Michele Malagola1, Benedetta Rambaldi1, Giuseppe Ravizzola2, Chiara Cattaneo3, Erika Borlenghi3, Nicola Polverelli1, Alessandro Turra1, Enrico Morello1, Cristina Skert1, Valeria Cancelli1, Federica Cattina1, Giorgio Giannetta1, Simona Bernardi, Simone Perucca, Camillo Almici4, Aldo Roccaro5, Liana Signorini6, Roberto Stellini6, Francesco Castelli6, Arnaldo Caruso2 and Domenico Russo1.
1 Chair
of Hematology, Clinical and Experimental Sciences Department,
University of Brescia, Bone Marrow Transplant Unit,ASST-Spedali Civili,
Brescia, Italy, Italy
2 Institute of Microbiology, Department of Molecular and Transplational Medicine, University of Brescia, Italy
3 Division of Hematology, ASST-Spedali Civili, Brescia, Italy
4 Laboratory for Stem Cells Manipulation and Cryopreservation, ASST-Spedali Civili, Brescia, Italy
5 ASST-Spedali Civili, Coordinamento e Progettazione Ricerca Clinica, CREA Laboratory, Brescia, Italy
6 Chair of Infectious Diseases, Division of Infectious and Tropical Diseases, University of Brescia, Italy
Corresponding
author: Michele Malagola, MD, Phd. Chair of Hematology, Bone Marrow
Transplant Unit, Clinical and Experimental Sciences Department,
University of Brescia, Italy. ASST-Spedali Civili, Brescia, Italy,
Italy. P.le Spedali Civili 1, 25123 Brescia, Italy. Tel:
+39/30/3996811, Fax: +39/30/3996021. E-mail:
michelemalagola@yahoo.it
Published: June 20, 2017
Received: February 8, 2017
Accepted: May 10, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017036 DOI
10.4084/MJHID.2017.036
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: Blood
stream infections (BSIs) represent a major complication of allo-SCT and
are a major cause of morbidity and mortality during and after bone
marrow aplasia.
Objectives:
The objective of this study was to describe the incidence and outcome
of BSIs in a cohort of patients submitted to allo-SCT, in order to
track changes of the epidemiology and bacteria resistance.
Methods:
We retrospectively analyzed the microbiological data of 162 patients
allotransplanted in Brescia University Hospital, over a period of 6
years.
Results: Eighty
patients experienced a BSIs for a total of 119 isolates. In 77 cases
(65%) a Gram positive bacterium was isolated, being coagulase negative Staphilococci
the most frequent species (77% of the cases). In 42 cases (35%) a Gram negative bacterium was isolated (E. coli 57% and
P. aeruginosa 24%). Fluoroquinolones resistance was frequent (90% for S. epidermidis, 92% for E. coli, 90% for P. aeruginosa). Methycillin resistance of S. epidermidis was 100%, 76% of E. coli were ESBL positive and among P. aeruginosa
resistance to carbapenems was 40%. The 2 years overall survival of
patients with BSIs vs patients without BSIs was 46% vs 60% (HR1,48,
p=0,07). P. aeruginosa and E. coli were the species with the highest mortality (50% and 33%, respectively).
Conclusions:
These data confirm that BSIs, mainly sustained by Gram positive
bacteria, are frequent in allotransplanted patients (50% of the cases)
and may influence the outcome of allotransplanted patients, being
antibiotics resistance highly frequent among these bacteria.
|
Introduction
Allogeneic
stem cell transplantation (allo-SCT) is widely considered a curative
option for many hematological malignancies, particularly for acute
leukemias. Morbidity and mortality of allo-SCT are mainly influenced by
relapse and transplant-related factors (e.g. conditioning toxicity),
infections and immunological events, such as graft versus host disease
(GVHD).[1-13] Blood stream infections (BSIs) represent the most
frequent infective event in allotransplanted patients, and their
incidence may vary from 20 to 70%.[1-12] Prolonged neutropenia,
gastro-intestinal mucosal damage and extensive use of central venous
catheters (CVC) are the major risk factors for BSIs.[3,5,7,9]
Usually
BSIs occur during the pre-engraftment phase, but they can occur in
later phases too.[5,10] Prophylactic antimicrobical therapy is
conventionally used during agranulocytosis, as well as an empirical use
of antibiotics in case of suspected BSI, despite it is well known that
multidrug resistant bacteria may emerge.[14-17] At present, a selective
gut decontamination, with the aim to reduce the translocation of
intestine Gram negative bacteria from the gut to peripheral
blood, is conventionally based on fuoroquinolones, but this policy,
during the last decade, induced the emergence of Gram negative
fluoroquinolones resistant bacteria and an increase in Gram positive
infections.[12,15]
In the event of a suspected bacterial
infection, the common clinical practice in patients submitted to
allo-SCT is to identify the specie involved in the infection as soon as
possible, and to use a targeted antibiotic therapy, based on the
antibiograms. However, it should be considered that only 30-40% of the
febrile episodes in patients submitted to allo-SCT can be defined as
BSIs. In fact, more than 50% of blood cultures (BCs) during fever do
not give rise to any bacteria, and, as a consequence, the
antimicrobical therapy is often empirical and, thus, the surveillance
of infections in a bone marrow transplant Unit is mandatory, in order
to correctly drive the use of empirical therapy.
The aim of this
retrospective study was to describe the incidence and outcome of BSIs
in a cohort of 162 patients submitted to allo-SCT, over a period of 6
years of transplant activity and to compare these data with the ones
reported in the litterature, in order to track changes of the
epidemiology and bacteria resistance.
Patients and Methods
This
retrospective analysis was pointed out on the BSIs occured from January
2010 to December 2015 in the Bone Marrow Transplant Unit of Brescia
University Hospital in Italy. Data were analysed from the computerized
database including all the informations of patients submitted to
