Ignazio Majolino1, Dosti Othman2, Attilio Rovelli1, Dastan Hassan2, Luqman Rasool2, Michele Vacca1, Nigar Abdalrahman2, Chra Abdullah2, Zhalla Ahmed2, Dlir Ali2, Kosar Ali2, Chiara Broggi1, Cinzia Calabretta1, Marta Canesi1, Gloria Ciabatti1, Claudia Del Fante1, Elisabetta De Sapio1, Giovanna Dore1, Andrea Frigato1, Marcela Gabriel1, Francesco Ipsevich1, Harem Kareem2, Dana Karim2, Rosa Leone1, Tavan Mahmood2, Annunziata Manna1, Maria Speranza Massei1, Andrea Mastria1, Dereen Mohammed2, Rebar Mohammed2, Khoshnaw Najmaddin2, Diana Noori2, Angelo Ostuni1, Angelo Palmas1, Marco Possenti1, Ali Qadir2, Giorgio Real1, Rebwar Shrif2, Caterina Valdatta1, Stefania Vasta1, Marta Verna1, Mariangela Vittori1, Awder Yousif2, Francesco Zallio1, Alessandro Calisti1 , Sergio Quattrocchi3 and Corrado Girmenia1.
1 Institute for University Cooperation (ICU), Rome Italy.
2 Hiwa Cancer Hospital (HCH), Sulaymaniyah, Iraqi Kurdistan.
3 Italian Agency for Development Cooperation (AICS), Rome, Italy.
Corresponding
author: Ignazio Majolino, Via Antonio Cerasi 22, 00152 Rome, Italy. Tel: +39-3381519277. E-mail:
ignazio.majolino@gmail.com
Published: April 15, 2017
Received: February 16, 2017
Accepted: March 20, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017031 DOI
10.4084/MJHID.2017.031
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
We
describe the entire process leading to the start-up of a hematopoietic
stem cell transplantation center at the Hiwa Cancer Hospital, in the
city of Sulaymaniyah, Kurdistan Iraqi Region. This capacity building
project was funded by the Italian Development Cooperation Agency and
implemented with the support of the volunteer work of Italian
professionals, either physicians, nurses, biologists and technicians.
The intervention started in April 2016, was based exclusively on
training and coaching on site, that represent a significant innovative
approach, and led to a first autologous transplant in June 2016 and to
the first allogeneic transplant in October. At the time of reporting, 9
months from the initiation of the project, 18 patients have been
transplanted, 15 with an autologous and 3 with an allogeneic graft. The
center at the HCH represents the first transplantation center in
Kurdistan and the second in wide Iraq. We conclude that international
development cooperation may play an important role also in the field of
high-technology medicine, and contribute to improved local centers
capabilities through country to country scientific exchanges. The
methodology to realize this project is innovative, since HSCT experts
are brought as volunteers to the center(s) to be started, while
traditionally it is the opposite, i.e. the local professionals to be
trained are brought to the specialized center(s).
|
Introduction
Hemopoietic
stem cell transplantation (HSCT), either autologous or allogeneic, is
an effective treatment for many hematologic disorders. On a global
basis, over 70.000 procedures are currently performed every year in
more than 70 countries.[1] Unfortunately, due to
economical and/or political constraints, not all the countries and
geographical areas have enough resources and expertise to establish a
HSCT program. This implies that in many countries patients are forced
to emigrate when a transplant is needed, with heavy social and economic
problems for their families and the governments.
The Hiwa Cancer
Hospital (HCH) of Sulaymaniyah is a leading oncology institution in
Iraqi Kurdistan. In 2015, the Institute for University Cooperation
(ICU) of Rome identified this center as a possible target for a project
of high-technology medical intervention addressed to the development of
a HSCT center devoted to the treatment of malignant and non-malignant
hematologic disorders, in particular thalassemia major, which
represents a major problem in the country. A transplantation expert
from Italy made a preliminary visit to the HCH, confirming the
feasibility of a stem cell transplantation program. A capacity-building
project was designed and submitted to the Italian Development
Cooperation Agency (AICS), which approved its funding on March 2016.
Capacity
building is the process by which individuals, organizations,
institutions and societies develop abilities to perform functions,
