-tositumomab
radioimmunotherapy. After a median follow-up of 4.9 years, the 2-year
estimate of PFS and OS were 76% and 97% in the CHOP-R and 80% and 93%
in the CHOP-RIT arm (p= 0.11 and 0.08, respectively).[48]
These results were partially contradicted by another study with a
longer patient follow-up. In a prospective, randomized international
first line FL treatment aimed at assessing the role of 90Y-Ibritumomab
tiuxetan as consolidation treatment after chemo-immunotherapy,
Morschhauser et al., after a follow-up spanning over 7 years, were able
to demonstrate a significant advantage of the arm randomized to receive
RIT consolidation compared to the arm addressed to no further treatment
(NFT). Patients receiving combination treatment had an 8-Y PFS of 48%
compared to patients receiving NFT (22% p < 0.001). This difference
remained significant in patients in CR/CRU (48% Vs. 32% p=0.008) and in
PR (33% Vs. 10% p <0.001) after chemo-immunotherapy.[49]
Importantly, in neither study, a significant and unexpected acute
toxicity was reported. In long-term follow-up, only a non-significant,
slight prevalence of secondary neoplasms and secondary myelodysplastic
syndrome (MDS) was observed in the RIT arm compared to controls (26 Vs.
14, p=.086 and 7 Vs. 1, p=.042, respectively). 90Y-Ibritumomab
tiuxetan in combination with Carmustine, Cytarabine, Etoposide and
Melphalan (so-called Z-BEAM conditioning regimen) followed by
autologous stem cell transplant (ASCT) has been selectively used in
transformed Follicular (TF) lymphoma. In a multicentre retrospective
clinical trial evaluating ASCT after Z-BEAM conditioning regimen in 63
TF enrolled from 4 U.S. centres from 2003 till 2011, Mei et al.
reported a very good long-term disease control, with a 2-Y PFS of 68%
and an OS of 90%. The median time of ASCT from diagnosis of TF was 7.5
months, and the 2-Y non-relapse mortality was 0.[50]
Due to a rather narrow therapeutic window, the therapeutic dose of both
drugs should be calculated in vivo by injecting a tracer dose of a
non-β emitting agent to predict the biodistribution of the drugs:
the 131I-labeled antibodies are γ-emitters and can be used for imaging and dosimetry. On the other hand, the 90Y-labeled monoclonal abs like Zevalin® are beta-emitters and cannot be used for imaging, thus 111In is used instead (in Europe) or 131I-Ibritumomab (In United States). When 111In-Ibritumomab
tiuxetan is injected in the patient images of the tumour and the normal
organs is produced. The therapeutic dose of 90Y-Ibritumomab
tiuxetan is determined by the patient’s weight and baseline platelet
count. Patients are first injected with Rituximab at the dose of 250
mg/m2 to saturate CD-20 receptors on B-cell precursors and then with 5 mCi of 111In
labeled Ibritumomab on day 1 followed by tumour imaging in the next few
days. Two or three whole-body images are required by the FDA to ensure
normal biodistribution. On the day 8, the patient receives another dose
of Rituximab, followed by 90Y-Ibritumomab tiuxetan at the dose mentioned above. 131I-Tositumomab can be used for imaging and treatment and has a more variable pharmacokinetic behaviour compared to 90Y-Ibritumomab: the γ-photons emitted by 131I (in addition to β particles) and the longer half-life of 131I
allow a more precise imaging definition and drug dosimetry in the
single-patient basis. The dose injected varies with differences in body
weight, tumour burden, and renal excretion of the radiotracer. The
therapeutic dose is administered within 7 to 14 days from tracer dose
and consists of 450 mg. of tositumomab saturating dose, followed by a
20-minute infusion of the patient specific 131I-tositumomab
dose. One day before tositumomab injection a saturating dose of
potassium iodide is also needed to avoid concentration of the
radiotracer in the thyroid. Thus, when a choice could be made between
the two drugs (as in the United States) and bone marrow toxicity could
be a concern, 131I tositumomab tiuxetan should be preferred.[51]
Tumour imaging pre-RIT could also be obtained with MoAbs conjugated
with other radiotracers. Biodistribution radiation dosimetry and
scouting of 90Y-Ibritumomab tiuxetan have been made with 89Zr-Ibritumomab.[52]
The highest absorbed dose was observed in liver (3.2 ± 1.8 mGy/MBq),
followed by spleen (2.9 ± 1.8 mGy/MBq), kidneys and lungs. The bone
marrow dose was lower (0.52 ± 0.04 mGy/MBq). The correlation between
predicted pre-therapy and therapy organ absorbed doses, based on 89Zr-Ibritumomab
tiuxetan images was very high (Pearson correlation coefficient r=0.97).
However, technical problems limiting the use of RIT still exist. The
first is the limited delivery into the tumour as the transport and
uptake of the Monoclonal Antibodies (MoAbs) by the tumour is variable,
and most of the injected dose still circulates in the plasma and
targets normal tissues.[53] The second problem is the
penetration in the tumour, which is variable and nonhomogeneous across
the different tumour regions. The steps associated with tumour
targeting by MoAbs are blood flow to the tumour extravasation across
the capillary wall, diffusion en the extracellular fluid and binding to
the tumour. Extravasation across the endothelium is the rate-limiting
step; the estimated permeability of extravasation is 1 μm/s for FDG and
0.003 μm/s for MoAbs.[54] Finally, tumours are
surrounded by a layer of extracellular matrix (ECM) proteins, such as
collagen, elastin, fibronectin, which inhibit the penetration and
dispersion of cancer therapeutic agents. ECM has been implicated
in the treatment resistance in solid tumours.[55] Thus, despite logistic and administrative problems for drug preparation and drug availability RIT with 90Y-labeled monoclonal Zevalin® in Europe and 131I-Tositumomab tiuxetan in U.S. remains an effective treatment for relapsed /refractory FL.
Conclusions
A
large body of evidence suggests today that FDG-PET/CT is indeed a new
paradigm for a modern FL management in clinical practice. It proved
very accurate in FL staging, restaging, prognostication and RIT
planning. Although informative on prognosis, MTV assessment at baseline
still remains an investigational tool as standardization problems and
unsettled thresholding still preclude its reproducibility in the daily
clinical care.
References
- Morton LM, Wang SS, Devesa SS et al.: Lymphoma
incidence patterns by WHO subtype in the United States, 1992-2001.
Blood 2006; 107: 265-76. https://doi.org/10.1182/blood-2005-06-2508 PMid:16150940 PMCid:PMC1895348

