Pulkit Rastogi, Sreejesh Sreedharanunni, Uday Yanamandra, Man Updesh Singh Sachdeva and Neelam Varma
Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh - 160012, India.
Corresponding
author: Dr. Sreejesh Sreedharanunni,
MD, DM. Assistant Professor, Dept of Hematology, 5th Floor, Research
Block A, Postgraduate Institute of Medical Education and Research,
Chandigarh – 160012, India. Tel: +91-9478053220, Fax: +91-172-2747124.
sreejesh.s@pgimer.edu.in
Published: May 1, 2017
Received: January 23, 2017
Accepted: April 1, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017033 DOI
10.4084/MJHID.2017.033
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Objectives: We
report a case of hairy cell leukemia (HCL) initially misdiagnosed as
plasma cell dyscrasia due to various clinical, morphological and
immunophenotypic confounders.
Methods and results:
In a patient diagnosed of marrow plasmacytosis and serum monoclonal
protein elsewhere and referred to our hospital, morphological
evaluation of bone marrow aspirate smears and trephine biopsy,
immunophenotyping, and molecular testing (BRAFV600E mutation) were
done. Clinically, the patient was asymptomatic; bone marrow revealed
plasmacytosis, mastocytosis, and lymphocytosis with a few "hairy"
cells. Immunophenotyping showed features of HCL with aberrant CD10
expression and a large subclone of CD19neg cells. A diagnosis of HCL
with reactive plasmacytosis and mast cell hyperplasia was made and
confirmed by immunophenotyping and molecular studies.
Conclusion:
Hematopathologists must be aware of various confounding factors and
should judiciously use flow cytometric and molecular studies for
attaining a proper diagnosis of HCL. We also report a very rare
immunophenotypic aberrancy (CD 19 negativity) in HCL.
|
Introduction
Hairy
cell leukemia (HCL) is an uncommon yet unique hematolymphoid neoplasm
exhibiting a characteristic cytomorphology, immunophenotype, and well
defined molecular features. It accounts for 2% of all lymphoid
leukemias.[1] Typically, it presents with splenomegaly, pancytopenia,
and monocytopenia; and shows a characteristic immunophenotype. The
cells are universally CD19positive with co-expression of CD25, CD11c,
CD103 and CD123. Patients of HCL can present with a spectrum of
different clinical and pathological characteristics often puzzling a
clinician or a pathologist. However, the clinical and pathological
findings often complement each other to clinch the diagnosis of HCL. A
correct diagnosis is mandatory as specific therapy in the form of
purine analogues can provide long-term remissions in such patients.[2]
We present a case of HCL with atypical clinical and laboratory features
confounding the primary hematological abnormality.
Case Reports
Clinical history:
A 69-year-old male with no known co-morbidities presented with
complaints of breathlessness on exertion for 15 days. It was not
associated with fever, cough, or purulent expectoration. He had
moderate pallor, tachycardia, and tachypnoea. There was no
lymphadenopathy or hepatosplenomegaly. Respiratory system evaluation
revealed features suggestive of consolidation confirmed by chest
roentgenogram. He improved with parenteral antibiotics. Meanwhile, he
was detected to have pancytopenia [Hemoglobin (Hb) – 91g/L, total
leukocyte count (TLC) – 2.3x109/L, and platelet count – 122 x109/L]
with the nadir absolute neutrophil count (ANC) of 252/µL. He was
subjected to bone marrow evaluation which revealed plasmacytosis
(plasma cells – 10%). A serum protein electrophoresis (SPEP)
performed subsequently, showed M-spike (0.3g/dL), though no
immunofixation studies were done. He was referred to our center for
further evaluation of suspected plasma cell dyscrasia.
On the
assessment at our center, he was asymptomatic. He did not complain of
bone pains. Physical examination was not contributory. His pancytopenia