solve problems and set and achieve objectives.[2] In
this paper we describe the entire process leading to the start-up of
the Center, the results obtained 9 months after the start of the
project and future perspectives. This is the first stem cell
transplantation center established in the Kurdistan Region, and the
second in Iraq. We conclude that international development cooperation
may be of great value in the field of high-technology medicine and
contribute to improved local centers’ capabilities, through
country-to-country scientific exchanges. Moreover, on-site training and
coaching proves an effective innovative method to establish a
sustainable activity in developing countries, as alternative to a more
traditional methodology, where local professionals to be trained are
brought to the specialized center(s) with higher expenditure and less
predictable final results.
Methods
Exploratory mission:
A relationship between the HCH and the ICU started in July 2015, when
an Italian HSCT expert conducted an exploratory mission on behalf of
ICU in order to ascertain the feasibility of a stem cell
transplantation project at the HCH. A transplantation unit (TU) with
positive pressure single rooms had been previously built in the HCH,
thanks to a donation of the Regione Toscana, Italy, but the unit was
never activated due in part to limited availability of adequate skills
and in part to economic problems.
During the first visit, an
appropriate grid, already successfully employed in other circumstances
and containing all the necessary questions, was applied to verify the
adequacy of the hospital itself and of all the necessary services that
are normally involved in HSCT. The areas involved in the process were
those listed in table 1. The
director of HCH and most of the single-sector responsible physicians or
administrators were interviewed. Inspections were also conducted to
better ascertain the availability and functioning of instrumentations
and devices. At the end of the visit, a positive evaluation was
released, confirming the feasibility of a capacity building project for
the start-up of a stem cell transplantation activity. A simplified
scheme of our project is shown in figure 1.
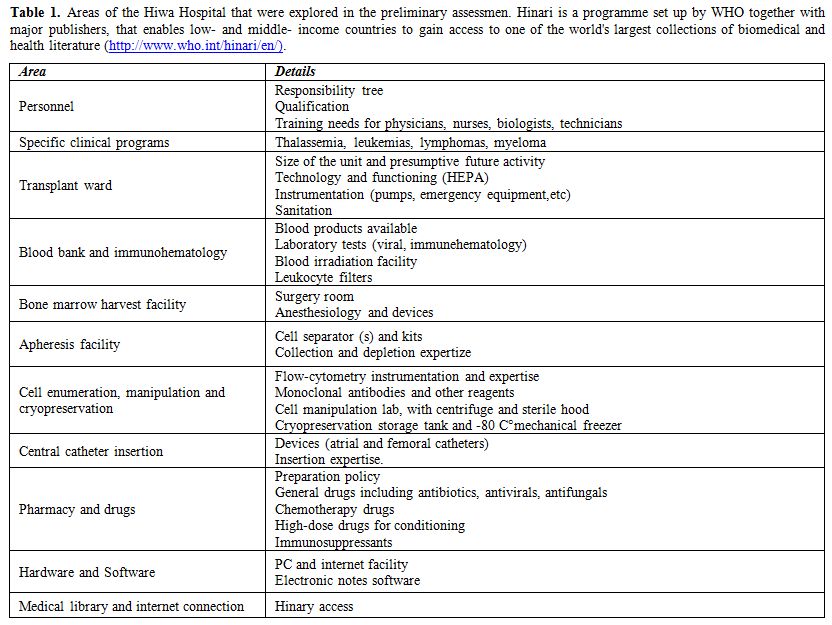 |
Table 1.
Areas of the Hiwa Hospital that were explored in the preliminary
assessmen. Hinari is a programme set up by WHO together with major
publishers, that enables low- and middle- income countries to gain
access to one of the world's largest collections of biomedical and
health literature (http://www.who.int/hinari/en/). |
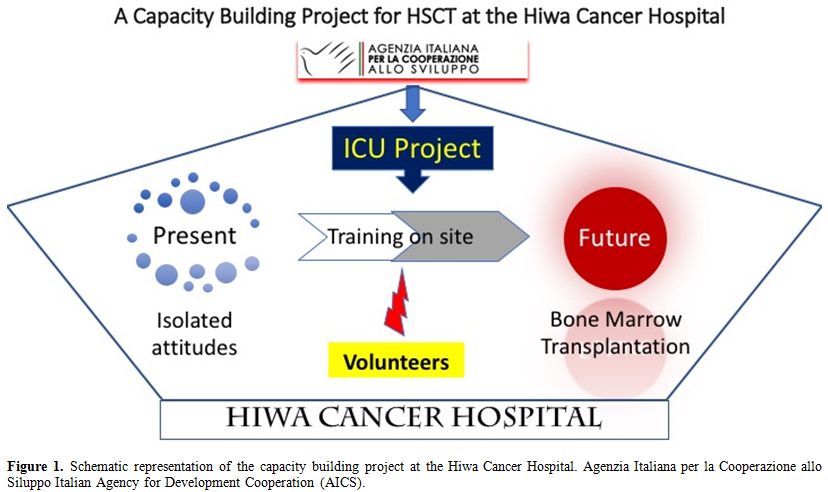 |
Figure 1. Schematic representation of the
capacity building project at the Hiwa Cancer Hospital. Agenzia Italiana
per la Cooperazione allo Siluppo Italian Agency for Development
Cooperation (AICS). |
Project definition and funding:
The second step was designing the project. This was done according to a
call for proposal (n. 10548/02/0) by the Italian Ministry for Foreign
Affairs – General Direction for Development Cooperation. A capacity
building project was submitted by ICU, approved and funded in December
2015. The assigned budget was € 329,000. The contribution of HCH itself
to the financial plan consisted of existing instrumentation and
laboratory facilities, but the HCH also provided for the accommodation
of the volunteers for the whole duration of the project.
Unfortunately,
still in the month of December 2015 a fire accident suddenly developed
in the TU, due to malfunctioning of the air-treatment unit, with severe
damage to the whole TU for a cost of approx. $ 200,000. Though this
obviously represented a factor for a possible delay or even suspension
of the project, the scientific advisor of ICU and the responsible for
AICS prompted for a rapid restoration of the TU, that HCH started in
April 2016. The delay was therefore minimal, and the team could begin
the training activity the same month, while the restoration works were
ended in July.
The capacity building process:
To reach the target of a self-sustainable HSCT activity at the HCH,
efforts were directed to the training of local personnel, in particular
to perform functions, solve problems and set and achieve objectives.[2]
The scientific advisor of the project also coordinated the volunteers
