Antonella Isgrò1, Marco Marziali1, Katia Paciaroni1, Gioia De Angelis1, Cecilia Alfieri1, Michela Ribersani1, Festus Olusola Olowoselu2, Guido Lucarelli1 and Javid Gaziev1
1LInternational
Center for Transplantation in Thalassemia and Sickle Cell Anemia.
Mediterranean Institute of Hematology, Policlinic of the University of
Rome “Tor Vergata”, Rome, Italy.
2 Dept. of Haematology and Blood Transfusion, College of Medicine, Lagos University Teaching Hospital, Lagos, Nigeria.
Corresponding
author: Antonella
Isgrò, M.D. International Center for Transplantation in Thalassemia and
Sickle Cell Anemia. Mediterranean Institute of Hematology. Policlinic
Tor Vergata, Rome, Italy. Viale Oxford, 81. Rome-00133, Italy. Phone:
(+39) 06 20661300. Fax: (+39) 06 20661302. E-Mail:
a.isgro@fondazioneime.org
Published: April 15, 2017
Received: January 8, 2017
Accepted: March 14, 2016
Mediterr J Hematol Infect Dis 2017, 9(1): e2017030 DOI
10.4084/MJHID.2017.030
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
Sickle
cell anemia (SCA) and its complications result in significant morbidity
and mortality, posing a significant public health challenge worldwide.
SCA and its societal costs disproportionally affect Africa. A strong
geographical link between the highest HbS allele frequencies and high
malaria endemicity was observed at the global scale, but this
observation is influenced primarily by the relationship found in
Africa.[1] Nigeria, in particular, has the largest population of
children and adults with SCA in the world. Approximately 150,000
children are born with SCA each year in Nigeria, compared to
approximately 1,100 children born in the US each year. A greater
understanding of the pulmonary factors contributing to morbidity and
mortality among children with SCA may lessen the public health burden
of SCA worldwide.
The prevalence of asthma in patients with SCA is
higher than in normal population (30-70% versus 20%).[2] Asthma
increases the risk of morbidity and mortality in patients with SCA.[3]
It has been shown that there is an association between the presence of
bronchial hyperactivity and the onset of acute chest syndrome
(ACS):[3-11] children with SCA and asthma have more frequent episodes
of ACS and/or veno-occlusive crisis (VOC). Asthma is also a risk factor
for early second hospitalization after discharge from hospital.[12]
Therefore, it seems that asthma is an additional factor that aggravates
some manifestations of SCA.
Often in a patient with SCA, it is
difficult to distinguish from the beginning the symptoms of an asthma
attack from that of an initial ACS. Children with SCA are prone to
invasive infections caused by S. pneumonia, H. influenzae, and Plasmodium falciparum.
Like thalassemia, allogeneic hematopoietic stem cell transplantation
(HSCT) is curative in most individuals with SCA.[13] We analyzed
pulmonary function in SCA patients underwent BMT through
high-resolution computed tomography (HRCT) scan and spirometry, before
and after transplant.
This study included 37 consecutive SCA
patients who underwent bone marrow transplantation from human leukocyte
antigen (HLA)-identical sibling donors between 2010 and 2015 following
a myeloablative conditioning regimen. The patients were referred to
Mediterranean Institute of Hematology for transplantation, and none of
them were followed previously in Italy. The median patient age was 10
years (range 2–17 years). Patient characteristics at the time of
