Francesco Zallio, Giulia Limberti and Marco Ladetto
Hematology Department, SS Antonio & Biagio and C. Arrigo Hospital, Alessandria, Italy.
Corresponding
author: Francesco Zallio, Hematology Department, SS Antonio & Biagio and C. Arrigo Hospital, Alessandria Italy; E-mail:
fazallio@ospedale.al.it
Published: May 1, 2017
Received: November 8, 2016
Accepted: March 17, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017035 DOI
10.4084/MJHID.2017.035
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Several
infectious agents appear to provide a proliferative signal --
“antigen-drive” – that could be implicated in the pathogenesis of
various type of Non-Hodgkin Lymphoma (NHL). A classical model of the
infection-driven lymphoproliferative disorder is Helicobacter
pylori-induced gastric MALT lymphoma, where antibiotic therapy allows
the eradication of both the infectious agent and the clonal B-cell
expansion. Following the footsteps of this example, several
retrospective studies have found a correlation with other pathogens and
B-cell Lymphomas, adding new relevant information about pathogenesis
and laying the groundwork for chemotherapy-free treatments.
Although
no clear association has been found between infectious agents and
Follicular Lymphoma (FL), a growing number of biological and clinical
observations suggests the interaction of physiological and pathological
microbial populations also in this subtype of lymphoma. In the
last few years, epidemiological studies investigating the association
of known risk factors and FL found a potential correlation with viral
or bacterial infections; moreover, recent findings of the stimulation
of FL clones support the importance of microbial exposure to
lymphomagenesis and disease progression.
In the following review
we make an attempt to find tangible evidence for a role of either
physiological and pathological exogenous microbial species in the
pathogenesis of FL, and try to integrate the findings coming from
epidemiological, biological and interventional studies to define future
novel treatment and prevention strategies for FL.
|
Introduction
FL
is the second most common form of NHL, accounting for approximately 30%
of NHL cases. The disease is characterized by a slow progression and
high response rates to therapy, that is the reason why it is considered
the prototype of indolent lymphomas; median survival is currently
around 14 years, with most patients displaying an indolent form of the
disease, slowly progressing over many years. Nonetheless, most patients
eventually develop increasingly resistant disease over time, and in up
to 45% of cases, the original indolent subtype transforms into an
aggressive subtype, an event that is associated with a poor outcome.[1,2,3]
The
first hit of the oncogenic cascade leading to FL is attributed to the
t(14;18) chromosomal translocation that occurs in an early B cell stage
in the bone marrow. Naive B cells, carrying the t(14;18), exit the bone
marrow and colonize secondary lymphoid tissue, where they undergo the
germinal center reaction but have a survival advantage due to their
constitutive expression of BCL2, which is not normally expressed in the
germinal center.[4]
Apart from the t(14;18),
recurrent secondary genetic alterations including genomic gains,
losses, and mutations (i.e. alterations in MLL2, EPHA7, TNFRSF14, and
EZH2) could provide a growth advantage to the neoplastic cells.
Moreover, the crosstalk between neoplastic B cells and the
microenvironment plays an important role in sustaining tumor cell
growth and eventually promoting transformation.[5]
Current treatment strategies vary from the classical watch and wait
approach to the use of anti CD20 monoclonal antibodies (labeled or
unlabeled with radioimmunoconjugates) in combination with chemotherapy,
while more aggressive treatment approaches including autologous or
allogeneic stem cell transplantation are reserved to patients with more
resistant disease.[6]
Recently a large bulk of
molecular and clinical research has been performed to better understand
the molecular mechanisms of lymphomagenesis and to develop
non-chemotherapeutic agents active in specific lymphoma subtypes; in
this field infectious agents could represent therapeutic targets for
lymphoma treatment toward chemotherapy-free therapeutic approaches.
Despite
huge advances in the comprehension of the genetic anatomy of FL, the
potential epidemiologic role of environmental stimuli has not been
clearly established; this is somehow in contrast to the huge bulk of
knowledge which has been accumulated in MALT lymphomas. Nevertheless, a
number of biological and clinical observations suggests that
interaction with physiological and pathological microbial populations
might play a role also in FL.
A classical model of the infection-driven lymphoproliferative disorder is Helicobacter pylori-induced
gastric MALT lymphoma where antibiotic therapy allows eradication of
both the infectious agent and the clonal B-cell expansion, leading to
long-term complete remissions (CR).[7]
The
identification of this pathogen as the causative agent in gastric MALT
lymphomas have resulted in substantial progress in understanding the
physiopathology of the disease permitting to develop new therapeutic
strategies. The list of lymphomas evolving in response to antigen
(bacterial or viral) has been growing rapidly in recent years,
associated in some cases with similar therapeutic success.[8]
Although no such association with infectious agents or other early
specific therapeutic target has yet been identified for FL, this
concept seems ideally suited to such an indolent disease.
This
review summarizes current evidence for a role of either physiological
and pathological exogenous microbial species in the pathogenesis of FL.
So, we underwent an extensive literature search focusing on clinical
observations suggesting such correlations, and we tried to underline
potential similarities between FL and other indolent
lymphoproliferative processes where the role of microbial organisms is
clearly established.
Role of Infection in other Lymphomas
In recent years, a growing number of exogenous microbial agents have been linked to NHL. The Helicobacter pylori (HP), Chlamydia psittaci and
hepatitis C virus are best-known examples, but other agents have been
identified in the pathogenesis of more rare subtypes of NHL; these
associations are important because they have clinical and therapeutic
implications and provide novel insights into the mechanisms that govern
lymphoma development.[9]
HP is a Proteobacteria
Epsilon bacterium known to cause stomach ulcers and chronic gastritis.
Its role in the pathogenesis of the majority of cases of gastric MALT
lymphoma was demonstrated nearly twenty years ago.
HP affects
about 50% of the world’s population even if only 1–2% of infected
individuals will develop a malignant disease. The pathogenetic role of
HP is related to the oncogenic properties of the cytotoxin-associated
antigen A (Cag-A), a protein that is able to activate the signaling
pathway leading to the activation and upregulation of the antiapoptotic
molecule BCL2.[10,11] Three major chromosomal