allo-SCT. At the same time, clinical charts of the patients who
experienced a BSIs were reviewed. All the BSIs occuring during the
patient hospitalization were recorded.
Definitions: BCs
were obtained from peripheral blood (PB) and CVC at fever onset
(defined as body temperature of at least 38°C) or whenever in suspicion
of infection. BSIs were defined as isolation of bacterial or fungal
pathogen from at least 1 blood culture, with the exception of bacteria
commonly considered skin contaminants (e.g. coagulase negative Staphylococci or Corynebacteria),
for whom at least two positivity were requested. The CVC related
infection was defined when a positive CVC-BC preceeded by two hours the
positivity of a PB-BC. CVC contamination was defined by the presence of
a positive CVC-BC and a negative PB-BC. For the porpuse of this study,
CVC contaminations were included in the analysis and considered as
bacteremia, considering that the clinical management of these cases is
not different with respect to the clinical management of any other BSI.
Subsequent positivity of a blood culture after at least 7 days
following first positivity was considered as a separate BSI, if blood
culture negativity was defined, in the meanwhile. Gram negative
bacteria were considered extended spectrum beta-lactamase (ESBL)
producers according to the published laboratory tests.[18,19]
Resistance to at least 3 antibiotics among ceftazidime or cefepime,
piperacillin/tazobactam, ciprofloxacin, gentamicin, imipenem or
meropenem were the basis for multi-drug resistance definition (MDR).
For the definition of resistance we referred to the European Committee
on Antimicrobial Susceptibility Testing (EUCAST) 2016.[20]
Briefly, the Minimal Inhibitory Concentrations (MIC) considered for
antibiotics resistance were the followings: ceftazidime or cefepime
> 8 mg/L for P. aeruginosa and
> 4 mg/L for enterobacteria, respectively; piperacillin/tazobactam
> 16 mg/L, cirpofloxacin > 1 mg/L, gentamicin > 4 mg/L and
carbapenems > 8 mg/L both for P. aeruginosa and for enetrobacteria.
Transplantation procedures:
The patients included in this analysis were transplanted during the
6-years period with either reduced intensity conditioning regimen (RIC)
or myeloablative conditioning regimen (MAC), depending on age, disease
type and comorbidity. All the RIC regimens were fludarabine and
thiotepa or busulfan-based, whereas the MAC regimens were busulfan or
total-body irradiation-based. GVHD prophylaxis consisted on
cyclosporine and methotrexate, with the addition of anti-thymocyte
globulin (ATG) in cases of matched unrelated donors (MUD). In the
haploidentical setting, GVHD prophylaxis consisted on cyclosporine,
mycophenolate and post-transplant cyclofosfamide.[21]
HLA matching was based on molecular four digits typing of a minimum of
4 loci (A, B, C, DRB1). Peripheral blood was used as the preferred stem
cells source. Acute and chronic GVHD were graded as previously
published.[22,23] All the patients had a Groshong CVC
at the time of conditioning regimen intiation. At hospital admittance,
mucosal swabs (nasal, mouth, axillary, rectal and genital) were
performed in all the patients, with the objective to detect bacterial
colonization. In case of positive coltures, antibiograms were included
in the laboratory report, with focus on vancomycin resistant Enterococci
(VRE), bacteria ESBL-producing or carbapenemase-producing
enterobacteriacae. Engraftment was defined as an absolute count of
neutrophils greater than 500/mmc for at least three consecutive days.
Porphylaxis and management of infections:
All the patients received standard antimicrobical prophylaxis with
levofloxacine, acyclovir and fluconazole from day 0. Levofloxacine was
discontinued at the engraftment. Patients with a previous history of
possible or probable invasive fungal infection (IFI) received secondary
anti-fungal prophylaxis with liposomal B-amphotericine. Pneumocystis carinii
prophylaxis with trimethoprim sulphametoxazole was started at the time
of neutrophil engraftment. Pre-emptive therapy for CMV with gancyclovir
was started in the event of CMV-DNA positivity as detected by
quantitative real-time PCR, in at least two examinations within 1 week.
As previosuly described, the diagnostic work up at fever onset,
or in case of any symptom of infection, consisted on blood cultures
collection, collection of culture samples from any site with suspect
infection and chest X-ray. Then, floroquinolone prophylaxis was stopped
and broad spectrum i.v. antibiotics were started. Empirical
antimicrobical therapy consisted on ceftazidime or piperacillin +
tazobactam with or without the addition of glycopeptide (e.g.
teicoplanine) depending on the clinical conditions of the patient (e.g.
hypotension, gastrointestinal mucositis). In case of fever persistance
after 72 hours of broad spectrum antibiotics with persistent negativity
of blood cultures, the diagnostic work-up for an IFI was started. This
consisted on a high-resolution CT scan of the lung, blood samples for
galactomannan dosage and broncoscopy with bronco-alveolar lavage and
tissue specimen whenever clinically possible. In case of possible IFI
according to the published criteria,[24] empirical therapy was started, using liposomal B-amphotericine or caspofungin.
Statistical analysis:
For the purpose of descriptive analysis, continuous variables were
summarized as median and range, categorical as frequencies and
percentages. Differences between groups were analyzed with Chi-square
and Mann-Whitney U tests for categorical and continuous variables,
respectively. Survival analysis was performed according to Kaplan-Meier
method and log-rank test was used to evaluate differences between
subgroups. All tests were 2-sided and p values below 0.05 were
considered statistically significant. Analyses were carried out with
EZR software version 1.33.[25] Results