- Smith
A, Crouch S, Lax S et al.: Lymphoma incidence, survival and prevalence
2004-2014: subtype analysis from the UK's Haematological Malignancy
Research Network. Bf. J. Cancer 2015; 112: 1575-84. https://doi.org/10.1038/bjc.2015.94 PMid:25867256 PMCid:PMC4453686

- Casulo
C, Byrtek M, Dawson KL et al.: Early relapse of follicular lymphoma
after rituximab plus cyclophosphamide, doxorubicin, vincristine and
prednisone defines patients at high risk for death: an analysis from
the National LymphoCare Study. J. Clin. Oncol. 2015; 33: 2516-22. https://doi.org/10.1200/JCO.2014.59.7534 PMid:26124482 PMCid:PMC4879714

- Solal-Celigny P. Roy P, Colombat P et al.: Follicular Lymphoma International Prognostic Index. Blood 2004; 104:1258-65. https://doi.org/10.1182/blood-2003-12-4434 PMid:15126323

- Federico
M, Bellei M, Marcheselli L et al.: Follicular Lymphoma International
Prognostic Index 2: a new prognostic index for follicular lymphoma
developed by the international Follicular Lymphoma prognostic factor
project. J Clin Oncol 2009; 27: 4555-62. https://doi.org/10.1200/JCO.2008.21.3991 PMid:19652063