translocations specific of MALT lymphomas are reported, i.e. t(11;18)
which is the most common (found in nearly 30% of the cases), t(14;18)
and t(1;14). HP eradication using a combination of antibiotics and
proton-pump inhibitors (PPI), represents the standard treatment of
HP-associated MALT-lymphomas, leading to lymphoma regression in about
75% of patients.[12]
Chlamydophila psittaci
belongs to the family of Chlamydiae and is the second most studied
among bacteria having a pathogenetic role in MALT-lymphomas. Chlamydophila psittaci
can cause a lung infection called psittacosis. DNA from this bacterium
has been found in biopsies of MALT lymphoma of the ocular adnexa. The
finding that C. psittaci
infection has been detected in up to approximately 80% of Italian
patients with ocular adnexa MALT lymphoma provided the rationale for
the antibiotic treatment of localized lesions. Moreover, the
eradication of C. psittaci
infection with doxycycline for patients with ocular adnexa MALT
lymphoma resulted in lymphoma regression in approximately 50% of
patients.[9]
Epstein-Barr virus (EBV) is the first Human Herpes
Virus found to be associated with the pathogenesis of cancer. EBV has a
worldwide distribution, being able to establish a lifelong infection in
more than 90% of individuals. Primary infection is usually asymptomatic
or could cause a benign lymphoproliferative disease, known as
infectious mononucleosis.
EBV has a successful strategy to
reside in the hematopoietic system, including the establishment of a
nonpathogenic latent infection of memory B lymphocytes that allows the
virus to persist for the lifetime. According to current knowledge,
latent antigens encoded by EBV interfere with a number of critical
cellular pathways, thereby promoting oncogenesis. Although human EBV
infection may lead to the development of a variety of hematopoietic and
epithelial cancers, most common cases result from the transformation of
infected B cells into lymphoproliferative disorders[13]
Hodgkin lymphomas, Diffuse large cell and Burkitt lymphomas. Moreover,
EBV can cause a rare but potentially fatal complication in
hematopoietic stem cell transplants, as well as in solid-organ
recipients, known as EBV-associated post-transplant Lymphoproliferative
disease (PTLD).[14,15,16]
Human Immunodeficiency
Virus (HIV) is a lentivirus of the retroviridae family that integrates
itself into host chromosomal DNA. The increased risk for lymphoma
appears related to multiple factors, including the transforming
properties of the virus itself, the immunosuppression and, most
importantly, opportunistic infections associated with other
lymphotropic herpes viruses such as EBV and human herpesvirus.[8]
Aggressive lymphomas account for the vast majority of cases. The
clinical outcome appears to be worse than in similar aggressive
lymphomas in the general population. However, following the
introduction of highly active antiretroviral therapy, the risk of
developing lymphoma in the context of HIV infection has decreased, and
the clinical outcome has improved.[17]
Hepatitis
C virus (HCV) is a small RNA virus of the Flaviviridae family; is
a hepatotropic and lymphotropic virus responsible for acute hepatitis
and chronic liver disease; the presence of HCV is associated with a
spectrum of lymphoproliferative disorders, ranging from polyclonal
B-cell expansion to overt malignant lymphoma. Indeed, as well as small
B-cell clones can be detected in bone marrow or liver biopsies, a
higher frequency of lymphoid malignancies has been reported in
HCV-positive patients. The association between HCV infection and NHL
has been demonstrated by epidemiological studies, in particular in
highly endemic geographical areas such as Italy, Japan, and southern
parts of United States. In these countries, together with diffuse large
B-cell lymphomas, marginal zone lymphomas are the histotypes most
frequently associated with HCV infection. The most convincing argument
for a causative link between HCV and lymphoproliferation is represented
by studies demonstrating the eradication of the neoplastic clone by the
antiviral treatment in HCV-positive patients affected by indolent NHL.[18] Analogous
to what has been observed in HP-associated gastric, the role of HCV
infection in lymphomagenesis may be related to the chronic antigenic
stimulation of B-cell immunologic response by the virus.[19,20]
Adult
T-cell leukemia-lymphoma (ATL) is an aggressive lymphoid proliferation
associated with the human lymphotropic virus type I (HTLV-I). ATL
usually occurs in people from HTLV-I endemic regions, such as southern
Japan and the Caribbean. HTLV-I causes transformation and clonal
expansion of T cells, resulting in ATL in approximately 1%-5% of the
infected hosts, with a mean latency period of > 50 years. ATL
carries a bad prognosis because of intrinsic chemoresistance and severe
immunosuppression. Recently, a worldwide meta-analysis revealed that
the combination of zidovudine and IFN-α is highly effective in the
leukemic subtypes of ATL and should be considered as standard
first-line therapy in this setting.[21]
Human
herpesvirus 8 (HHV8) is a gammaherpesvirus associated with primary
effusion lymphoma, a lymphoproliferative disease that is rarely
observed in immunocompromised individuals. These neoplastic disorders
that result from HHV8 infection are most commonly related to
immunodeficiency states, including HIV infection and EBV infection. The
lymphoma is characterized by the localization in one of the body
cavities (pleural, pericardial, or peritoneal cavity), without lymph
node enlargement and lymphadenopathy. Prognosis is very poor, with a
median survival of 6 months.[22]
Epidemiological Evidence
Given
the heterogeneous nature of lymphoma subtypes and their different
clinical behavior, it is intriguing to identify the risk factors
potentially responsible for the occurrence of NHL, so various
occupational, environmental and chemical agents have been claimed by
several epidemiological studies. However, although for some factors the
correlation seems to exist, definite conclusions have not been drawn.
Several reports have also investigated the possible association between
infection-related conditions and the occurrence of NHL; in fact,
several infectious agents have been identified as causative factors for
the development of NHL, most likely due to their induction of DNA
damage, inflammatory cells proliferation, and cytokine release.
To
address this issue the International Lymphoma Epidemiology Consortium
(InterLymph), an open scientific forum for epidemiological research
funded in 2001, undertook the NHL Subtypes Project. The aim of
this an international group of multidisciplinary specialists, who have
worked together, is identifying associations of several risk factors
across different Lymphoma subtypes.[23]
Regarding
FL, in 2013 a large pooled analysis carried on by the Interlymph
Consortium made an attempt to assess associations between medical,
hormonal, family history, lifestyle and occupational factors with the
risk of developing FL. The incidence rate of FL was reported as higher
in western countries, which comprises ~30% of NHL, with a white to