who delivered training with lectures and seminars, and were also in
charge of editing and verifying protocols, as well as of steering
clinical work and coaching the local personnel. This was done by
attending the morning patient tour and the afternoon outpatient clinic,
or participating to the laboratory activities. The personnel involved
in the HSCT program, either Italian and Kurdish, also attended the
regular weekly activities, such as the morning briefings (every
working-day), the weekly seminars on clinical and scientific issues,
the transplantation meetings (once a week) with discussion of all the
transplantation cases. The chairman of the transplantation meeting
regularly took minutes that were regularly distributed to all
participants. Results
Project start-up:
In April 2016, we decided to hold the preliminary training course
addressed to doctors, nurses, biologists and technicians of the HCH.
The course took approximately 3 weeks. A list of the covered subjects
is reported in table 2. Editing and verification of clinical and laboratory protocols dedicated to the transplantation program (Table 3)
were conducted during the same period. The hospital provided the
dedicated staff, and the director also drew an organigram depicting the
responsibility tree. This led to a substantial modification of the
organization, also to cope with existing international standards, as
those defined by JACIE at http://www.jacie.org/standards.
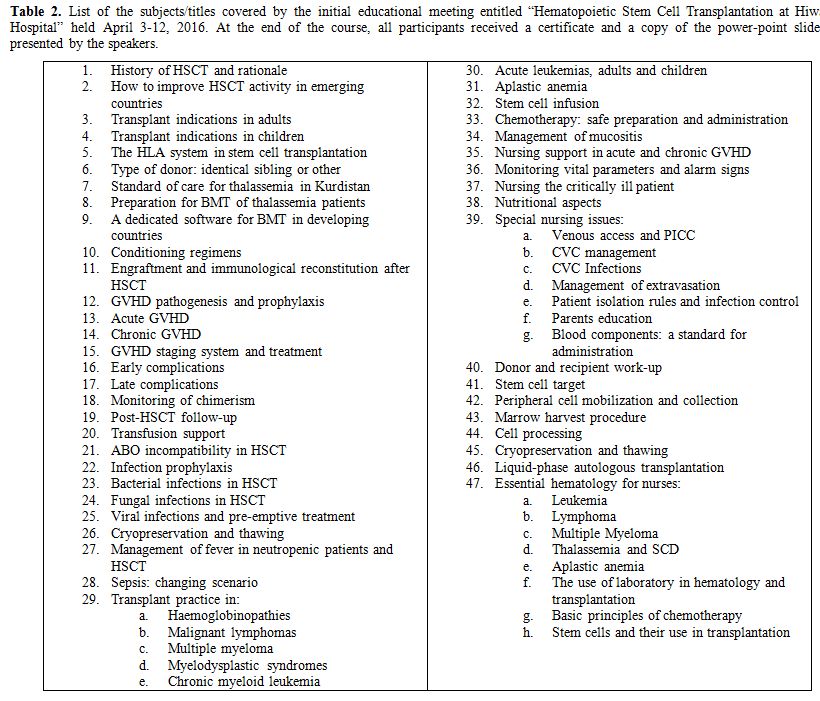 |
Table 2.
List of the subjects/titles covered by the initial educational
meeting entitled “Hematopoietic Stem Cell Transplantation at Hiwa
Hospital” held April 3-12, 2016. At the end of the course, all
participants received a certificate and a copy of the power-point
slides presented by the speakers. |
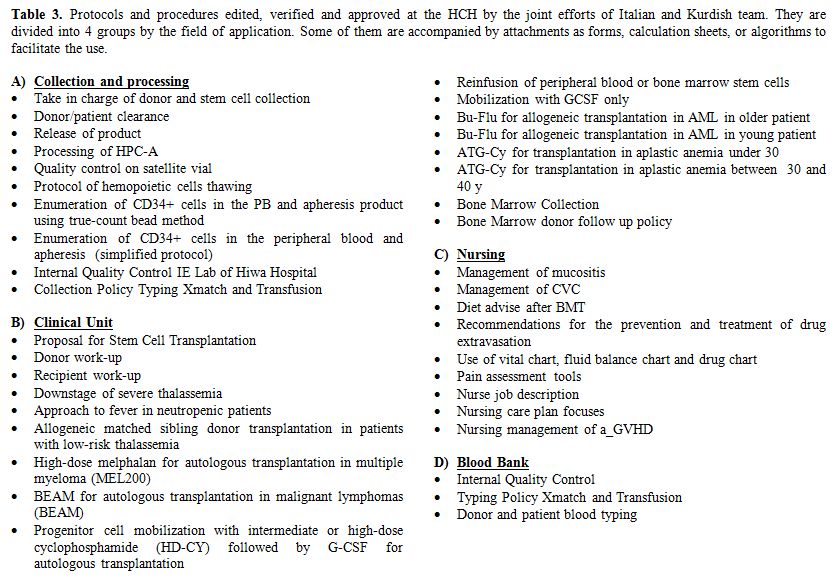 |
Table 3. Protocols and procedures edited,
verified and approved at the HCH by the joint efforts of Italian and
Kurdish team. They are divided into 4 groups by the field of
application. Some of them are accompanied by attachments as forms,
calculation sheets, or algorithms to facilitate the use. |
The
apheresis facility was the first to be started with 2 new-generation
cell separators, a Fresenius Comtec, and an Amicus Fenwall device. A
reliable and easy to use flow-cytometry double platform technique for
CD34+ cell enumeration was assessed, based on a well established
methodology.[3] The manipulation laboratory and a
technique for cell cryopreservation were set up by the Italian team and
implanted in the HCH. A well-equipped transplantation sterile ward,
with 6 HEPA-filtered, positive pressure, conditioned-air single rooms
was already present and ready for use. At the beginning the
cryopreservation was carried out by means of a -80°C mechanical freezer
alone,[4] but later a fully equipped liquid nitrogen
tank was supplied and cells were initially freezed in the -80°C to be
later stored in the liquid phase of liquid nitrogen.
One month
later, a series of patients underwent clinical selection procedures
based on previously approved criteria and including age, general
performance, organ function, disease phase and informed consent, and
some of them were finally admitted for the stem cell collection and
cryopreservation in view of the autologous transplantation. For stem
cell mobilization, in patients with multiple myeloma we used a protocol
with G-CSF alone (G-CSF 10 µg/kg/day
until the CD34+ cell collection target was achieved, usually day 5),
while in lymphoma patients harvest was done in the context of the
advanced disease protocol itself. This was BeGeV[5] in Hodgkin lymphoma, DHAP in non-Hodgkin’s lymphomas, always with addition of G-CSF 10 µg/kg/day
since the end of chemotherapy to the day when CD34+ cell collection
target was achieved. In some cases, also the intermediate-dose (2 to 4
g/m2) cyclophosphamide mobilization protocol[6,7] was employed. Since in this phase of the program only single transplants were planned, a target of 5 x 106/Kg CD34+ cells was set, using an algorithm to have an accurate collection prediction,[8]
with an intention to increase the value as soon as the preliminary
results would confirm us of the adequacy of the procedures in view of a
double autologous transplantation program.