- Cheson
BD, Fisher RI, et al. Recommendations for Initial Evaluation, Staging,
and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano
Classification. J Clin Oncol 2014; 32(27): 3059-68 https://doi.org/10.1200/JCO.2013.54.8800 PMid:25113753 PMCid:PMC4979083

- Barrington
SF, Mikhaeel NG, et al. Role of Imaging in the Staging and Response
Assessment of Lymphoma: Consensus of the International Conference on
Malignant Lymphomas Imaging Working Group. J Clin Oncol 2014; 32 (27):
3048-58. https://doi.org/10.1200/JCO.2013.53.5229 PMid:25113771 PMCid:PMC5015423

- Weiler-Sagie
M, Buschelev O, Epelbaum R et al 18F-FDG avidity in lymphoma
readdressed: a study of 766 patients. J Nucl Med. 201; 51: 25-30. https://doi.org/10.2967/jnumed.109.067892

- Tsukamoto
N, Kojima N, Hasegawa M et al.: The usefulness of
18F-Fluorodeoxyglucose Positron Emission Tomography (18F-FDG PET) and a
comparison with 67Gallium scintigraphy in the evaluation of lymphoma.
Cancer 2007; 110(3): 652-9. https://doi.org/10.1002/cncr.22807 PMid:17582800

- Elstrom R, Guan L, Baker G et al.: Utility of FDG-PET scanning in lymphoma by WHO classification. Blood 2003; 101: 3875-76. https://doi.org/10.1182/blood-2002-09-2778 PMid:12531812

- Le
Dorz L, De Guibert S, Bayat S et al.: Diagnostic and prognostic impact
of 18F-FDG PET/CT in follicular lymphoma. J Nucl. Med. Mol. Imaging
2010; 37: 2307-14. https://doi.org/10.1007/s00259-010-1539-5 PMid:20717826

- Luminari
S, Biasoli I, Arcaini L et al.: the use of FDG-PET in the initial
staging of 142 patients with follicular lymphoma: a retrospective study
from the FOLL 05 randomized trial of the Fondazione Italiana Linfomi.
Ann Oncol 2013; 24, 2108-2112. https://doi.org/10.1093/annonc/mdt137 PMid:23585513

- Wohrer
S, Jaeger U, Kletter K et al.: 18F-fluoro-deoxy-glucose positron
emission tomography (18F-FDG-PET) visualizes follicular lymphoma
irrespective of grading. Ann Oncol 2016; 17:780-84. https://doi.org/10.1093/annonc/mdl014 PMid:16497824

- Perry
C, Lerman H, Joffè L et al.: The value of PET/CT in detecting bone
marrow involvement in patients with Follicular Lymphoma. Medicine 2016;
95(9) e2910. https://doi.org/10.1097/MD.0000000000002910 PMid:26945387 PMCid:PMC4782871

- Bastion
Y, Sebban C, Berger F.: In cadence, predictive factors and outcome of
lymphoma transformation in follicular lymphoma patients. J clin Oncol
1997; 15: 1587-94. https://doi.org/10.1200/JCO.1997.15.4.1587 PMid:9193357

- Montoto
S, Davies AJ, Matthews J et al.: Risk and clinical implications of
transformation of follicular lymphoma to diffuse large B-cell Lymphoma.
J Clin Oncol 2007; 25: 2426-33. https://doi.org/10.1200/JCO.2006.09.3260 PMid:17485708

- Youen
AR, Kamel OW, Halpern J and Horning S.: Long-term survival after
histologic transformation of low-grade follicukar lymphoma. J Clin
Oncol 1995; 13 (7): 1726-33. https://doi.org/10.1200/JCO.1995.13.7.1726 PMid:7602362

- Bodet-Milin
C, Kraeber-Bodéré F, Moreau P et al.: Investigation of FDG-PET/CT
imaging to guide biopsies in the detection of histological
transformation of indolent lymphoma. Haematologica 2008; 93(3): 471-2. https://doi.org/10.3324/haematol.12013 PMid:18310543