black ratio of 2:3, and relatively rare in developing and Far Eastern
countries. Moreover, FL risk was increased in subjects with a
first-degree relative with non-Hodgkin lymphoma in spray painters among
women with Sjögren and among cigarette smokers and obese
subjects. No specific observation mentioned a link between
infection and risk of FL.[24]
Another large
retrospective case–control study using SEER and Medicare database
investigated the role of infection-related conditions and different NHL
subtypes.[25] Cases were defined as individuals with
a SEER diagnosis of primary lymphoid malignancy between 1992 and 2005.
The database identified respiratory and skin infections to be
associated with an increased risk of NHL in individuals aged more than
66 years. Claims for sinusitis, laryngitis and herpes zoster were
present in the history of FL patients, sinusitis, laryngitis and herpes
zoster were significant at longer latencies. Most FL cases carried the
t(14;18), which was hypothesized to be transformed by exogenous antigen
stimulation, such as from a viral infection.[26]
Antigenic stimulation and/or subclinical immune deficiency,
predisposing patients to both infections and lymphoma, were claimed as
possible association between infection-related conditions and lymphoma. Biological Evidence
The
gene sequence for the immunoglobulin (Ig) heavy-chain (H) and
light-chain (L) variable regions are assembled in the early stages of
B-cell development in the bone marrow from distinct variable (V),
diversity (D) and joining (J) segments through a process of somatic DNA
rearrangement known as V(D)J recombination. In later stages, which take
place in the germinal center (GC) of the secondary lymphoid tissues,
naive B-cells with low-affinity functional surface Ig (sIg) are induced
to proliferate. The high proliferation rate is associated with somatic
hypermutation of Ig genes, a process that introduces a high incidence
of mutations within the V region of genes. Somatic hypermutation is
thought to be a prerequisite for affinity maturation of antibody
response. At this stage, normal B cells that are specific for an
antigen are induced to operate a selection process that expands the
population of B cells with an optimal binding affinity for the antigen.
Also, B cells carrying the t(14;18) exit the bone marrow and
colonize the GC of secondary lymphoid tissue; subsequently they undergo
somatic hypermutations of IgVH-genes, with mutational patterns very
similar to their normal counterpart, but with a survival advantage due
to the constitutive expression of BCL2.
Recent studies by
Schneider et al. demonstrated that somatic hypermutations occurring in
FL cells could introduce sugar moieties, like high-mannose-terminated
glycan, into the variable domain of the surface Ig antigen-binding
sites, which create potential novel binding sites to mannose-specific
lectins. In FL cells, B-cell receptor (BCR) expression is retained,
despite the characteristic chromosomal translocation t(14;18), because
BCR is fundamental for the transduction of the signals that maintain
the survival and growth of FL clones. BCR variable-region mannoses in
FL are recognized by lectins of common opportunistic bacteria, such as Burkholderia cenocepacia and Pseudomonas aeruginosa,
that are usually found in soil and water; these lectins represent a
potent stimulus for the proliferation of B cells expressing this kind
of glycan-terminated glycan.[27,28,29] Therefore,
these studies directly support the potential importance of microbial
exposure in the proliferation and survival of FL clones, and they might
be a key to a better understanding of the pathogenesis of FL.
Clinical Evidence
Although
an expanding literature has examined several risk factors potentially
correlated with the occurrence of FL, the etiology of the proliferative
stimulus is generally unknown, and the few relationships observed
suggest a complex multifactorial etiology. A
recent meta-analysis, selecting more than 20 articles, showed a more
than two-times increase in the odds of developing NHL in patients with
HBV infection. Interestingly, regarding FL subtype, a trend toward
statistical risk was observed in countries with a high prevalence of
HBV infection while no statistical risk was seen in countries with a
low prevalence of HBV infection.[30]The
conclusion of the study was that was difficult to determine if the
increased risk of FL in areas of high prevalence of HBV infections is
due to simply to a larger number of HBV infections or a true causal
relationship. In the latter case, HBV might be responsible for
lymphomagenesis through a chronic stimulation of B-cells which may
predispose to DNA damage and transformation into neoplastic cells, or
through an immunologic response to chronic local antigenic stimulation.As
opposed to HBV, a case-control study carried on by the InterLymph did
not show an increased association between HCV infection and FL, that
was restricted to other specific B-NHL subtypes like diffuse large
B-cell lymphoma (DLBCL), marginal zone lymphoma, and lymphoplasmacytic
lymphoma.[19]An
interesting clinicopathological finding came up from a Spanish study
that analyzed a retrospective series of 58 patients with a diagnosis of
HCV-positive B-cell lymphoproliferative disorder; eight of them were
affected by FL, and at least half of them expressed BCL2 and p53.Interestingly,
the authors reported a cohort of 11 patients in which a clonal B cell
expansion in the peripheral blood, bone marrow could be revealed, in
the absence of conclusive histological evidence of neoplastic
infiltration. These expanded clones make up a definite group of
HCV-associated monoclonal B-cell Lymphocytosis that should be monitored
because at 10% risk of evolution to overt lymphoma.[31]Currently,
the association between EBV and follicular lymphoma is reported only in
the form of isolated case reports in patients with various form of
immunodeficiency or in the context of transformation to diffuse large
cell lymphoma or classical Hodgkin lymphoma. Mackrides et al. analyzed
382 cases of FL consecutively diagnosed at the University of Miami and
Stanford, in order to provide an estimated prevalence of EBV-positive
FL (ref); all the cases were tested for the expression of EBV-encoded
small RNA (EBER) as determined by in situ hybridization. They
identified 10 cases of EBV-positive FL (prevalence=2.6%) with a
significant prevalence of grades 3A-3B FL (9 out of 10) and frequent
strong coexpression of CD30; all cases demonstrated progression of the
disease to a higher grade FL or diffuse large B-cell lymphoma. Given
the increased incidence of EBV in high-grade FL and the fact that the
cases are clinically and morphologically indistinguishable from
EBV-negative FL patients, the authors suggested the screening for EBER
in all high-grade cases.[32]Recently an intriguing association between Coxiella burnetii/Q fever and the incidence of B-cell lymphomas was proposed by Melenotte et al. in a large scale study.[33]
Starting from the observation of the occurrence of lymphoma in a
patient with Q fever, they screened over 1000 consecutive patients of