First autologous transplants:
A first autologous transplant was carried out 3 months after the
program was started in a multiple myeloma patient, using melphalan 140
mg/m2 as high-dose regimen and
peripheral blood stem cells (PBSC) as autograft. The engraftment was
prompt without major complications. The following month another myeloma
patient was successfully autografted, and the program was therefore set
out with a series of candidates either with myeloma or malignant
lymphoma. Since July, when the HSCT Unit restoration was completed, the
transplants were all carried out in the new ward. The clinical
characteristics of the patients and the data of stem cell collection,
transplantation and engraftment are reported in table 4.
There were 15 patients, 11 males and 4 females. Median age was 40 years
(range 20 to 60). MM patients were 7, HL were 6 and NHL were 2. Status
of disease was CR1 in 4, CR2 in 5, PR1 in 3 and SR in 2. Following the
high-dose therapy, all the patients received G-CSF 5 mcg/kg to speed
engraftment. All received antiviral and antifungal
prophylaxis. The median number of CD34+ cells infused was 5.5 x 106/Kg
(range 4.6 to 20.0). All patients fully engrafted but one who died with
an acute heart failure on day+19 with granulocyte engraftment but
without platelet engraftment. In this series, granulocyte engraftment
(≥0.5 x 109/L) occurred on (median) day +11 with a narrow range from 9 to 12. Platelet engraftment (≥20.0 x 109/L)
occurred on (median) day +12, range 10 to 17. Thirteen out of the 15
patients underwent a febrile complication, with or without bacterial
isolation. Only one patient had a life-threatening complication, with
intestinal perforation, but underwent a successful surgical
intervention. Data on disease reevaluation are not presented as
follow-up is currently too short. All patients but one are alive at a
median of 75 days from transplant (range 15-223).
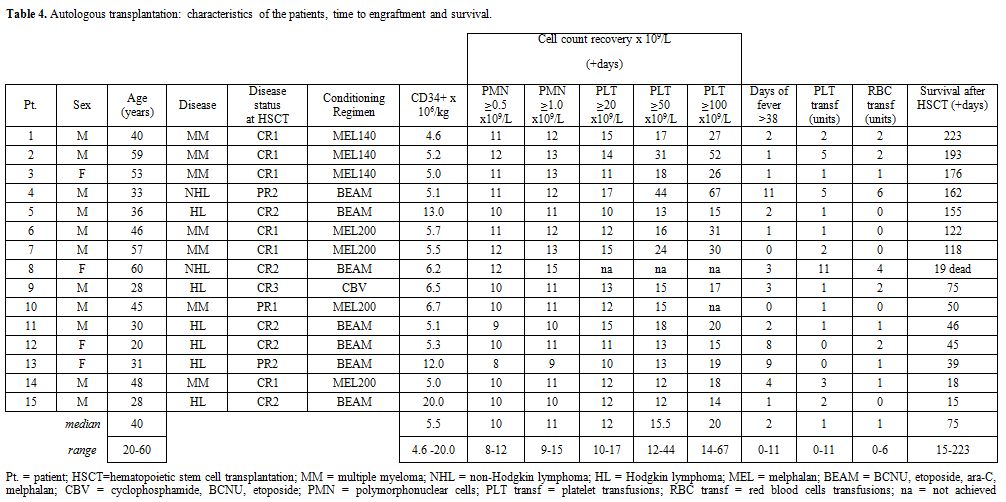 |
Table 4.
Autologous transplantation: characteristics of the patients, time to engraftment and survival. |
First allogeneic transplants:
The initial allogeneic program was set up with the aim to offer a cure
to the most frequent hematological disease in the region,[9] i.e. thalassemia.[10]
More than one thousand patients with thalassemia live in the area of
Sulaymaniyah, most of them are children belonging to large families and
having therefore a high probability of a matched family donor. Patients
eligible to transplant were considered those with low-risk
characteristics (age ≤ 7 years, liver size ≤ 2 cm below costal margin)
and a HLA matched sibling donor.[11,12] A downstaging
protocol with hydroxyurea and deferoxamine or deferasirox was adopted
in cooperation with the Thalassemia & Congenital Blood Diseases
Center in Sulaymaniyah directed by LR. Conditioning regimen included iv
busulfan and cyclophosphamide.[13] GvHD and rejection prophylaxis included ATG,[14]
from day -12 to -10, and cyclosporin, methotrexate and
methylprednisolone. The first allogeneic HSCT was performed on October
8th, 2016 and up to now overall 3 patients (2 females, 1 male)
underwent HSCT. All of them received GCSF-primed bone marrow[15] from
an HLA matched sibling. All donor/recipient couples shared the
same blood group and were CMV concordant, i.e. all CMV positive.
Engraftment occurred at a median time of 17 days. No major
complications were observed in the early aplastic phase after HSCT. One
patient developed grade II aGvHD and other potentially life-threatening
complications (CMV enterocolitis, low grade microangiopathy, PRES)
which resolved with proper treatment. All three patients have been
already discharged at home (on day +25, +27 and +96, respectively);
they are alive and well, continuing immunosuppression. Two of them are
already transfusion independent, the third, though full donor chimera,
having just recovered from many complications, not yet.
Discussion
Iraqi
Kurdistan first gained autonomous status in 1970 following an agreement
with the Iraqi government, and was re-confirmed as an autonomous entity
in 2005. The region has considerable oil and mineral resources.
However, due to the current conflict with the Islamic State, with more
than a million Syrian and Iraqi refugees seeking shelter in the Kurdish