Patients' characteristics:
From 2010 to 2015, 162 patients with hematological malignancies were
submitted to allo-SCT. Among these, 80 patients (49%) experienced a
BSI, for a total of 119 isolates. Eighty-two (51%) out of 162 patients
had no BSIs. Patient demografics are reported in Table 1.
Briefly, the median age was 48 years (17-68), approximately two thirds
of the patients were transplanted for acute leukemias (AL) (93/162,
57%) and 70/162 (43%) patients were transplanted in 1st complete
remission (CR). Seventy-one (44%) out of 162 patients received a MAC,
122 (76%) were transplanted using peripheral blood stem cells (PBSC)
and 86 (53%) received a transplant from a MUD. During patients' follow
up the incidence of aGVHD grade >= 2 was 35% (56/162 cases) and
the incidence of extensive cGVHD was 12% (19/162 cases). The incidence
of possible/probable IFI was 35% (57/162 cases) and the incidence of
CMV reactivation was 59% (95/162 cases). The median follow 14 months
(range 0-68). No significant differences were observed comparing the
clinical and biological characteristics of patients with BSIs (BSI-pos)
and patients without BSIs (BSI-neg) (data not shown). As a consequence
we were not able to identify any factor possibly correlated with the
risk of developing a BSI.
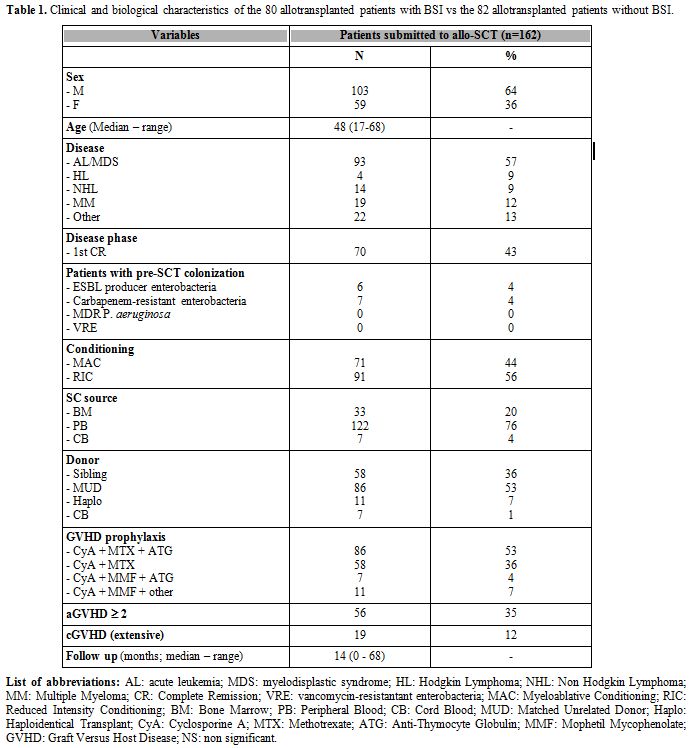 |
Table 1. Clinical and biological
characteristics of the 80 allotransplanted patients with BSI vs the 82
allotransplanted patients without BSI. |
Bloodstream infections:
One-hundred nineteen isolates were obtained from the blood samples of
80 patients with BSI (BSI-pos). The median time of positivity of BCs
was 19 days from transplant (range day -4 to day +921). In 42/80
patients (52%) the positive blood culture was detected before day +19
from allo-SCT. Half (n° 59) of the positive BCs derived from PB samples
and half (n° 60) could be considered CVC-related. Thirty-five out of
119 (29%) positive BCs were considered as contamination as previously
defined. Therefore, 84/119 (71%) of the BSIs could be considered real
bacteremia according to microbiological criteria. In 27/84 cases (24%)
the BSI could be defined CVC-related, according to the above reported
criteria.
Considering epidemiology, 77/119 (65%) and 42/119 (35%)
BCs were positive for Gram positive and Gram negative bacteria,
respectively. Data on the different species distribution is reported in
Table 2. Polimicrobical BSIs were found in 11/119 (9%). In one patient the BCs were positive for a Gram positive bacteria (S. heamolyticus) toghether with a Candida parapsilosis.
Antibiotics resistance is reported in Table 2. S. epidermidis was resistant to methycillin in all the cases and to fluoroquinolones in 90% of the cases. Sixty-seven percent of E. coli were ESBL producers and 92% were resistant to fluoroquinolones. Moreover, 40% and 90% of P. aeruginosa were resitant to carbapenems and fluoroquinolones, respectively. No carbapenemase-producing K. pneumoniae (KPC) was isolated in our series.
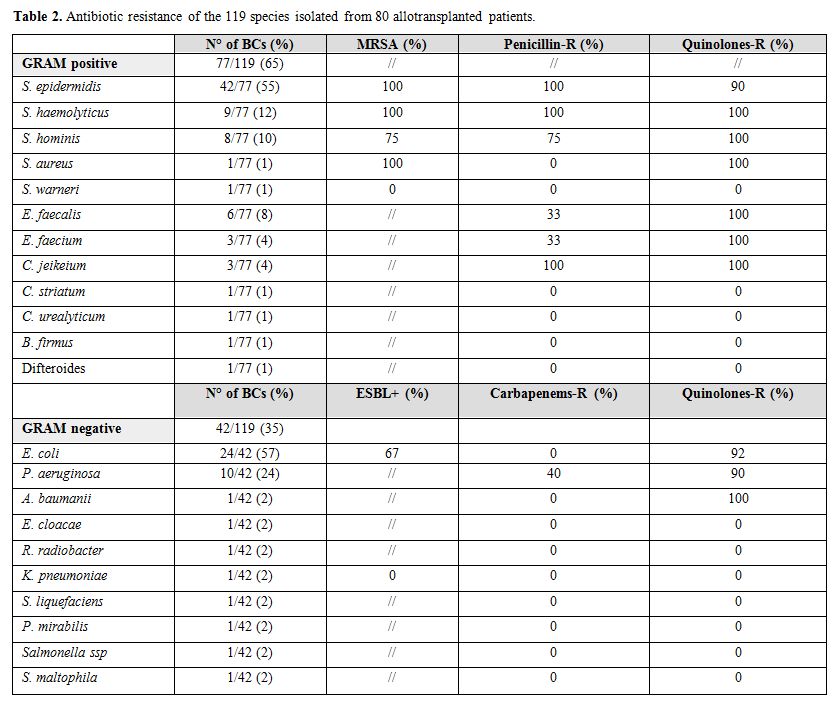 |
Table 2. Antibiotic resistance of the 119 species isolated from 80 allotransplanted patients. |
When
we analyzed the clinical and transplant characteristics of the patients
who experienced a Gram positive or a Gram negative BSI, we found that:
patients with Gram negative BSIs were more frequently affected by acute
leukemia/myelodisplastic syndrome (83% vs 54%, p=0,006) and were more
frequently transplanted from cord blood (14% vs 3%, p=0,04). On the
other hand, patients with Gram positive BSIs were more frequently
allotransplanted using a RIC regimen (69% vs 51%, p=0,004).
Gram
positive / Gram negative ratio was 1 and 3,2 considering early BSIs
(before day +19) vs late BSIs (after day +19) (p= 0,004). This
difference was related to the reduction of Gram negative bacteremia
after recovery from agranulocytosis (p=0,001). When we separately
analyzed the clinical and biological characteristics and the different
species distribution according to the time of positivity of the blood
culture (before or after day +19 from allo-SCT), we were not able to
identify any significant difference (data not shown). With the aim to
identify factors correlated with the time of development of BSIs
(during or after aplasia), we analyzed the clinical and biological
variables of patients with positive BCs, according to the time of BC
positivity [before engraftement (n=47) and after engraftment (n=33)].
We found that BSIs in patients transplanted from a sibling donor were
more frequent after engraftement (45% vs 23%; p=0,05) and that patients
who developed a BSI during aplasia had a higher incidence of IFI (47%
vs 24%; p=0,04).
Forty-four (55%) out of 80 patients had an
organ involvement together with the BSI. This was the lung in 57% of
the cases (25/44 cases), and the gut in 14% (6/44 cases). Within the 6
years of the observation time, we found a homogeneous Gram positive /
Gram negative ratio in all the years, with the exception of 2012 and
2013, where we observed a reduction in the number of positive BCs (11
in 2012 and 13 in 2013) and a reduction in Gram positive / Gram
negative ratio (1,2 in 2012 and 0,6 in 2013). No statistical
significant differences were observed comparing the number of positive
BCs and the Gram positive / Gram negative ratio in the single years
(data not shown).
Pre-transplant microbiological-history:
Twenty-nine out of 80 (36%) patients with BSIs experienced other
microbiological isolates during the treatment of the hematological
disease before allo-SCT, for a total of 55 isolates. In 12/29 cases
(34%) the specie responsible of the BSI before and after the transplant
treatment phase was the same, and the antibiograms were comparable. In
27 (49%) and 28 (51%) out of 55 BSIs a Gram positive and Gram negative
bacteria was isolated, respectively. Among Gram positive and Gram