the French National Referral Center for Q fever database and examined
if there was an association between the two diseases. An excess risk of
DLBCL and FL was found in individuals who had Q fever compared with the
general population and above all patients with a persistent localized
infection were found to have a greater risk of lymphoma. These results
support the evidence that a novel factor should be added to the list of
bacteria that promote human B-cell lymphomas, in particular, FL. The most relevant studies reporting a link between Follicular lymphoma and infectious agents are summarized in Table 1.
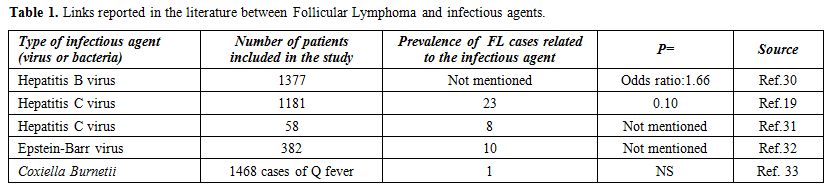 |
Table 1. Links reported in the literature between Follicular Lymphoma and infectious agents. |
Taken
in mind the gastric MALT as a well-accepted example of antigen-driven
neoplastic cell proliferation, Portlock et al. explored the association
between infectious agents and NHL in a cohort of 56 patients with an
untreated advanced non-bulky indolent lymphoma.[34] All patients were tested for HP, HCV, Borrelia burgdorferi, Chlamydia psittaci
and small bowel bacterial overgrowth; in this series, a documented
infection was found in 37% of the patients, with a prevalence of HP.
Starting from the observation of anecdotal lymphoma remissions after
antibiotic therapy in a series of patients not requiring chemotherapy,
they speculated that a prevention strategy would decrease the risk of
future lymphoma progression driven by such antigens. Therefore in 2007,
they launched a prospective clinical trial testing the role of
prolonged clarithromycin antibiotic therapy as a first treatment in the
same category of indolent advanced-stage lymphoma patients. Although
the small sample size, they reported lymphoma objective responses in 9
of 32 patients (28.1%) with a long treatment-free survival for patients
responding to antibiotics.[35]The
association between infectious agents and FL added new important
information about the role played by the antigen stimulation in FL;
moreover, the possibility to treat the neoplastic disease in a simple
and efficient way could be considered a step toward developing a
lymphoma preventive strategy by reducing the “antigen drive”.
Discussion
In the last years, several infectious agents, like Hepatitis C, Human Immunodeficiency, and Epstein-Barr viruses, and Helicobacter Pylori, Chlamydia psittaci, and Coxiella Burnetii
bacteria have been reported as involved in the malignant transformation
of B or T lymphocytes, and therefore associated with the pathogenesis
of lymphoproliferative disorders. While this hypothesis has been
demonstrated for some rare subtypes of NHL, for the majority the
evidence is uncertain. Regarding FL, based on available data, evidence
linking this lymphoma subtype and exogenous infectious agents are weak,
and currently, FL cannot be considered as an infection-driven disease.
However, some clinical, epidemiologic studies and case reports indicate
that it is still somehow premature to conclude that exogenous agents
have a negligible role in the genesis of FL.It
has been estimated that chronic infections caused by viruses, bacteria,
and parasites are the causative agents of nearly 10-15% of global
cancers burden.[36,37] These infectious agents
promote a cascade of events culminating in chronic inflammatory
responses. Chronic antigenic stimulation has been postulated as a
potential mechanism for carcinogenesis, thus predisposing target
tissues to increased cancer susceptibility. In particular, in
antigen-driven hematologic malignancies, like HP-associated with MALT,
the chronic stimulation of the innate immune system causes a clonal
expansion of B-lymphocytes, which leads to the production of oxidative
reactions; these events result in genetic alterations, which eventually
result in the development of a neoplastic monoclonal
lymphoproliferation. As well as for MALT lymphomas, also for FL could
be postulated an inflammatory response secondary to an
infectious-driven chronic antigenic stimulation, inducing t(14:18)
translocation, leading to the transformation of a germinal
center-derived B-cell.Apart
from HP and other few microorganisms that colonize the gastrointestinal
tract, it should be kept in mind that little is known about the complex
community of human microbiota which includes more than 109
procaryotic cells per individual. Modern next generation sequencing
tools for microbiome analysis are becoming widely available and
intriguing correlations between the type of bacterial colonization in
multiple districts, and some diseases have been established. Of note,
human microbiota has some well-established differences among different
world areas as also observed in several indolent lymphoid disorders. It
is, therefore, advisable to further investigate this potential link by
performing careful case-control or population analyses aiming at
verifying whether specific pathological or nearly physiological
microbiota patterns might be responsible for a chronic antigen
stimulation in those lymphomas where a clearly responsible
microorganism has still not been identified. Intestinal
microbiota either directly or indirectly through the immune system can
lead to aberrant DNA replication, particularly in some B lymphocytes
which are vulnerable to genetic instability and activation, eventually
affecting several pathways associated with lymphomagenesis.[38]Finally,
a chronic antigenic infectious stimulation was shown to be fundamental
also in the cell perturbation of the microenvironment in sustaining the
neoplastic cell growth. Indeed also this pathway could play a role in
the oncogenic cascade leading to FL,[39,40] and the
encouraging results obtained by a novel panel of inhibitors of the
signal transduction of the BCR, has led to further investigate the
crosstalk between the downstream BCR signaling cascade and the
microenvironment. The Btk inhibitor ibrutinib and the PI3Kδ inhibitor
idelalisib have demonstrated good safety profile and promising clinical
efficacy, affecting the survival of neoplastic B cells by preventing
lymphocyte adhesion and homing, and inhibiting the microenvironment
signals that commonly sustain the malignant clone.[41,42]
Conclusions
The
pathogenesis of FL is a multistep process in which the t(14;18)
translocation in a B lymphocyte appears to be fundamental for the
initiation of the neoplastic cascade. Even if still unclear, infectious
agents could play a role as a first hit responsible for the B-cell
malignant transformation and growth. Precise elucidation of the
mechanisms underlying lympho-proliferations may provide important clues
for understanding how immune disturbance contributes to the development
of this subtype of lymphoma. Moreover, the responses shown by BCR
inhibitors and by antibacterial treatments, which can have
cytoprotection properties like Rifaximin, considered a gut
microenvironment modulator, provide an intriguing argument for a
causative link between infectious agents and B-cell lymphoproliferation.
References
- Freedman A. Follicular lymphoma: 2012 update on diagnosis and management. Am J Hematol. 2012;87(10):988–995. http://dx.doi.org/10.1002/ajh.23313 PMid:23001911