territory, and also due to the fall of oil price, since 2012 the
country entered a deep economic crisis that also involved the health
system. The Italian Ministry of Foreign Affairs, through the AICS, is
regularly supporting the Kurdish population also with health and social
projects.
We decided to dedicate our efforts to the
development of HSCT at the HCH of Sulaymaniyah mainly for two reasons.
First, HCH is today the main center in the Kurdish territory treating
hematologic malignancies and congenital disorders as thalassemia major,
the latter occurring at high frequency in Kurdistan. Second, at the
time of our first visit the HCH counted already with most of the
facilities necessary for an HSCT program, nevertheless an external
support would be needed.
Specifically, in the project we
developed at the HCH, the capacity-building methodology addressed the
implementation of a sustainable HSCT program through the collaboration
with experts in the field of adult hematology, pediatric
hemato-oncology, transfusion medicine, apheresis, infectious diseases,
nursing, cell manipulation, molecular biology and biophysics coming
from different Italian institutions. Almost all these experts had a
specific and long-lasting experience in the field of HSCT, and were
selected not only on the basis of their competence, but also of their
previous experience of cooperation with developing countries. All of
them were volunteers, while the non-governmental organization ICU
provided funds administration and reporting.
It is a common
belief that among the main obstacles in the implementation of
technically sophisticated procedures, as it is the case for HSCT, the
most important are the frequent lack of a priority scale, the absence
of teamwork as well as of appropriate methodology for problem-solving,
decision-sharing and quality management. A tendency not to establish a
transparent and effective responsibility tree is another factor.
All these issues are more prominent in developing countries, where also
procurement of resources and consequently of instrumentation and
reagents is often critical.
Since the beginning our efforts were
dedicated to training. Different techniques were used, not only the
traditional lectures and seminars, but principally the coaching method.
Written protocols and procedures were developed, and the method of
shared decisions was adopted to solve the clinical and laboratory
problems. This is a key function not only for the start-up but also for
quality control and improvement.
On-site training and coaching
represent an innovative method to establish a sustainable activity in
developing countries, as alternative to a more traditional one, where
local professionals to be trained are brought to the specialized
center(s) with higher expenditure and less predictable final results.
At present, we have no evidence that on-site training has more efficacy
compared with the traditional methodology, and what are the situations
where it would be more appropriate. With all the current limitations
for immigration policies, in the future more projects based on capacity
building on-site will probably be developed, and more data will be
available.
With the start-up of the autologous transplantation
program in June 2016 the HCH progressively developed an autonomous
capacity, and consolidated the technical skills not only in the fields
of apheresis, cell manipulation and immunohematology, but also in the
infection control.[16] In fact, by the end of 2016
among the 15 patients autografted, only one developed a
life-threatening infectious complication, but was eventually rescued.
Another patient died, due to sudden heart failure following initial
engraftment, a complication likely to be in part linked to age and a
borderline cardiac function. More severe criteria for admission were
consequently setup. Overall, the preliminary results seem encouraging
with prompt and stable hematologic recovery in all and few severe
complications.
The allogeneic transplantation program for
thalassemia at HCH, carries many advantages for the country: it reduces
psychosocial and financial burden for families and allows significant
saving for the government.[17] The estimated costs of
performing locally HSCT are lower than in the countries where patients