transplantation are summarized in Table 1.
Patients Lansky/Karnofsky performance score varied between 90-100% at
the time of transplantation. None of these patients had a splenectomy
before transplantation. Only two patients received chronic blood
transfusions, and the serum ferritin level before transplantation was
278 + 231 ng/mL (mean + SD). Before transplantation, eighteen patients
had recurrent, painful, vaso-occlusive crisis (VOC); thirteen patients
had VOC in association with acute chest syndrome (ACS); five patients
experienced ischemic stroke with or without association with
VOC; two patients exhibited leukocytosis, and dactylitis and one
patient exhibited priapism. Twelve patients were on hydroxyurea therapy
before transplantation. Repeated and severe VOC, stroke, acute chest
syndrome, in association with a not easy availability of hydroxyurea
and/or transfusion therapy in their Country, were indications for HSCT.
The patients were subjected to instrumental examinations in
pre-transplant phase with HRCT and spirometry. Patients received
fludarabine (30 mg/m2/d) for 5 days
followed by conditioning regimen including targeted intravenous
busulfan and cyclophosphamide (200 mg/kg total dose). They received
cyclosporine A, low-dose methylprednisolone, and a short course of
methotrexate as graft-versus-host disease (GVHD) prophylaxis. All
patients received BM from HLA-identical sibling donors 36 h after the
final dose of cyclophosphamide. HRCT scan revealed parenchymal
consolidation with nodular thickening in 19 out 37 patients, as active
or residual pneumonia. Twenty-five out 37 patients had evaluable
standard spirometry before transplantation. The following parameters
were included: forced vital capacity, forced expiratory volume in the
first second (FEV1), the ratio FEV1/FVC, and the total lung capacity (TLC). Spirometry results (FVC, FEV1, FEV1/FVC,
TLC) were expressed as percent of the predicted value based on gender,
age, and ethnic appropriate reference standards. Spirometry was
performed according to standard protocols using European Respiratory
Society/American Thoracic Society acceptability and repeatability
criteria, adapted for children where appropriate (normal value:
FVC>75; FEV1/FVC>80).[14]
Eleven out of 25 patients had a restrictive respiratory pattern (FVC
<75%), one patient had a restrictive/obstructive pattern (FVC
<75% and FEV1/FVC
<80%) and 13 of 25 patients had normal respiratory function tests.
Six out 12 patients with restrictive respiratory pattern had ACS and
bronchial hyperactivity (Table 1).
All 37 patients had sustained engraftment after transplant. After 3-6
months of transplantation, we found no significant changes in
spirometry values (Table 2). In
particular, four out of nine patients had unchanged respiratory
pattern, three patients experienced worsening, probably due to
post-transplant infectious complications and/or the occurrence of acute
GVHD (UPN 207, acute GVHD; UPN 213 and UPN 234, Aspergillus fumigatus pneumonia; UPN 237, Klebsiella pneumonia pneumonia) and two patients showed an initial amelioration.
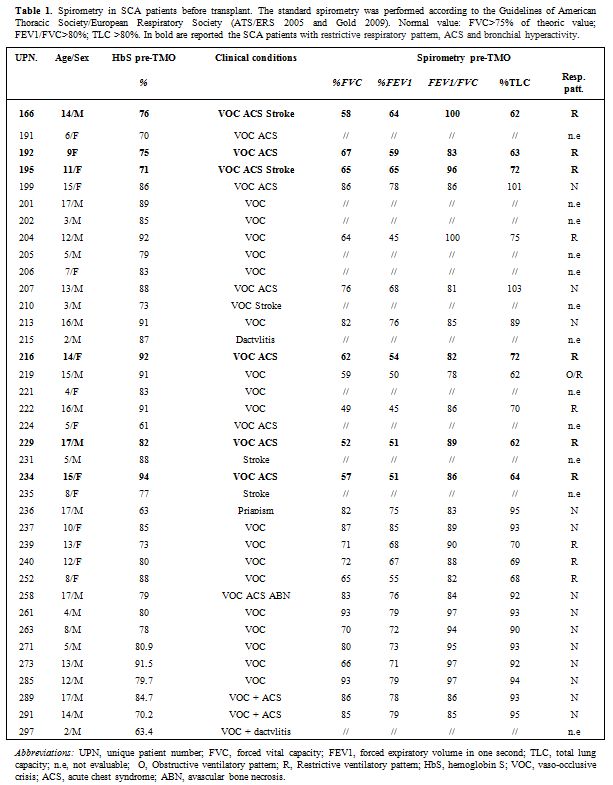 |
Table 1.
Spirometry in SCA patients before transplant. The standard
spirometry was performed according to the Guidelines of American
Thoracic Society/European Respiratory Society (ATS/ERS 2005 and Gold
2009). Normal value: FVC>75% of theoric value; FEV1/FVC>80%;
TLC >80%. In bold are reported the SCA patients with restrictive