- Bastion
Y, Sebban C, Berger F, Felman P, Salles G, Dumontet C, et al.
Incidence, predictive factors, and outcome of lymphoma transformation
in follicular lymphoma patients. J Clin Oncol. 1997;15(4):1587–1594. http://jco.ascopubs.org/content/15/4/1587.long PMid:9193357

- Link
BK, Maurer MJ, Nowakowski GS, Ansell SM, Macon WR, Syrbu SI, et al.
Rates and outcomes of follicular lymphoma transformation in the
immunochemotherapy era: a report from the University of Iowa/MayoClinic
Specialized Program of Research Excellence Molecular Epidemiology
Resource. J Clin Oncol. 2013;31(26):3272–3278. http://dx.doi.org/10.1200/JCO.2012.48.3990 PMid:23897955

- Stamatopoulos
K, Kosmas C, Belessi C, Stavroyianni N, Kyriazopoulos, Papadaki
T. Molecular insights into the immunopathogenesis of follicular
lymphoma. Immunol Today. 2000;21(6):298-305. http://dx.doi.org/10.1016/S0167-5699(00)01650-9 PMip:10825742

- Kridel R, Sehn LH, Gascoyne RD. Pathogenesis of follicular lymphoma. J Clin Invest. 2012;122(10):3424–3431. http://dx.doi.org/10.1172/JCI63186 PMid:23023713