recovered (Hb-112 g/L, TLC-3.2 x109/L, ANC-1.4 x109/L, platelets-522 x109/L).
A repeat bone marrow examination was performed to evaluate suspected
plasma cell dyscrasia. Bone marrow aspirate (BMA) revealed 5% plasma
cells; however showed 36% larger lymphoid cells with clumped chromatin
and a moderate amount of pale basophilic cytoplasm. A few cells had
grooved/reniform nucleus or cytoplasmic projections (Figure 1A). An increase in mast cells was also noted. Trephine biopsy showed an interstitial infiltrate, typical of hairy cell leukemia (Figure 1B, C) along with an increase in mast cells confirmed by mast cell tryptase immunohistochemistry (Figure 1D).
No significant clusters of mast cells were highlighted. Repeat SPEP and
immunofixation study, done at our center, revealed polyclonal
hypergammaglobulinemia.
Multiparametric flow cytometry (Figure 2)
was performed on the BMA using four/six color antibody panels by
lyse-wash-stain method (antibodies from BD Biosciences, San Jose, CA).
One tube containing unstained leukocytes was used as negative control.
A minimum of one lakh events was acquired on dual laser BD FACS Canto
II and analyzed using BD FACS Diva software. Bright CD19positive low
side scatter events (5.3% of viable gated leucocytes) were gated which
were positive for CD10, CD20, CD22, CD79b, surface Igκ, CD25, CD11c,
CD103, and CD123. Serendipitously, we found a large subclone of cells
(15% of viable gated leucocytes) expressing exactly the same
immunophenotype markers except for CD19, indicating its loss of
expression from hairy cells. The fluorochrome related technical issues
were ruled out as the cells showed similar profile using both
anti-CD19PECy7 and anti-CD19APC-H7 (clone SJ25C1, BD Biosciences). The
CD19 negative cells had an immune profile exactly similar to CD19+ve
cells and revealed expression of hairy cell markers along with CD20,
CD22, CD79b, CD45, and CD10. The plasma cells (CD38pos/CD138pos/CD19pos/CD81pos/CD56neg and no light chain restriction – Figure 2) and mast cells (CD117pos/CD33pos/CD2neg/CD25neg)
showed normal immunophenotype indicating reactive plasmacytosis and
mast cell hyperplasia. A diagnosis of hairy cell leukemia with atypical
features (CD19 negative subclone, CD10 positivity, reactive
plasmacytosis and mast cell hyperplasia) was made which was
subsequently confirmed by amplification-refractory mutation system
polymerase-chain-reaction (ARMS-PCR) for BRAF V600E mutation (Figure 1E).
The
patient remained asymptomatic, and his laboratory parameters remained
normal; not warranting purine analogue therapy. He has been keeping
under close medical observation.
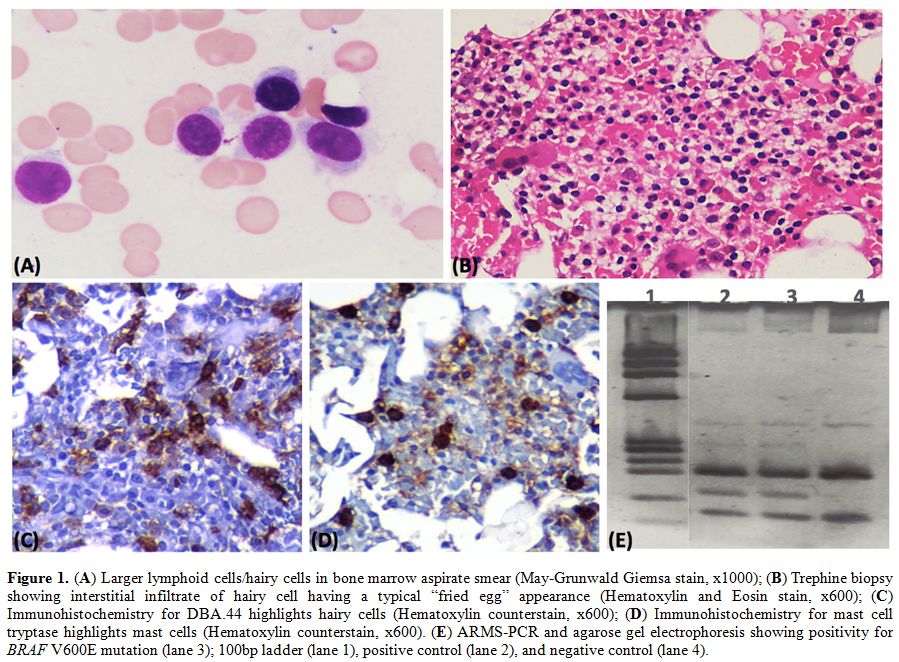 |
Figure 1. (A) Larger lymphoid cells/hairy
cells in bone marrow aspirate smear (May-Grunwald Giemsa stain, x1000);
(B) Trephine biopsy showing interstitial infiltrate of hairy cell
having a typical “fried egg” appearance (Hematoxylin and Eosin stain,
x600); (C) Immunohistochemistry for DBA.44 highlights hairy cells
(Hematoxylin counterstain, x600); (D) Immunohistochemistry for mast
cell tryptase highlights mast cells (Hematoxylin counterstain, x600).
(E) ARMS-PCR and agarose gel electrophoresis showing positivity for
BRAF V600E mutation (lane 3); 100bp ladder (lane 1), positive control
(lane 2), and negative control (lane 4). |
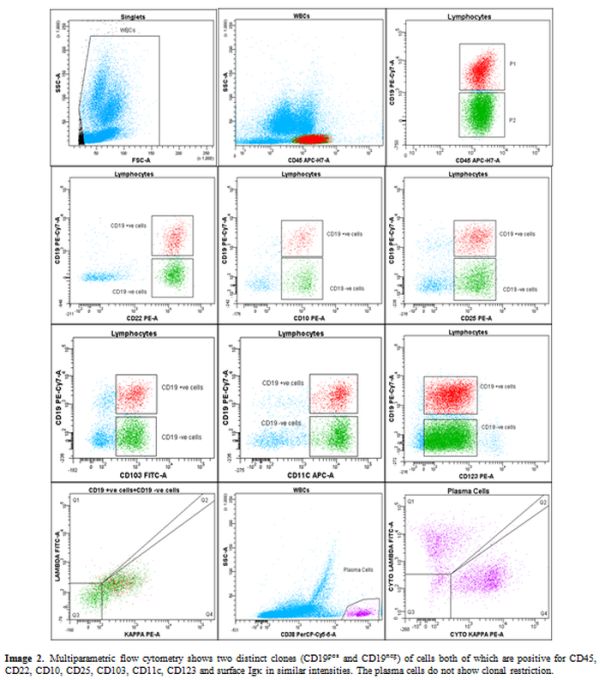 |
Figure 2. Multiparametric flow cytometry
shows two distinct clones (CD19pos and CD19neg) of cells both of which
are positive for CD45, CD22, CD10, CD25, CD103, CD11c, CD123 and
surface Igκ in similar intensities. The plasma cells do not show clonal
restriction. |
Discussion
HCL
is a unique B-cell non-Hodgkin lymphoma (NHL) characterized by
splenomegaly, cytopenias affecting two or more lineages and
morphologically by typical hairy cells. Though the majority of cases
have this typical presentation, there are scenarios in which the
clinical, morphological or immunophenotypic features are atypical,