respiratory pattern, ACS and bronchial hyperactivity. |
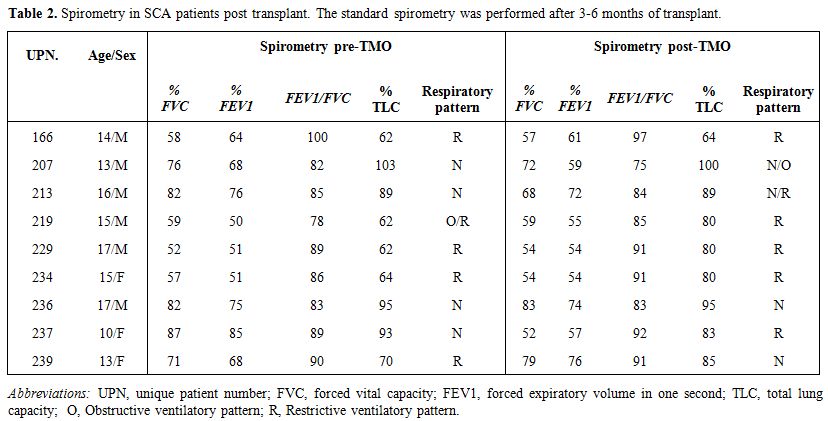 |
Table 2. Spirometry in SCA patients post transplant. The standard spirometry was performed after 3-6 months of transplant. |
Pulmonary complications are leading causes of morbidity and mortality in SCA.
Airway
obstructions and repeated pulmonary infections are among the primary
causes of pulmonary involvement, and they result in obstructive or
restrictive respiratory disorders, which can result in pulmonary
hypertension. Moreover, this population often presents with acute chest
syndrome, which is characterized by chest pain, prostration, cough,
dyspnea and hypoxia.[2]
When assessing pulmonary function,
individuals may exhibit either normal function or altered ventilatory
patterns, which are classified as obstructive, restrictive or mixed.
Obstructive ventilatory patterns (OVPs) are characterized by
disproportionately decreased peak flows (PEF) when compared to the
volume that can be eliminated, and the FEV1 and FEV1/FVC
are the major measures by which to characterize OVPs. Restrictive
pulmonary patterns (RVPs) are characterized by decreased FVC. It should
be noted that RVP cannot be measured by spirometry. However, RVP values
can be inferred when the vital capacity (VC) and FVC are decreased, and
the FEV1/FVC
ratio is normal or increased. Finally, mixed ventilatory pattern (MVP)
is characterized by having both obstruction and restriction
simultaneously. In the present study, the lung function tests (LFTs)
obtained from 48% of the patients revealed changes in the pulmonary
function (i.e., RVP or MVP). Our results indicate that RVP is a common
finding in this disease. The presence of RVP in this population might
result from episodes of vaso-occlusion in the lung, which is an organ
that is prone to suffer from this condition because of its anatomical
characteristics. This can result in pulmonary infarctions, necrosis of
the alveolar wall with consequent airway remodeling, pulmonary fibrosis
and progressive loss of lung function. In addition, RVP may be a result
of fibrosis after many infection diseases in pulmonary districts.[15]
In fact, the HRCT often in these patients showed fibrotic outcomes,
expression of recent or past lung infections. RVP has been observed in
our patients with bronchial hyperactivity instead of a typical asthma
attack. Airway hyper-responsiveness is another feature of sickle cell
lung disease. Various studies have defined hyper-responsiveness either
as a decrease in lung function after exercise, cold air, or
methacholine challenges or increase after administration of a
bronchodilator and up to 70% of children with sickle cell disease have
airway hyperresponsiveness.[4] After 3-6 months of transplant, we do
not observe significant changes in spirometry value. In such a short
time from the transplant, lung function may still be influenced by
infectious events during transplant or the possible occurrence of acute
GVHD. Our findings indicate that routine spirometry, before and after
transplantation, is an important adjunct to the clinical in this
patient population with a high prevalence of pulmonary disease and lung
dysfunction.
References
- Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething
PW, Williams TN, Weatherall DJ, Hay SI. Global distribution of the
sickle cell gene and geographical confirmation of the malaria
hypothesis. Nat Commun. 2010;1:104. https://doi.org/10.1038/ncomms1104 PMid:21045822 PMCid:PMC3060623