- Dreyling
M, Ghielmini M, Rule S, Salles G, Vitolo U, Ladetto M. Newly Diagnosed
and Relapsed Follicular Lymphoma: ESMO Clinical Practice Guidelines
Published in 2016. 2016;27(suppl5):v83-v90 http://dx.doi.org/10.1093/annonc/mdw400 PMid:27664263

- Zucca
E, Bertoni F, Vannata B, Cavalli F. Emerging role of infectious
etiologies in the pathogenesis of marginal zone B-cell lymphomas. Clin
Cancer Res. 2014;20(20):5207-16. http://dx.doi.org/10.1158/1078-0432.CCR-14-0496 PMid:25320370

- Mamessier
E, Broussais-Guillaumot F, Chetaille B, Bouabdallah R, Xerri L, Jaffe
ES, et al. Nature and importance of follicular lymphoma precursors.
Haematologica. 2014;99(5):802-10. http://dx.doi.org/10.3324/haematol.2013.085548 PMid:24790058

- Ferreri
AJ, Ernberg I, Copie-Bergman C. Infectious agents and lymphoma
development: molecular and clinical aspects. J Intern Med.
2009;265(4):421-38. http://dx.doi.org/10.1111/j.1365-2796.2009.02083.x PMid:19298458

- Wotherspoon
AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter
pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet
1991;338:1175–6. PMid:1682595