were previously referred; a systematic analysis of this costs will soon
be performed. Moreover, the new skills acquired together with the
continuation of cooperation are paramount for further implementing the
activity and extending the transplantation accessibility to children
with other disorders, as leukemias, bone marrow failures,
immune-deficiencies and others.
Here only the initial results of
the HSCT activity at the HCH are reported. We are aware that, after
start-up, transplantation activity needs resources and organization
over the medium and long term to ensure full autonomy of the Center. To
that purpose we also introduced the center to the international context
registering it as full member in the EBMT, and promoted the search for
scientific grants in order to allow medical doctors and other
professionals to visit other centers in Europe and the US. In addition,
a new project on pediatric hematology was submitted to the AICS and
recently funded. This new project, managed by the NGO AVSI, is aimed at
improving biological and clinical aspects of childhood leukemia
management at the HCH, but also to strengthen the transplantation
program, especially in the allogeneic field.
Conclusions
Thanks
to the cooperation initiative we described, the HCH is the only center
performing also allogeneic HSCT in the Iraqi Kurdistan Region, and in
the whole Iraq. We conclude that international cooperation may be of
great value also in the field of high-technology medicine, and may
contribute to improve the capabilities even of centers in critical
contexts, representing a valuable instrument also in fostering
country-to-country scientific exchanges.
Acknowledgements
We
are greatly indebted to Miss Sham O. Hamawandi, of the HCH secretary,
who made an incredible work coordinating the efforts of the Italian and
Kurdish teams. We also thank Dr. Francesca Bonifazi (President of
GITMO) for facilitating the recruitment of volunteer medical doctors.
Dr. Aleksandra Babic (President of the EBMT nursing board) and Dr.
Giampaolo Gargiulo (Responsible for the GITMO nurse group) contributed
substantially to the success of the project by selecting the best
professionals among the nurses in Italy and other countries in
Europe. Dr. Giorgio Dini was of great help in the construction of
the training program. A number of institutions provided supplementary
resources to the project. Dr. Roberto Nannerini (Arcobaleno
Association) and Prof. Alberto Ciferri (Jepa-Limmat Foundation) gave a
fundamental contribution with dedicated grants for the stages of two
biologists in Italian institutions. Under its Visitor Training Program
the American Society of Hematology also funded a stage in Italy of a
Kurdish medical doctor. We would never have succeeded without the
help of many Kurdish authorities, in particular Dr. Rekawt H. Rashid
Karim (Minister of Health) and Dr. Meran Mohamad Abas (Director of
Health, Sulaimanyah). The Iraq Program Coordinator of the Italian NGO
“Emergency”, Eng. Hawar Mustafa, provided us a with a fraternal support
and was often of incredible help with bureaucracy. We wish to thank him
warmly. We want to tribute a special acknowledgment to the Italian
Consul in Erbil, Dr Alessandra Di Pippo, and the Italian Ambassador in
Baghdad Dr Marco Carnelos who assumed the project as a priority for the
Italian diplomacy and personally did any effort to sustain its
development also contributing to pose the cornerstone of the first
Kurdish HSCT center at the Hiwa Cancer Hospital in Sulaymaniyah. A
special thank for Prof Eduardo Missoni (Global Health and Development,
Bocconi Management School) who reviewed the manuscript, we feel
indebted for his criticism and encouragement.
References
- Gratwohl A, Pasquini MC, Aljurf M, Atsuta Y,
Baldomero H, Foeken L, Gratwohl M, Bouzas LF7, Confer D, Frauendorfer
K, Gluckman E, Greinix H, Horowitz M, Iida M, Lipton J, Madrigal A,
Mohty M, Noel L, Novitzky N, Nunez J, Oudshoorn M, Passweg J, van Rood
J, Szer J, Blume K, Appelbaum FR, Kodera Y, Niederwieser D; Worldwide
Network for Blood and Marrow Transplantation (WBMT). One million
haemopoietic stem-cell transplants: a retrospective observational
study. Lancet Haematol. 2015 Mar;2(3): e91-100. Epub 2015 Feb 27. https://doi.org/10.1016/S2352-3026(15)00028-9