negative bacteria, coagulase negative Staphylococci (14/27, 52%) and E. coli (22/28, 76%) were mostly represented.
Thirteen
out of 162 (8%) patients of this series were colonized by resistant
microorganisms (ESBL producers, carbapenem-resistant enterobacteria,
MDR P. aeruginosa and VRE). In particular, 5/80 (6%) of patients who developed a BSI were colonized by carbapenem-resitant E. coli. All these patients experienced a BSI caused by carbapenem-resistant E. coli. Moreover, 8/82 (10%) patients who did not experienced a BSI were colonized by resistant microorganisms (E. coli ESBL producers in 5 cases, E. coli carbapenem-resistant in 2 cases and K. pneumonia ESBL-producer in 1 case). No MDR P. aeruginosa or VRE were isolated.
Outcome: The overall survival of the 80 BSI-pos patients compared to the 82 BSI-neg patients is reported in Figure 1A.
Fourty-seven out of 80 BSI-pos patients (59%) vs 35/82 BSI-neg patients
(43%) died during follow up (p=0,2). Considering the outcome according
to the distribution of BSIs before (n=47) or after (n=33) the
engraftement, no statistical significant differences were observed
(data not showed). Thirty out of 47 (64%) and 17/33 (52%) patients with
BSIs before and after engraftement died, respectively (p=0,27). The
projected 2 years OS for patients with BSI vs patients without BSI is
46% vs 60% (HR 1.48, 95% CI: 0,96-2,29; p=0,07). Major causes of death
in the BSI-pos vs BSI-neg groups were: disease relapse (42% vs 55%; p=
0,37), infections (42% vs 17%; p= 0,02), aGVHD (2% vs 8%; p= 0,36),
cGVHD (5% vs 6%; p= 1,00) and toxixity (2% vs 6%; p= 0,30). As reported
in Figure 1B the transplant related mortality (TRM) among BSI-pos and BSI-neg patients was comparable (p=0,22).
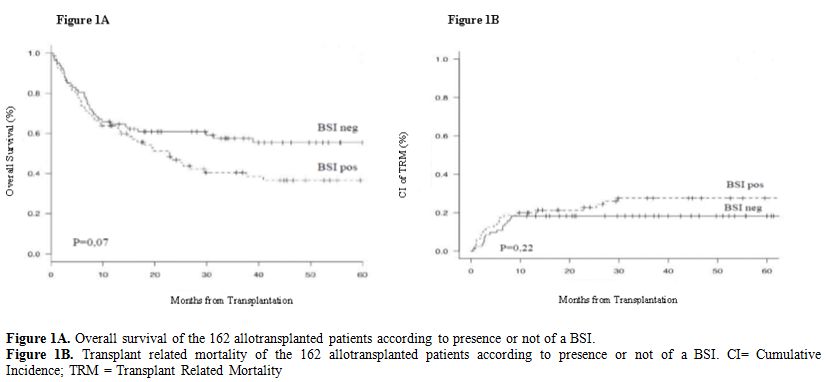 |
Figure 1A. Overall survival of the 162 allotransplanted patients according to presence or not of a BSI.
Figure
1B. Transplant related mortality of the 162 allotransplanted patients
according to presence or not of a BSI. CI= Cumulative Incidence; TRM =
Transplant Related Mortality
|
Fifteen
out of 80 patients with positive BSI (19%), died because of the
bacterial infection. Considering the 162 allotransplanted patients, the
BSI related mortality was 9% (15/162 cases). Nine out of these 15
deaths (60%) were related to Gram positive bacteria, leading to a Gram
positive related mortality of 12% (9/77 Gram + isolates). Six out of 15
infection-related deaths (40%) were caused by a Gram negative agent,
leading to a Gram negative related mortality of 14% (6/42 Gram negative
isolates). Interestingly, we observed that among the Gram positive
bacteria the mortality rate was 33% for Enterococci (3/9 isolates), 22% for S. haemolyticus (2/9 isolates) and 10% for S. epidermidis (4/42 isolates).
Considering Gram negative bacteria, the mortality rate was 50% for P. aeruginosa (5/10 isolates, in 1 case MDR, in 4 cases multisensible) and 4% for E. coli (1/24 isolates, ESBL producer).
Discussion
Infective
complications are commonly considered the most relevant event
associated with increased morbidity and mortality in allotransplanted
patients (1 - 12), and the microbiological surveillance of bacterial
isolates in Transplant Units is a mainstay of good clinical practice.
In this view, we collected the data on 162 allotransplanted patients,
80 of whom (49%) experienced a BSI, over a period of 6 years of
transplant activity. The total number of positive BCs in the period of
observation was 119, 84 of whom (71%) could be considered as real
bacteremia.
Overall, our data are in line with previous reports,
that cover a longer period (10-15 years), showing a predominance of
Gram positive over Gram negative bacteria (65% vs 35%), being S. epidermidis (55%) and E. coli (57%) the predominant species among Gram positive and Gram negative BSIs (Table 2).[4,5,6,8,12,16,17]
We were not able to identify clinical and transplant variables
significantly associated with the development of BSIs, and this is
probably related to the relatively low number of patients in each
subgroup. Interestingly, patients with a Gram negative BSI were more
frequently affected by acute leukemia or myelodisplastic syndrome
(p=0,006) and were more frequently transplanted from cord blood
(p=0,04). Although the number of patients in each group is relatively
small to draw final conclusions, we can speculate that these
differences may be caused by the intensive pre-tranplant treatement, by
the high prevalence of refractory disease among acute leukemia patients
and by the delayed neutrophil recovery observed when a cord blood is
used as stem cell source, respectively. On the other hand, a BSI
sustained by a Gram positive bacteria was more frequent in patients
allotransplanted using a RIC regimen (p=0,04). This may partially
reflect the characteristics of these patients (e.g. frail and elderly
patients).
One point of interest in the field of bacterial infections is antimicrobical resistance.[14-17]
Our data confirm that this problem has now reached the highest level of
criticism, as we observed fluoroquinolones resistance both among Gram
positive (roughly 100%) and Gram negative (between 90 and 100%)
bacteria, toghether with methicillin resistance among Gram positive
bacteria (100% of the S. aureus, epidermidis and haemolyticus and 75% of the S. hominis) (Table 2). This is in line with previously reported series[12,17]
and it is strongly associated with the large use of fluoroquinolones
for prophylaxis during the aplastic phase, although this practice is
commonly suggested by the most recently published guidelines.[26]
Considering these data, the debate on the utility of fluoroquinolones
prophylaxis is still opened. This policy, indeed, may reduce the
mortality of Gram negative bacteria, but other propylaxis are under
investigation. Recently, Pohlen and Colleagues, reported on a study
comparing ciprofloxacin versus colistin prophylaxis in AML patients