- DeBaun
MR, Strunk RC. The intersection between asthma and acute chest syndrome
in children with sickle-cell anaemia. Lancet. 2016;387(10037):2545-53. https://doi.org/10.1016/S0140-6736(16)00145-8

- Boyd
JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with
increased mortality in individuals with sickle cell anemia.
Haematologica.2007;92:1115-8. https://doi.org/10.3324/haematol.11213 PMid:17650441

- Sylvester
KP, Patey RA, Rafferty GF, Rees D, Thein SL, Greenough A. Airway
hyperresponsiveness and acute chest syndrome in children with sickle
cell anemia. Pediatr Pulmonol. 2007;42:272-6. https://doi.org/10.1002/ppul.20571 PMid:17262858

- Sylvester
KP, Patey RA, Broughton S, Rafferty GF, Rees D, Thein SL, Greenough A.
Temporal relationship of asthma to acute chest syndrome in sickle cell
disease. Pediatr Pulmonol. 2007;42:103-6. https://doi.org/10.1002/ppul.20430 PMid:17186507

- Boyd
JH, Macklin EA, Strunk RC, DeBaun MR. Asthma is associated with acute
chest syndrome and pain in children with sickle cell anemia. Blood.
2006;108:2923-7. https://doi.org/10.1182/blood-2006-01-011072 PMid:16690969 PMCid:PMC1892235

- Bryant R. Asthma in the pediatric sickle cell patient with acute chest syndrome. J Pediatr Health Care. 2005;19:157-62. https://doi.org/10.1016/j.pedhc.2004.12.003 PMid:15867831

- Knight-Madden
JM, Forrester TS, Lewis NA, Greenough A. Asthma in children with sickle
cell disease and its association with acute chest syndrome. Thorax.
2005;60:20610 https://doi.org/10.1136/thx.2004.029165 PMid:15741436

- Nordness
ME, Lynn J, Zacharisen MC, Scott PJ, Kelly KJ. Asthma is a risk factor
for acute chest syndrome and cerebral vascular accidents in children
with sickle cell disease. Clin Mol Allergy. 2005;3:2 https://doi.org/10.1186/1476-79612

- Boyd JH, Moinuddin A, Strunk RC, DeBaun MR. Asthma and acute chest in sickle-cell disease. Pediatr Pulmonol. 2004;38:229-32. https://doi.org/10.1002/ppul.20066 PMid:15274102

- Bernaudin
F, Strunk RC, Kamdem A, Arnaud C, An P, Torres M, Delacourt C, DeBaun
MR. Asthma is associated with acute chest syndrome, but not with an
increased rate of hospitalization for pain among children in France
with sickle cell anemia: a retrospective cohort study. Haematologica.
2008;93:1917-8 https://doi.org/10.3324/haematol.13090 PMid:18815195

- Frei-Jones
MJ, Field JJ, DeBaun MR.Risk factors for hospital readmission within 30
days: a new quality measure for children with sickle cell disease.
Pediatr Blood Cancer. 2009;52:481-5. https://doi.org/10.1002/pbc.21854 PMid:19058209 PMCid:PMC2730199

- Isgrò
A, Paciaroni K, Gaziev J, Sodani P, Gallucci C, Marziali M, Angelis GD,
Alfieri C, Ribersani M, Roveda A, Akinyanju OO, Wakama TT, Olowoselu
FO, Adediran A, Lucarelli G. Haematopoietic stem cell transplantation
in Nigerian sickle cell anaemia children. Niger Med J.
2015;56(3):175-9. https://doi.org/10.4103/0300-1652.160355 PMid:26229224 PMCid:PMC4518332

- Lum
S, Bountziouka V, Sonnappa S, Wade A, Cole TJ, Harding S, Wells JC,
Griffiths C, Treleaven P, Bonner R, Kirkby J, Lee S, Raywood E, Legg S,
Sears D, Cottam P, Feyeraband C, Stocks J. Lung function in children in
relation to ethnicity, physique and socioeconomic factors. Eur Respir
J. 2015;46(6):1662-71. https://doi.org/10.1183/13993003.00415-2015 PMid:26493801

- Walters
MC, Hardy K, Edwards S, Adamkiewicz T, Barkovich J, Bernaudin F,
Buchanan GR, Bunin N, Dickerhoff R, Giller R, Haut PR, Horan J, Hsu LL,
Kamani N, Levine JE, Margolis D, Ohene-Frempong K, Patience M,
Redding-Lallinger R, Roberts IA, Rogers ZR, Sanders JE, Scott JP,
Sullivan KM; Multicenter Study of Bone Marrow Transplantation for
Sickle Cell Disease. Pulmonary, Gonadal, and Central Nervous System
Status after Bone Marrow Transplantation for Sickle Cell Disease. Biol
Blood Bone Marrow Transplant. 2010; 16(2):263-272. https://doi.org/10.1016/j.bbmt.2009.10.005


