- Garriga M (2013): The Capacity Building Concept. Available from http://www.coastalwiki.org/wiki/The_Capacity_Building_Concept

- Brando
B, Siena S, Bregni M, Gianni AM, Grillo P, Sommaruga E. A standardized
flow cytometry protocol for mobilized peripheral CD34+ cells estimation
and collection for autotransplantation in cancer patients. Eur J
Histochem 1994; 38: 21-6. PMid:8547706

- Calvet
L, Cabrespine A, Boiret-Dupré N, Merlin E, Paillard C, Berger M, Bay
JO, Tournilhac O, Halle P. Hematologic, immunologic reconstitution, and
outcome of 342 autologous peripheral blood stem cell transplantations
after cryopreservation in a -80°C mechanical freezer and preserved less
than 6 months. Transfusion. 2013 Mar;53(3):570-8. https://doi.org/10.1111/j.1537-2995.2012.03768.x

- Santoro
A, Mazza R, Pulsoni A, Re A, Bonfichi M, Zilioli VR, Salvi F, Merli F,
Anastasia A, Luminari S, Annechini G, Gotti M, Peli A, Liberati AM, Di
Renzo N, Castagna L, Giordano L, Carlo-Stella C. Bendamustine in
combination with gemcitabine and vinorelbine is an effective regimen as
induction chemotherapy before autologous stem-cell transplantation for
relapsed or refractory Hodgkin lymphoma: final results of a multicenter
phase II study. J Clin Oncol. 2016 Sep 20;34(27):3293-9. https://doi.org/10.1200/JCO.2016.66.4466