during neutropenia.[28] Although this was not a
randomized trial, ciprofloxacin prophylaxis was confirmed highly
effective in reducing the incidence of infections (69% vs 79% for
colistin; p=0,07), but was confirmed to be associated with
fluoroquinolone resistance, as expected. Moreover, 67% of E. coli was ESBL producer and 40% of P. aeruginosa was resistant to carbapenems, and this is similar to what previously reported.[12,15]
Interestingly, no KPC was isolated. This point is of interest, because
currently data from the litterature suggest that
carbapenemase-producing enterobacteriacae (namely KPC) is an emerging
problem in hematological patients, particularly challenging among
allotransplanted patients. As reported by Girmenia and Colleagues in a
retrospective italian survey, the incidence of KPC infections in
allotransplanted patients was 2%, with a high-risk of infections in
colonized patients. Moreover, the infection related mortality was 64%.[27]
One possible explanation for the absence of KPC in our series may be
related to the fact that we did not observe any pre-transplant
colonization sustained by this bacteria. Alltogether these data enhance
the importance of microbiobiological surveillance with the aim to
promptly start patients' isolation and reduce MDR bacteria spreading.
Looking
at the species distribution per year in our series, the Gram positive /
Gram negative ratio, as well as the isolated species, remained costant,
with the exception of 2 years (2012 and 2013), in which an overall
reduction in BSIs and a reduction of the Gram positive / Gram negative
ratio was observed. Although these differences do not reach the level
of significance, they may be partially explained considering the
different management of CVC in the years 2012 and 2013, when we had a
single nurse dedicated to CVC medications. Considering the high
incidence of CVC-related infections (24%), the adoption of
clinical-care strategies such as CVC medication under optimal asepsis
and by dedicated nurses may be the best way to prevent BSIs.
Colonization
by multiresistant microorganisms was detected in a small proportion of
patients (13/162, 8%), and was mainly sustained by ESBL-producer or
carbapenem-resistant enterobacteria (namely E. coli).
Due to the relatively small number of cases we could not compare the
outcome according to colonization. We indeed observed that all the
patients colonized by carbapenem-resistant E. coli experienced a BSI caused by a similar resistant microorganism.
Moving
from epidemiology to outcome, we observed that 15/162 allotransplanted
patients (9%) died because of BSIs and this mortality rate is
comparable to the one observed in other reports.[7,8,29] P. aeruginosa can
be still considered the major killer, irrespective of its resistance
profile (5 deaths out of 10 cases – 50%, similarly to what reported by
Collin et al - in one case only MDR). Enterococci, coagulase negativeStaphylococci, and E. coli
showed a mortality of 33% (3/9 cases), 12% (6/51 cases) and 4% (1/24
cases), respectively. It should be stressed that in 12/15 (80%) of
these bacteremia-related deaths other factors, such as GVHD or active
disease at transplant, were present at the time of BSI. Moreover, we
observed that the long term outcome of patients who experience a BSI
was impaired with respect to those who do not experience this
complication (2 years OS for patients with BSI vs patients without BSI:
46% vs 60%; p=0,07). Although the TRM of the two groups is comparable (Figure 1B),
the difference in mortality among patients with BSI and those without
BSI is more evident after at least 12 months from transplant. This may
be partially related to the fact that bacteremia are present in the
late phase of the transplant too and usually affects extremely frail
patients, such as those with GVHD and chronic steroid treatment. In
such critically hill patients, a bacteremia may rapidly and negatively
influence the outcome.
In conclusion, BSIs continue to be a
significant event in allotransplanted patients, with Gram positive
bacteria being the species at highest incidence and P. aeruginosa
being the specie with the highest mortality. Routinely use of
fluoroquinolone prophylaxis and prompt empirical antimicrobical therapy
significantly reduces the mortality related to bacterial infections,
but emergence of quinolones resistance in the great majority of Gram
positive and negative species remain an unsolved issue. Even though
further studies on prophylaxis in allotransplanted patients during
neutropenia are warranted, we have no data to change our policy,
considering the outcome of the reported cases of BSIs. A modern
approach to the problem of BSIs is probably the investigation of the
patients' microbioma before allo-SCT. Some data from the literature
reported that intestinal domination, defined as occupation of at least
30% of the microbiota by a single bacterial taxon, is associated with
BSI in patients undergoing allo-SCT, that the gut microbiota can
identify high-risk patients before allo-SCT and that manipulation of
the gut microbiota for prevention of BSIs in high-risk patients may be
a useful direction for future research.[30,31,32]
Thus, in the near future, we will probably need to include this
analysis in the baseline work up and in the follow up of patients
addressed to allo-SCT.
Authors' Contributions
MM,
BR and DR designed the study. All the Authors collected the data. MM,
BR, GR, CC, NP and DR analyzed the results. All the Authors gave their
final approval to the Manuscript.
References
- Williamson EC, Millar MR, Steward CG, Cornish
JM, Foot AB, Oakhill A, Pamphilon DH, Reeves B, Caul EO, Warnock DW,
Marks DI. Infections in adults undergoing unrelated donor bone marrow
transplantation. Br J Haematol 1999; 104:560-8. https://doi.org/10.1046/j.1365-2141.1999.01229.x PMid:10086795