leading to diagnostic confusion. The case presented here exemplifies
this intriguing situation where the patient on evaluation for a lower
respiratory tract infection was found to have cytopenias, had no
palpable splenomegaly and the bone marrow showed only a few "hairy
cells" along with confounders in the form of plasmacytosis and
mastocytosis. All these together with a minor quantity of serum
“M” protein led to initial misdiagnosis.
Splenomegaly is an
important feature seen in up to 90% of patients with HCL. However, its
absence should not exclude a diagnosis of HCL.[3] And more importantly,
a changing trend has been observed in the symptomatology of HCL over
the past 30 years. Number of cases are being diagnosed at an early
stage with a less marked splenomegaly.[4]
A co-existence of plasma
cell myeloma with HCL as well as the development of myeloma in patients
with HCL has been reported in the literature.[5] At times, plasma cell
myeloma/leukemia may mimic HCL also.[6-7] Clonal plasma cells were
excluded by flow cytometry and SPEP studies. The initial report of
small monoclonal band in SPEP from outside our institute might
represent a transient monoclonal gammopathy, as has been reported
previously with several infections.[8-9] However, a wrong
interpretation could not be conclusively resolved in the absence of
immunofixation studies. The association of mast cell hyperplasia with
HCL has been well characterized by Macon et al.[10] This has been
attributed to the angiogenesis and further progression of the disease,
confirmed by a latter study.[11] There has also been a case report of
systemic mastocytosis associated with a clonal hematopoietic
non-mastcell lineage disease (SM-AHNMD) where the coexisting neoplasms
were of both lymphoid and myeloid origin.[12] Our case shows a striking
mast cell hyperplasia, however, a systemic mastocytosis has been ruled
out based on immunophenotype studies.
Immunophenotype
aberrancies have been well described in HCL, like negativity for CD103
or CD25; and positivity for CD10 or CD23.[13] In our case, the cells
showed positivity for CD10, and there was a subclone with absence of
CD19 expression. While CD10 expression is relatively common (5-26% of
cases) and explained by alternate origin of leukemic cells from
germinal center,[13] the absence of CD19 expression in HCL has not been
previously reported in the literature. CD19 plays an important role in
B-cell growth and differentiation and its expression increases as a
B-cell matures. This characteristic is often the basis of using it in
flow cytometry as a gating marker for the diagnosis and for minimal
residual disease (MRD) testing in various B-cell malignancies. In fact,
of all the B-NHLs, HCL cases show the maximum level of expression of
CD19.[14] The abnormal immunophenotypic pattern should be borne in mind
while performing the MRD analysis during follow-up. An alternate marker
(CD20) should also be considered for gating leukemic cells in these
patients.[15]
Conclusions
We
report a case of HCL with unique clinical, morphological and
immunophenotypic features. A hematopathologist must be aware of these
confounding factors and must deal such cases with a high index of
suspicion and a supportive armamentarium of flow cytometry and
molecular studies.
Acknowledgement
The authors are
thankful to Mrs. Jasbir Kaur Hira and Mrs Praveen Bose for the
technical help in performing molecular and immunophenotypic studies
respectively.
References
- Foucar K, Falini B, Catovsky D, Stein H. Hairy cell
leukaemia. In: Swerdlow SH, Campo E, Harris NL, et al. eds. WHO
classification of tumours of haematopoietic and lymphoid tissues. Lyon,
France: IARC press; 2008:188-190.
- Else
M, Dearden CE, Catovsky D. Long-term follow-up after purine analogue
therapy in hairy cell leukaemia. Best Pract Res Clin Haematol
2015;28:217-29. https://doi.org/10.1016/j.beha.2015.09.004 PMid:26614900