- Noy
A, Schoder H, Gonen M et al.: The majority of transformed lymphomas
have high standardized uptake values (SUVs) on positron emission
tomography (PET) szcanning similar to diffuse large B-cell Lymphoma
(DLBCL). Ann Oncol 2009; 20(3) : 508-12. https://doi.org/10.1093/annonc/mdn657 PMid:19139176 PMCid:PMC4542578

- Kharam
M, NovaK L, Cyhriac J et al.: Role of Fluoridne-18 Fluoro-Deoxyglucose
positron emission tomography scan in the evaluation and follow-up of
patients with low-grade lymphomas. Cancer 2006; 107: 175-83. https://doi.org/10.1002/cncr.21967 PMid:16721817

- Novelli
S, Briones J, Flotats A et al.: PET/CT assessment of follicular
lymphoma and high grade B cell lymphoma – Good correlation with
clinical and histological features at diagnosis. Adv. Clin Exp Med
2015; 24: 325-30. https://doi.org/10.17219/acem/31804 PMid:25931367

- Wondergem
M, Rizvi SNF, Jauw Y et al. 18F-FDG or 3'-Deoxy-3'-18F-Fluorothymidine
to detect transformation of Follicular Lymphoma. J Nucl Med 2015; 56:
216-21. https://doi.org/10.2967/jnumed.114.149625 PMid:25593118

- Lepage
E, Sebban C, Gisselbrecht C et al.: Treatment of low-grade non-Hodgkin
lymphomas: assessment of doxorubicin in a controlled trial. Haematol
Oncol 1990; 8(1): 31-9. https://doi.org/10.1002/hon.2900080105

- Bai B, Bading J Conti PS. Tumor quantification in clinical Positron Emission Tomography. Theranostics 2013; 3(10): 787-801. https://doi.org/10.7150/thno.5629 PMid:24312151 PMCid:PMC3840412

- Song
MK, Chung JS, Lee JJ et al.: Metabolic tumor volume by positron
emission tomography / computed tomography as a clinical parameter to
determine therapeutic modality for early stage Hodgkin's lymphoma.
Cancer Sci. 2013; 104: 1656-61. https://doi.org/10.1111/cas.12282 PMid:24033666

- Mikhaeel
NG, Smith D, Dunn JT et al.: Combination of baseline metabolic tumor
volume and early response on PET/CT improves progression-free survival
prediction in DLBCL. Eur. J. Nucl. Mol. Imaging 2016; 43 (7):1209-19. https://doi.org/10.1007/s00259-016-3315-7 PMid:26902371 PMCid:PMC4865540

- Ceriani
L, Martelli M, Zinzani PL et al.: Utility of baseline 18FDG-PET/CT
functional parameters in defining prognosis of primary mediastinal
(thymic) large B-cell lymphoma. Blood 2015; 126(8): 950-56. https://doi.org/10.1182/blood-2014-12-616474 PMid:26089397

- Cottereau
AS, Becker S, Broussais F et al.: Prognostic value of baseline total
metabolic tumor volume (TMTV0) measured on FDG-PET/CT in patients with
peripheral T-cell lymphoma (PTCL). Ann Oncol. 2016; 27(4): 719-24. https://doi.org/10.1093/annonc/mdw011 PMid:26787236

- Meignan
M, Cottereau AS, Versari A et al.: Baseline Metabolic Tumor Volume
predicts outcome in high-tumor-burden follicular lymphoma: a pooled
analysis of three multicentre studies. J Clin Oncol 2016 in press. https://doi.org/10.1200/JCO.2016.66.9440

- Juweid ME and Cheson BD: Positron-Emission Tomography and assessment of Cancer Therapy. N Engl J Med 2006; 354: 496-507. https://doi.org/10.1056/NEJMra050276 PMid:16452561