- Parsonnet
J, Hansen S, Rodriguez L, Gelb AB, Warnke RA, Jellum E, et al.
Helicobacter pylori infection and gastric lymphoma. N Engl J Med.
1994;330:1267–71. http://dx.doi.org/10.1056/NEJM199405053301803 PMid:8145781

- Levy
M, Copie-Bergman C, Traulle C et al. Conservative treatment of primary
gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue:
predictive factors of response and outcome. Am J Gastroenterol
2002;97:292–7. http://dx.doi.org/10.1111/j.1572-0241.2002.05460.x PMid:11866264

- Grywalska E, Rolinski J. Epstein-Barr Virus–Associated Lymphomas. Semin Oncol. 2015;42(2):291-303. http://dx.doi.org/10.1053/j.seminoncol.2014.12.030 PMid:25843733

- Roschewski M, Wilson WH. EBV-associated lymphomas in adults. Best Pract Res Clin Haematol. 2012;25:75–89. http://dx.doi.org/10.1016/j.beha.2012.01.005 PMid:22409825

- Bechtel
D, Kurth J, Unkel C, Küppers R. Transformation of BCR-deficient
germinal-center B cells by EBV sup- ports a major role of the virus in
the pathogenesis of Hodgkin and post transplantation lymphomas. Blood.
2005;106:4345–50. http://dx.doi.org/10.1182/blood-2005-06-2342 PMid:16131568

- Zallio
F, Primon V, Tamiazzo S, Pini M et al. Epstein-Barr virus reactivation
in allogeneic stem cell transplantation is highly related to
cytomegalovirus reactivation., Clin Transplant. 2013 Jul-Aug;27(4). http://dx.doi.org/10.1111/ctr.12172. PMID:23781897

- Martis N, Mounier N. Hodgkin lymphoma in patients with HIV infection: a review. Curr Hematol Malig Rep. 2012;7:228–34. http://dx.doi.org/10.1007/s11899-012-0125-2 PMid:22547166

- Arcaini
L, Besson C, Frigeni M, Fontaine H. Interferon-free antiviral treatment
in B-cell lymphoproliferative disorders associated with hepatitis C
virus infection. Blood. 2016 Nov 24;128(21):2527-2532. PMID:27605512

- De
Sanjose S, Benavente Y, Vajdic CM. Hepatitis C et al. Non-Hodgkin
Lymphoma Among 4784 Cases and 6269 Controls From the International
Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol.
2008;6(4):451–458. http://dx.doi.org/10.1016/j.cgh.2008.02.011 PMid:18387498

- Mihăilă RG. Hepatitis C virus - associated B cell non-Hodgkin's lymphoma. World J Gastroenterol. 2016;22(27):6214-23. http://dx.doi.org/10.3748/wjg.v22.i27.6214 PMid:27468211

- Mahieux R, Gessain A. Adult T-cell leukemia/lymphoma and HTLV-1. Curr Hematol Malig Rep. 2007 Oct;2(4):257-64. http://dx.doi.org/10.1007/s11899-007-0035-x PMid:20425378

- Kaplan
LD. Human herpesvirus-8: Kaposi sarcoma, multicentric Castleman
disease, and primary effusion lymphoma. Hematology Am Soc Hematol Educ
Program. 2013;2013:103-8. http://dx.doi.org/10.1182/asheducation-2013.1.103 PMid:24319170

- Morton
LM, Slager SL, Cerhan JR, Wang SS, Vajdic CM, Skibola CF, et al.
Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: the
InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst
Monogr. 2014;2014(48):130-144. http://dx.doi.org/10.1093/jncimonographs/lgu013 Pmid:25174034

- Linet
MS, Vajdic CM, Morton LM, de Roos AJ, Skibola CF, Boffetta P, et al.
Medical history, lifestyle, family history, and occupational risk
factors for follicular lymphoma: the InterLymph Non- Hodgkin Lymphoma
Subtypes Project. J Natl Cancer Inst Monogr. 2014;2014(48):26-40. http://dx.doi.org/10.1093/jncimonographs/lgu006 PMid:25174024

- Anderson
LA, Atman AA, McShane CM, Titmarsh GJ, Engels EA, Koshiol J. Common
infection-related conditions and risk of lymphoid malignancies in older
individuals. Br J Cancer. 2014;110(11):2796-803. http://dx.doi.org/10.1038/bjc.2014.173 PMid:24691420

- Roulland
S, Navarro J-M, Grenot P, Milili M, Agopian J, Montpellier B, et al.
Follicular lymphoma-like B cells in healthy individuals: a novel
intermediate step in early lymphomagenesis. J Exp Med.
2006;203(11):2425-31. http://dx.doi.org/10.1084/jem.20061292 PMid:17043145

- Chiorazzi N. A spoonful of sugar helps lymphoma cells go up. Blood. 2015;125(21):3215-6. http://dx.doi.org/10.1182/blood-2015-04-636209 PMid:25999440