- Johnston
JB, Grever MR. Hairy Cell Leukemia. In: Greer JP, Arber DA, Glader B et
al. eds. Wintrobe's Clinical Hematology. Philadelphia; Lippincott
Williams & Wilkins; 2014:4395-4443.
- Frassoldati
A, Lamparelli T, Federico M, Annino L, Capnist G, Pagnucco G, Dini E,
Resegotti L, Damasio EE, Silingardi V. Hairy cell leukemia: a clinical
review based on 725 cases of the Italian Cooperative Group (ICGHCL).
Italian Cooperative Group for Hairy Cell Leukemia. Leuk Lymphoma
1994;13:307-16. https://doi.org/10.3109/10428199409056295 PMid:7519510

- Saif
MW, Greenberg BR. Multiple myeloma and hairy cell leukemia: a rare
association or coincidence? Leuk Lymphoma 2001;42:1043-8. https://doi.org/10.3109/10428190109097724 PMid:11697621

- Hanbali A, Alrajeh A, Rasheed W. Plasma cell leukemia mimicking hairy cell leukemia. Hematol Oncol Stem Cell Ther 2015;8:91-2. https://doi.org/10.1016/j.hemonc.2015.05.001 PMid:26013472

- Lesesve JF, Broseus J. Confusing Hairy Cells in a Case of IgG Kappa Plasma Cell Leukemia. Clin Lab 2016;62:749-50. https://doi.org/10.7754/Clin.Lab.2015.150834 PMid:27215099

- Stoimenis
D, Spyridonidou C, Papaioannou N. Transient Monoclonal Gammopathy
Induced by Disseminated Staphylococcus aureus Infection. Case Rep Med
2012;2012:607104.

- Seve
P, Turner R, Stankovic K, Perard L, Broussolle C. Transient monoclonal
gammopathy in a patient with Bartonella quintana endocarditis. Am J
Hematol 2006;81:115-7. https://doi.org/10.1002/ajh.20499 PMid:16432867

- Macon
WR, Kinney MC, Glick AD, Collins RD. Marrow mast cell hyperplasia in
hairy cell leukemia. Mod Pathol 1993;6:695-8. PMid:8302811

- Ribatti D, Crivellato E, Molica S. Mast cells and angiogenesis in haematological malignancies. Leuk Res 2009;33:876-9. https://doi.org/10.1016/j.leukres.2009.02.028 PMid:19324412

- Gülen
T, Sander B, Nilsson G, Palmblad J, Sotlar K, Horny HP, Hägglund H.
Systemic mastocytosis: progressive evolution of an occult disease into
fatal mast cell leukemia: unique findings on an unusual hematological
neoplasm. Med Oncol 2012;29:3540-6. https://doi.org/10.1007/s12032-012-0261-5 PMid:22661384

- Chen
YH, Tallman MS, Goolsby C, Peterson L. Immunophenotypic variations in
hairy cell leukemia. Am J Clin Pathol 2006;125:251-9. https://doi.org/10.1309/PMQXVY619Q8Y43AR PMid:16393677

- Ginaldi
L, De Martinis M, Matutes E, Farahat N, Morilla R, Catovsky D. Levels
of expression of CD19 and CD20 in chronic B cell leukaemias. J Clin
Pathol 1998;51:364-9. https://doi.org/10.1136/jcp.51.5.364 PMid:9708202 PMCid:PMC500695

- Sausville
JE, Salloum RG, Sorbara L, Kingma DW, Raffeld M, Kreitman RJ, Imus PD,
Venzon D, Stetler-Stevenson M. Minimal residual disease detection in
hairy cell leukemia. Comparison of flow cytometric immunophenotyping
with clonal analysis using consensus primer polymerase chain reaction
for the heavy chain gene. Am J Clin Pathol 2003;119:213-7. https://doi.org/10.1309/G6299513NGLCUB1K PMid:12579991

[TOP]