- Juweid
ME, Wiseman GA, Vose JM et al.: Response assessment of aggressive
non-Hodgkin lymphoma by integrated International Workshop criteria and
fluorine-18-fluorodeoxyglucose positron emission tomography. J clin
Oncol 2005; 23: 4652-4661. https://doi.org/10.1200/JCO.2005.01.891 PMid:15837965

- Nogami
M, Naamoto Y, Sakamoto S et al.: Diagnostic performance of CT, PET,
side-by-side, and fused image interpretation for restaging of non
Hodgkin lymphoma. Ann Nucl Med 2007; 21: 189-96. https://doi.org/10.1007/s12149-007-0015-1 PMid:17581717

- Trotman
J, Fournier M, Lamy T, et al. Positron emission tomography-computed
tomography (PET-CT) after induction therapy is highly predictive of
patient outcome in follicular lymphoma: analysis of PET-CT in a subset
of PRIMA trial participants. J Clin Oncol. 2011; 29:3194–200.https://doi.org/10.1200/JCO.2011.35.0736 PMid:21747087

- Dupuis
J, Berriolo-Riedinger A, Julian A, et al. Impact of
[18F]fluorodeoxyglucose positron emission tomography response
evaluation in patients with high-tumor burden follicular lymphoma
treated with immunochemotherapy: a prospective study from the Groupe
d'Etudes des Lymphomes de l'Adulte and GOELAMS. J Clin Oncol.
2012;30:4317–22. https://doi.org/10.1200/JCO.2012.43.0934 PMid:23109699

- Luminari
S, Biasoli I, Versari A, et al. The prognostic role of post- induction
FDG-PET in patients with follicular lymphoma: a subset analysis from
the FOLL05 trial of the Fondazione Italiana Linfomi (FIL). Ann Oncol.
2014; 25: 442–7. https://doi.org/10.1093/annonc/mdt562 PMid:24412823

- Meignan
M. Gallamini A, Haioun C.: Report of the first international workshop
on PET scan in lymphoma. Leukemia and Lymphoma 2009; 50(8): 1257-60. https://doi.org/10.1080/10428190903040048 PMid:19544140

- Trotman
J, Luminari S, Boussetta S et al.: Prognostic value of PET-CT after
first-line therapy in patients with follicular lymphoma: a pooled
analysis of central scan review in three multicentric studies. Lancet
Haematol. 2014; Oct 1(1): e17-27. https://doi.org/10.1016/S2352-3026(14)70008-0

- Lu
Z, Lin M, Downe P, Chong S, Ling S (2014) The prognostic value of mid-
and post-treatment [(18)F] fluorodeoxyglucose (FDG) positron emission
tomography (PET) in indolent follicular lymphoma. Ann Nucl Med 2014;
28(8): 805–811. https://doi.org/10.1007/s12149-014-0874-1 PMid:25008291

- Cheson BD, Pfistner B, Juweid ME, et al: Revised response criteria for malignant lymphoma. J Clin Oncol 25:579-586, 2007. https://doi.org/10.1200/JCO.2006.09.2403 PMid:17242396

- Rambaldi
A, Carlotti E, Oldani E et al.: Quantitative PCR of bone marrow
BCL2/IgH+ at diagnosis predicts treatment response and long term
outcome in follicular non-Hodgkin lymphoma. Blood 2005; 105: 3428-33. https://doi.org/10.1182/blood-2004-06-2490 PMid:15637137

- Federico
M, Luminasi S, Dondi A et al.: R-CVP versus R-CHOP versus R-FM for the
initial treatment of patients with advanced-stage follicular lymphoma:
results of the FOLL05 trial conducted by the Fondazione Italiana
Linfomi. J Clin Oncol 2013; 31: 1506-13.https://doi.org/10.1200/JCO.2012.45.0866 PMid:23530110

- Gribben
JG, Neuberg D, Freedman AS et al.: Detection of polymerase chain
reaction of residual cells with the bcl-2 translocation is associated
with increased risk of relapse after autologous bone marrow
transplantation for B-cell lymphoma. Blood 1993; 81(12): 3449-57.
PMid:8507880

- Davis
TA, Kaminsky MS, Leonard JP et al.: The radioisotope contributes
significantly to the activity of radioimmunotherapy. Clin Cancer Res.
2004; 10:7792-98. https://doi.org/10.1158/1078-0432.CCR-04-0756 PMid:15585610

- Kaminsky
MS, Estes J, Zasadny KR et al. Radioimmunotherapy with Iodine 131I
tositumomab for relapsed or refractory B-cell on-Hodgkin lymphoma:
updated results and long-term follow-up of the University of Michigan
experience. Blood 2000; 96: 1259-1266.