- Indovina
A, Liberti G, Majolino I, Buscemi F, Scimè R, Gentile S, Vasta S,
Pampinella M, Cappuzzo V, Santoro A. Cyclophosphamide 4 g/m2 plus
rhG-CSF for mobilization of circulating progenitor cells in malignant
lymphomas. Int J Artif Organs. 1993 Dec;16 Suppl 5:30-4.
PMid:7516915

- Bashey
A, Donohue M, Liu L, Medina B, Corringham S, Ihasz A, Carrier E, Castro
JE, Holman PR, Xu R, Law P, Ball ED, Lane TA. Peripheral blood
progenitor cell mobilization with intermediate-dose cyclophosphamide,
sequential granulocyte-macrophage-colony-stimulating factor and
granulocyte-colony-stimulating factor, and scheduled commencement of
leukapheresis in 225 patients undergoing autologous transplantation.
Transfusion. 2007 Nov;47(11):2153-60. https://doi.org/10.1111/j.1537-2995.2007.01440.x PMid:17958545

- Pierelli
L, Maresca M, Piccirillo N, Pupella S, Gozzer M, Foddai ML, Vacca M,
Adorno G, Coppetelli U, Paladini U. Accurate prediction of autologous
stem cell apheresis yields using a double variable-dependent method
assures systematic efficiency control of continuous flow collection
procedures. Vox Sang. 2006 Aug;91(2):126-34. PubMed PMID: 16907873. https://doi.org/10.1111/j.1423-0410.2006.00796.x PMid:16907873

- Hamamy
HA, Al-Allawi NA. Epidemiological profile of common haemoglobinopathies
in Arab countries. J Community Genet. 2013, 4:147-67. https://doi.org/10.1007/s12687-012-0127-8 PMid:23224852 PMCid:PMC3666833

- Lucarelli
G, Galimberti M, Polchi P, Angelucci E, Baronciani D, Giardini C,
Andreani M, Agostinelli F, Albertini F, Clift RA. Marrow
transplantation in patients with thalassemia responsive to iron
chelation therapy. N Engl J Med. 1993; 329: 840-4. https://doi.org/10.1056/NEJM199309163291204 PMid:8355742

- Angelucci
E, Matthes-Martin S, Baronciani D, Bernaudin F, Bonanomi S, Cappellini
MD, Dalle JH, Di Bartolomeo P, de Heredia CD, Dickerhoff R, Giardini C,
Gluckman E, Hussein AA, Kamani N, Minkov M, Locatelli F, Rocha V,
Sedlacek P, Smiers F, Thuret I, Yaniv I, Cavazzana M, Peters C; EBMT
Inborn Error and EBMT Paediatric Working Parties. Hematopoietic stem
cell transplantation in thalassemia major and sickle cell disease:
indications and management recommendations from an international expert
panel. Haematologica. 2014, 99:811-20. https://doi.org/10.3324/haematol.2013.099747 PMid:24790059 PMCid:PMC4008115

- Sabloff
M, Chandy M, Wang Z, Logan BR, Ghavamzadeh A, Li CK, Irfan SM, Bredeson
CN, Cowan MJ, Gale RP, Hale GA, Horan J, Hongeng S, Eapen M, Walters
MC. HLA-matched sibling bone marrow transplantation for ß-thalassemia
major. Blood. 2011, 117:1745-50. https://doi.org/10.1182/blood-2010-09-306829 PMid:21119108 PMCid:PMC3056598

- Mathews V, Savani BN. Conditioning regimens in allo-SCT for thalassemia major. Bone Marrow Transplant, 2014; 49:607-10. https://doi.org/10.1038/bmt.2013.216 PMid:24442250

- Goussetis
E, Peristeri I, Kitra V, Vessalas G, Paisiou A, Theodosaki M, Petrakou
E, Dimopoulou MN, Graphakos S. HLA-matched sibling stem cell
transplantation in children with ß-thalassemia with anti-thymocyte
globulin as part of the preparative regimen: the Greek experience. Bone
Marrow Transplant, 2012;47:1061-6. https://doi.org/10.1038/bmt.2011.219 PMid:22080966

- Deotare
U, Al-Dawsari G, Couban S, Lipton JH. G-CSF-primed bone marrow as a
source of stem cells for allografting: revisiting the concept. Bone
Marrow Transplant, 2015; 50:1150-6 https://doi.org/10.1038/bmt.2015.80 PMid:25915812

- Girmenia
C, Viscoli C, Piciocchi A, Cudillo L, Botti S, Errico A, Sarmati L,
Ciceri F, Locatelli F, Giannella M, Bassetti M, Tascini C, Lombardini
L, Majolino I, Farina C, Luzzaro F, Rossolini GM, Rambaldi A.
Management of carbapenem resistant Klebsiella pneumoniae infections in
stem cell transplant recipients: an Italian multidisciplinary consensus
statement. Haematologica. 2015 Sep;100(9):e373-6. doi:
10.3324/haematol.2015.125484 https://doi.org/10.3324/haematol.2015.125484

- Faulkner
LB, Uderzo C, Masera G. International cooperation for the cure and
prevention of severe hemoglobinopathies. J Pediatr Hematol Oncol. 2013;
35: 419-23. doi: 10.1097/MPH.0b013e31829cd920. Review. https://doi.org/10.1097/MPH.0b013e31829cd920

[TOP]





