- Coelho
V, Krysov S, Ghaemmaghami AM, Emara M, Potter KN, Johnson P, et al.
Glycosylation of surface Ig creates a functional bridge between human
follicular lymphoma and microenvironmental lectins. Proc Natl Acad Sci
U S A. 2010;107(43):18587-92. http://dx.doi.org/10.1073/pnas.1009388107 PMid:20937880

- Schneider
D, Dühren-von Minden M, Alkhatib A, Setz C, van Bergen CA,
Benkißer-Petersen M, et al. Lectins from opportunistic bacteria
interact with acquired variable-region glycans of surface
immunoglobulin in follicular lymphoma. Blood. 2015;125(21):3287-96. http://dx.doi.org/10.1182/blood-2014-11-609404 PMid:25784678

- Dalia
S, Chaveza J, Castillob JJ, Sokol L. Hepatitis B infection increases
the risk of non-Hodgkin lymphoma: A meta-analysis of observational
studies. Leuk Res. 2013;37(9):1107-15. http://dx.doi.org/10.1016/j.leukres.2013.06.007 PMid:23809055

- Mollejo
M, Menárguez J, Guisado-Vasco P. Bento L, Algara P, Montes-Moreno S et
al. Hepatitis C virus-related lymphoproliferative disorders encompass a
broader clinical and morphological spectrum than previously recognized:
a clinicopathological study. Mod Pathol. 2014 Feb;27(2):281-93

- Mackrides
N, Campuzano-Zuluaga G, Maque-Acosta Y, Moul A, Hijazi N, Ikpatt FO et
al. Epstein-Barr virus-positive follicular lymphoma. Mod Pathol. 2017
Apr;30(4):519-529.

- Melenotte
C, Million M, Audoly A, Gorse A, Dutronc H, Roland G,et al. B-cell non-
Hodgkin lymphoma linked to Coxiella burnetii. Blood.
2016;127(1):113-121. http://dx.doi.org/10.1182/blood-2015-04-639617 PMid:26463422

- Portlock
CS, Hamlin P, Noy A, Chey W, Gaydos CA, Palomba L, et al. Infectious
disease associations in advanced stage,indolent lymphoma (follicular
and nonfollicular): developing a lymphoma prevention strategy. Annals
of Oncology. 2008;19(2):254-8. http://dx.doi.org/10.1093/annonc/mdm484 PMid:17965114

- Portlock
CS, Hamlin PA, Gerecitano JF, Noy A, Palomba ML, Walkley J, et al. A
Positive Prospective Trial of Antibiotic Therapy in Advanced Stage,
Non-Bulky Indolent Lymphoma Tumor Microenviron Ther. 2015;2(1):14–18. http://dx.doi.org/10.1515/tumor-2015-0001 PMid: 26798624

- Bouvard
V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A
review of human carcinogens-Part B: biological agents. WHO
International Agency for Research on Cancer Monograph Working Group.
Lancet Oncol. 2009;10(4):321-2. PMid:19350698

- Samaras
V, Petros I, Rafailidis PI, Eleni G, Peppas G, Falagas ME. Chronic
bacterial and parasitic infections and cancer: a review. J Infect Dev
Ctries. 2010;4(5):267-81. PMid:20539059

- Yamamoto
ML, Maier I, Dang AT, Berry D, Liu J, Ruegger PM, et al. Intestinal
bacteria modify lymphoma incidence and latency by affecting systemic
inflammatory state, oxidative stress, and leucocyte genotoxicity.
Cancer Res. 2013;73(14):4222–4232. http://dx.doi.org/10.1158/0008-5472 PMid:23860718

- Dave
SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, et al.
Prediction of survival in follicular lymphoma based on molecular
features of tumor-infiltrating immune cells. N Engl J Med.
2004;351(21):2159–2169. http://dx.doi.org/10.1056/NEJMoa041869 PMid:15548776

- Glas
AM, Knoops L, Delahaye L, Kersten MJ, Kibbelaar RE, Wessels LA, et al.
Gene-expression and immunohistochemical study of specific T-cell
subsets and accessory cell types in the transformation and prognosis of
follicular lymphoma. J Clin Oncol. 2007;25(4):390–398. http://10.1200/JCO.2006.06.1648 PMid:17200149

- Gopal
AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, et
al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent
lymphoma. N Engl J Med. 2014;370:1008-18. http://dx.doi.org/10.1056/NEJMoa1314583 PMid: 24450858

- Novero A, Ravella PM, Chen Y, Dous G, Liu D. Ibrutinib for B cell malignancies. Exp Hematol Oncol. 2014;3:4. http://dx.doi.org/10.1186/2162-3619-3-4 PMid: 24472371













