- Witzig
TE, Flinn IW, Gordon LI et al.: Treatment with ibritumomab tiuxetan
radioimmunotherapy in patients with rituximab-refractory follicular
non-Hodgkin Lymphoma. J Clin Oncol 2002; 20: 3262-69.https://doi.org/10.1200/JCO.2002.11.017 PMid:12149300

- Kaminsky
MS, Zasadny KR, Francis IR et al.: Radioimmunotherapy of B-cell
lymphoma with [131I] anti –B1 (Anti CD20) antibody. N Engl J Med 1993;
329: 459-65. https://doi.org/10.1056/NEJM199308123290703 PMid:7687326

- Kaminsky
MS, Tuck M, Estes J et al.: 131I-Tositumomab therapy as initial
treatment for follicular lymphoma. N Engl J Med 2005; 352: 441-49. https://doi.org/10.1056/NEJMoa041511 PMid:15689582

- Press
OW, Unger JM, Rimsza LM et al.: Phase III randomized intergroup trial
of CHOP plus Rituximab compared with CHOP chemotherapy plus 131Iodine
–Tositumomab for previously untreated follicular non-Hodgkin lymphoma:
SWOG S0016. J Clin Oncol 2012; 31:314-20.https://doi.org/10.1200/JCO.2012.42.4101 PMid:23233710 PMCid:PMC3732010

- Morchhauser
F, Radford J, Van Hoof A et al.: 90Yttrium-Ibritumomab Tiuxetan
consolidation of first remission in advanced-stage follicular
non-Hodgkin lymphoma: updated results ofter a median follow-up of 7.3
years from the international randomized, phase III first-line indolent
trial. J Clin Oncol 2013; 31:1997-83. https://doi.org/10.1200/jco.2012.45.6400

- Mei
M, Wondergrem MJ, Palmer JM et al.: Autologous transplantation for
transformed non-Hodgkin lymphoma using an Yttrium-90 Ibritumomab
Tiuxetan conditioning regimen. Biol Blood Marrow Transplant 2014; 20:
2056-75. https://doi.org/10.1016/j.bbmt.2014.07.028 PMid:25079874

- Jacene
HA, Ross F, Kasecamp W et al.: Comparison of 90Y-Ibritumomab Tiuxetan
and 131I-Toszitumomab in Clinical Practice. J Nucl Med 2007;
48:1767-76. https://doi.org/10.2967/jnumed.107.043489 PMid:17942813

- Rizvi
SNF, Visser OJ, Voszjan MJVD et al.: Biodistribution, radiation
dosimetry and scouting of 90Y-Ibritumomab tiuxetan therapy in patients
with relapsed B-cell non-Hodgkin's lymphoma using 89Zr-Ibritumomab
tiuxetan and PET. Eur. J Nucl Med Mol Imaging 2012; 39: 512-20. https://doi.org/10.1007/s00259-011-2008-5 PMid:22218876 PMCid:PMC3276758

- Thurdber
GM, Schmidt MM, Wittrup KD: Factors determining antibody distribution
in tumors. Trends Pharmacol Sci. 2008; 29: 57-61.

- Kim SJ: Combination radioimmunotherapy approaches and quantification of immune-PET. Nucl Mol Imaging 2016; 50: 104-11.

- Choi
IK; Strauss R, Richter M: Strategies to increase drug penetration in
solid tumours. Front. Oncol. 2013; 26 (63): 193-8.

[TOP]


























