- Ninin
E, Milpied N, Moreau P, André-Richet B, Morineau N, Mahé B, Vigier M,
Imbert BM, Morin O, Harousseau JL, Richet H. Longitudinal study of
bacterial, viral, and fungal infections in adult recipients of bone
marrow transplants. Clin Infect Dis 2001; 33:41-7. https://doi.org/10.1086/320871 PMid:11389493

- Marena
C, Zecca M, Carenini ML, Bruschi A, Bassi ML, Olivieri P, Azzaretti S,
Locatelli F. Incidence of, and risk factors for, nosocomial infections
among hematopoietic stem cell transplantation recipients, with impact
on procedure-related mortality. Infect Control Hosp Epidemiol 2001;
22:510-7. https://doi.org/10.1086/501942 PMid:11700879

- Poutsiaka
DD, Price LL, Ucuzian A, Chan GW, Miller KB, Snydman DR. Blood stream
infection after hematopoietic stem cell transplantation is associated
with increased mortality. Bone Marrow Transplant 2007 ;40:63-70. https://doi.org/10.1038/sj.bmt.1705690 PMid:17468772

- Almyroudis
NG, Fuller A, Jakubowski A, Sepkowitz K, Jaffe D, Small TN, Kiehn TE,
Pamer E, Papanicolaou GA. Pre- and post-engraftment bloodstream
infection rates and associated mortality in allogeneic hematopoietic
stem cell transplant recipients. Transpl Infect Dis 2005 ;7:11-7. https://doi.org/10.1111/j.1399-3062.2005.00088.x PMid:15984943

