Received: April 22, 2017
Accepted: July 19, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017048 DOI 10.4084/MJHID.2017.048
This article is available on PDF format at:

Ruchi Gupta, Khaliqur Rahman, Manish Kumar Singh, Surabhi Kumari, Geeta Yadav and Soniya Nityanand

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Background:
Myelodysplastic syndrome (MDS) is a heterogeneous disorder
characterized clinically by the presence of cytopenia/s. Limited data
are available about the morphological spectrum and cytogenetic profile
of Indian MDS patients. The aim of the study was to ascertain the
clinico-pathological, morphological and cytogenetic spectrum of Indian
MDS patients. |
Introduction
Materials and Methods
Results
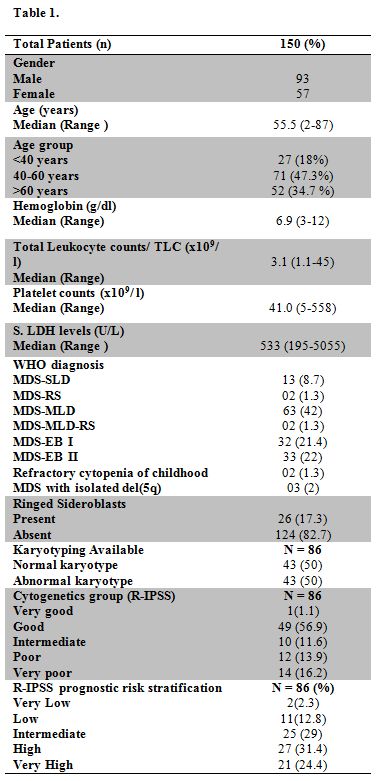 |
Table 1 |
Discussion
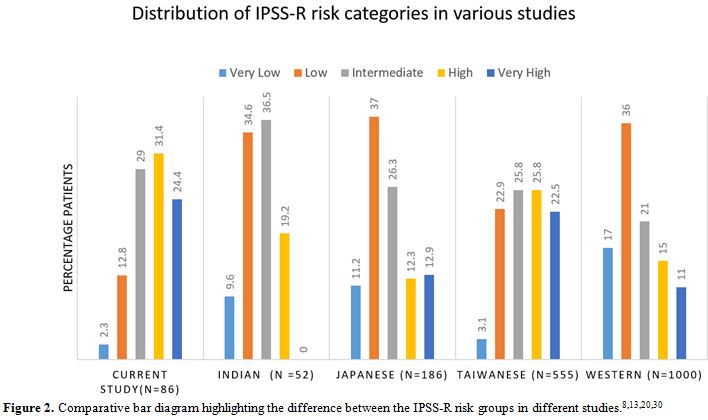 |
Figure 2. Comparative bar diagram highlighting the difference between the IPSS-R risk groups in different studies.[8,13,20,30] |
References






























 |
Supplementary Table 1. Cytogenetic profile of MDS patients in different sub groups. |
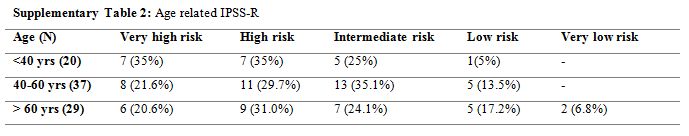 |
Supplementary Table 2. Age related IPSS-R |
[TOP]