- Collin
BA, Leather HL, Wingard JR, Ramphal R. Evolution, incidence, and
susceptibility of bacterial bloodstream isolates from 519 bone marrow
transplant patients. Clin Infect Dis 2001 ;33:947-53. https://doi.org/10.1086/322604 PMid:11528564

- Cappellano
P, Viscoli C, Bruzzi P, Van Lint MT, Pereira CA, Bacigalupo A.
Epidemiology and risk factors for bloodstream infections after
allogeneic hematopoietic stem cell transplantion. New Microbiol
2007;30:89-99. PMid:17619251

- Ortega
M, Rovira M, Almela M, Marco F, de la Bellacasa JP, Martínez JA,
Carreras E, Mensa J. Bacterial and fungal bloodstream isolates from 796
hematopoietic stem cell transplant recipients between 1991 and 2000.
Ann Hematol 2005 ;84:40-6. https://doi.org/10.1007/s00277-004-0909-0 PMid:15480665

- Lukenbill
J, Rybicki L, Sekeres MA, Zaman MO, Copelan A, Haddad H, Fraser T,
DiGiorgio MJ, Hanna R, Duong H, Hill B, Kalaycio M, Sobecks R, Bolwell
B, Copelan E. Defining incidence, risk factors, and impact on survival
of central line-associated blood stream infections following
hematopoietic cell transplantation in acute myeloid leukemia and
myelodysplastic syndrome. Biol Blood Marrow Transplant 2013; 19:720-4. https://doi.org/10.1016/j.bbmt.2013.01.022 PMid:23380342

- Shigematsu
A, Yamamoto S, Sugita J, Kondo T, Onozawa M, Kahata K, Endo T,
Shiratori S, Ota S, Yamaguchi K, Wakasa K, Takahata M, Goto H, Ito S,
Takemura R, Tanaka J, Hashino S, Nishio M, Koike T, Asaka M, Imamura M.
Increased risk of bacterial infection after engraftment in patients
treated with allogeneic bone marrow transplantation following
reduced-intensity conditioning regimen. Transpl Infect Dis 2010
;12:412-20. https://doi.org/10.1111/j.1399-3062.2010.00560.x PMid:20738830

- Liu
CY, Lai YC, Huang LJ, Yang YW, Chen TL, Hsiao LT, Liu JH, Gau JP, Chen
PM, Tzeng CH, Chiou TJ. Impact of bloodstream infections on outcome and
the influence of prophylactic oral antibiotic regimens in allogeneic
hematopoietic SCT recipients. Bone Marrow Transplant 2011
;46:1231-9. https://doi.org/10.1038/bmt.2010.286 PMid:21113186

- Busca
A, Cavecchia I, Locatelli F, D'Ardia S, De Rosa FG, Marmont F, Ciccone
G, Baldi I, Serra R, Gaido E, Falda M. Blood stream infections after
allogeneic stem cell transplantation: a single-center experience with
the use of levofloxacin prophylaxis. Transpl Infect Dis 2012 ;14:40-8. https://doi.org/10.1111/j.1399-3062.2011.00650.x PMid:21599817

- Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet 2009; 373:1550-61. https://doi.org/10.1016/S0140-6736(09)60237-3

- El-Mahallawy
H, Samir I, Abdel Fattah R, Kadry D, El-Kholy A. Source, pattern and
antibiotic resistance of blood stream infections in hematopoietic stem
cell transplant recipients. J Egypt Natl Canc Inst 2014; 26:73-7. https://doi.org/10.1016/j.jnci.2013.12.001 PMid:24841157

- Therriault
BL, Wilson JW, Barreto JN, Estes LL. Characterization of bacterial
infections in allogeneic hematopoietic stem cell transplant recipients
who received prophylactic levofloxacin with either penicillin or
doxycycline. Mayo Clin Proc 2010; 85:711-8. https://doi.org/10.4065/mcp.2010.0006 PMid:20675508 PMCid:PMC2912731

- Oliveira
AL, de Souza M, Carvalho-Dias VM, Ruiz MA, Silla L, Tanaka PY, Simões
BP, Trabasso P, Seber A, Lotfi CJ, Zanichelli MA, Araujo VR, Godoy C,
Maiolino A, Urakawa P, Cunha CA, de Souza CA, Pasquini R, Nucci M.
Epidemiology of bacteremia and factors associated with
multi-drug-resistant gram-negative bacteremia in hematopoietic stem
cell transplant recipients. Bone Marrow Transplant 2007; 39:775-81. https://doi.org/10.1038/sj.bmt.1705677 PMid:17438585

- Mikulska
M, Del Bono V, Raiola AM, Bruno B, Gualandi F, Occhini D, di Grazia C,
Frassoni F, Bacigalupo A, Viscoli C. Blood stream infections in
allogeneic hematopoietic stem cell transplant recipients: reemergence
of Gram-negative rods and increasing antibiotic resistance. Biol Blood
Marrow Transplant 2009; 15:47-53. https://doi.org/10.1016/j.bbmt.2008.10.024 PMid:19135942

- Rupp
ME, Fey PD. Extended spectrum beta-lactamase (ESBL)-producing
Enterobacteriaceae: considerations for diagnosis, prevention and drug
treatment. Drugs 2003; 63:353-65. https://doi.org/10.2165/00003495-200363040-00002

- Harada
S, Ishii Y, Yamaguchi K. Extended-spectrum beta-lactamases:
implications for the clinical laboratory and therapy. Korean J Lab Med
2008; 28:401-12. https://doi.org/10.3343/kjlm.2008.28.6.401 PMid:19127103

- The European Committee on Antimicrobial Susceptibility Testing. (EUCAST). http://www.eucast.org/
- Slade
M, Fakhri B, Savani BN, Romee R. Halfway there: the past, present and
future of haploidentical transplantation. Bone Marrow Transplant 2016;
e-pub ahead of print Jul 25; doi: 10.1038/bmt.2016.190. https://doi.org/10.1038/bmt.2016.190

- Rowlings
PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ,
Cahn JY, Calderwood S, Gratwohl A, Socié G, Abecasis MM, Sobocinski KA,
Zhang MJ, Horowitz MM. IBMTR Severity Index for grading acute
graft-versus-host disease: retrospective comparison with Glucksberg
grade. Br J Haematol 1997; 97:855-64. https://doi.org/10.1046/j.1365-2141.1997.1112925.x PMid:9217189

- Filipovich
AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P,
Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A,
Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B,
Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M,
Vogelsang G, Flowers ME. National Institutes of Health consensus
development project on criteria for clinical trials in chronic
graft-versus-host disease: I. Diagnosis and staging working group
report. Biol Blood Marrow Transplant 2005; 11:945-56.https://doi.org/10.1016/j.bbmt.2005.09.004 PMid:16338616

- Pagano
L, Caira M, Nosari A, Van Lint MT, Candoni A, Offidani M, Aloisi T,
Irrera G, Bonini A, Picardi M, Caramatti C, Invernizzi R, Mattei D,
Melillo L, de Waure C, Reddiconto G, Fianchi L, Valentini CG, Girmenia
C, Leone G, Aversa F. Fungal infections in recipients of hematopoietic
stem cell transplants: results of the SEIFEM B-2004 study--Sorveglianza
Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect
Dis 2007; 45:1161-70. https://doi.org/10.1086/522189 PMid:17918077

- Kanda
Y. Investigation of the freely available easy-to-use software 'EZR' for
medical statistics. Bone Marrow Transplant 2013; 48:452-8. https://doi.org/10.1038/bmt.2012.244 PMid:23208313 PMCid:PMC3590441

- Bucaneve
G, Micozzi A, Menichetti F, Martino P, Dionisi MS, Martinelli G,
Allione B, D'Antonio D, Buelli M, Nosari AM, Cilloni D, Zuffa E,
Cantaffa R, Specchia G, Amadori S, Fabbiano F, Deliliers GL, Lauria F,
Foà R, Del Favero A; Gruppo Italiano Malattie Ematologiche dell'Adulto
(GIMEMA) Infection Program. Levofloxacin to prevent bacterial infection
in patients with cancer and neutropenia. N Engl J Med 2005; 353:977-87.
https://doi.org/10.1056/NEJMoa044097 PMid:16148283

- Girmenia
C, Rossolini GM, Piciocchi A, Bertaina A, Pisapia G, Pastore D, Sica S,
Severino A, Cudillo L, Ciceri F, Scimè R, Lombardini L, Viscoli C,
Rambaldi A; Gruppo Italiano Trapianto Midollo Osseo (GITMO); Gruppo
Italiano Trapianto Midollo Osseo GITMO. Infections by
carbapenem-resistant Klebsiella pneumoniae in SCT recipients: a
nationwide retrospective survey from Italy. Bone Marrow Transplant
2015;50:282-8. https://doi.org/10.1038/bmt.2014.231 PMid:25310302

- Pohlen
M, Marx J, Mellmann A, Becker K, Mesters RM, Mikesch JH, Schliemann C,
Lenz G, Müller-Tidow C, Büchner T, Krug U, Stelljes M, Karch H, Peters
G, Gerth HU, Görlich D, Berdel WE. Ciprofloxacin versus colistin
prophylaxis during neutropenia in acute myeloid leukemia: two parallel
patient cohorts treated in a single center. Haematologica 2016;
101:1208-1215. https://doi.org/10.3324/haematol.2016.147934 PMid:27470601 PMCid:PMC5046650

- Orasch
C, Weisser M, Mertz D, Conen A, Heim D, Christen S, Gratwohl A,
Battegay M, Widmer A, Flückiger U. Comparison of infectious
complications during induction/consolidation chemotherapy versus
allogeneic hematopoietic stem cell transplantation. Bone Marrow
Transplant 2010; 45:521-6. https://doi.org/10.1038/bmt.2009.187 PMid:19668238

- Taur
Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, Lee YJ, Dubin
KA, Socci ND, Viale A, Perales MA, Jenq RR, van den Brink MR, Pamer EG.
Intestinal domination and the risk of bacteremia in patients undergoing
allogeneic hematopoietic stem cell transplantation. Clin Infect Dis
2012; 55:905-14. https://doi.org/10.1093/cid/cis580 PMid:22718773 PMCid:PMC3657523

- Montassier
E, Al-Ghalith GA, Ward T, Corvec S, Gastinne T, Potel G, Moreau P, de
la Cochetiere MF, Batard E, Knights D. Pretreatment gut microbiome
predicts chemotherapy-related bloodstream infection. Genome Med 2016;
8:49. https://doi.org/10.1186/s13073-016-0301-4 PMid:27121964 PMCid:PMC4848771

- Laterza
L., Rizzatti G., Gaetani E., Chiusolo P., Gasbarrini A. The gut
microbiota and immune system relationship in human graft-versus-host
disease. Mediterr J Hematol Infect Dis 2016, 8(1): e2016025,
DOI:10.4084/MJHID.2016.025 https://doi.org/10.4084/mjhid.2016.025

[TOP]


































