Lassina Traore1,2, Ouéogo Nikiema1,2, Abdoul Karim Ouattara1,2, Tegwindé Rébéca Compaore1,2, Serge Théophile Soubeiga1,2, Birama Diarra1,2, Dorcas Obiri-Yeboah3, Pegdwendé Abel Sorgho1,2, Florencia Wendkuuni Djigma1,2, Cyrille Bisseye1,2, Albert Théophane Yonli1,2 and Jacques Simpore1,2
1 Biomolecular Research Center Pietro Annigoni (CERBA)
2 LABIOGENE UFR/SVT, University Ouaga I Prof. Joseph KI-ZERBO 01 BP 364 Ouagadougou, Burkina Faso.
3 Deparment of Microbiology and Immunology, School of Medical Sciences, University of Cape Coast, Ghana
Corresponding
author: Lassina Traore. Biomolecular
Research Center Pietro Annigoni (CERBA)/LABIOGENE UFR/SVT, University
Ouaga I Prof. Joseph Ki-Zerbo, BP 364 Ouagadougou, Burkina Faso.
Burkina Faso, West Africa. Tel: +226 76 50 37 05. E-mail:
ttl.lass@yahoo.fr
Published: September 1, 2017
Received: June 13, 2017
Accepted: August 5, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017049 DOI
10.4084/MJHID.2017.049
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Epstein
Barr Virus (EBV) and Human Herpes Virus 6 (HHV-6) are responsible for
severe diseases, particularly in immunocompromised persons. There is
limited data of the infection of these opportunistic viruses in Burkina
Faso.
The purpose of this study was to characterize EBV and HHV-6
subtypes and to assess their impact on CD4 T cell count, HIV-1 viral
load and antiretroviral treatment in people living with HIV-1.
The study population consisted of 238 HIV-positive patients with
information on the CD4 T cell count, HIV-1 viral load and HAART. Venous
blood samples collected in EDTA tubes were used for EBV and HHV-6 Real
Time PCR subtyping.
An infection rate of 6.7% (16/238) and 7.1%
(17/238) were found respectively for EBV and HHV-6 in the present
study. Among EBV infections, similar prevalence was noted for both
subtypes (3.9% [9/238] for EBV-1 vs 4.6% [11/238] for EBV-2) with 2.1%
(5/238) of co-infection. HHV-6A infection represented 6.3% (15/238) of
the study population against 5.0% (12/238) for HHV-6B. EBV-2 infection
was significantly higher in patients with CD4 T cell count ≥ 500
compared to those with CD4 T cell count less than 500 cells (1.65% vs
8.56%, p = 0,011). The prevalence of EBV and HHV-6 infections was
almost similar in HAART-naive and HAART-experienced patients.
The
present study provides information on the prevalence of EBV and HHV-6
subtypes in people living with HIV-1 in Burkina Faso. The study also
suggests that HAART treatment has no effect on infection with these
opportunistic viruses in people living with HIV-1.
|
Introduction
In
the human species, there are eight (8) herpes viruses, including herpes
simplex types 1 and 2 (HSV1 and HSV2), Varicella Zoster Virus (VZV),
cytomegalovirus (CMV), Epstein-Barr Virus (EBV) and herpesviruses
(HHV6, HHV7 and HHV8 associated with Kaposi's sarcoma).[1]
They belong to the human herpesviridae family, and the high similarity
between these viruses shows that they have a common origin.[1]
EBV
is one of the most common human viruses, and it is found all over the
world. Recent studies have shown that EBV seroprevalence is estimated
to be present in more than 90% of adults older than 35 years of age
worldwide.[2] Each year, new infections are estimated at 200,000.[3]
The Epstein-Barr virus is commonly acquired during childhood in
developing countries (more than 90% of pre-school children). In
developed countries, many people are not infected in childhood but are
rather infected in adolescence or during adulthood. Variations in the
EBV genome made it possible to distinguish two (02) subtypes of the
virus: EBV-1 and EBV-2 (or EBV-A and EBV-B types).[4]
Seroprevalence studies have shown that EBV-1 strain predominates in
western countries, whereas EBV-2 strain is only common in some areas of
Equatorial Africa and New Guinea.[4] Primary infection with EBV is often asymptomatic or is responsible for infectious mononucleosis[5] and generally with no serious complications.[6]
On the other hand, chronic infection is reported in many cases of
cancers (gastric carcinoma, Burkitt's lymphoma [BL to nasopharyngeal
carcinoma, Hodgkin's classic lymphoma (LH), gastric carcinoma][7-10] and oral hairy leukoplakia.[11] Furthermore, it is suggested that EBV is associated with brain cancer, salivary gland tumors, hepatocellular carcinoma[4] and also with certain autoimmune diseases such as multiple sclerosis,[12] especially in immunocompromised individuals.[13]
In
most HIV-infected persons with progressive immunodeficiency, the number
of EBV infected B cells increases in blood circulation[14]
and can develop opportunistic lymphomas (Burkitt's lymphoma, lymphomas
that diffuse to large cells and primitive cerebral lymphomas) all
associated with EBV.
Human herpesvirus 6 (HHV-6) is a member of
the beta-herpes virus family, genetically close to cytomegalovirus
(CMV) and human herpesvirus 7 (HHV-7).[15] It is
responsible for infection of the vast majority of children in the early
years of life and persists like the majority of the others herpes
viruses in the latent form after the primary infection. It is a
ubiquitous virus that infects T lymphocytes, monocytes, macrophages,
certain epithelial cells and central nervous system cells. It early
appeared that there were two HHV-6 variants or subtypes, subtype A
(HHV-6A) and subtype B (HHV-6B) defined according to antigenic, genetic
and potential differences in their respective pathogenicity.[16]
The seroprevalence of HHV-6 infection is estimated to be between 70 and
100% in the human population and varies according to geographic
location.[17] In the United States and Japan, primary
HHV-6 infection affects children between 6 and 12 months, and it is
estimated to be between 97 and 100%. This primary infection is due to
HHV-6B subtype,[18] and HHV-6B viral DNA is also frequently detected in children in parts of Sub-Saharan Africa where HIV-1 is endemic.[19]
There are limited data available on HHV-6A prevalence in sub-Saharan
Africa. Studies have shown that HHV-6A is acquired late in life with a
primary infection, generally asymptomatic.[17]
However, more recent studies have described symptomatic primary
infection in American and African children including roseola and
febrile diseases.[19] HHV-6B is the causative agent
of a very young child benign disease, exanthema subitum, still called
infantile roseola or sixth disease.
HHV-6A has mainly a
neurological tropism. HHV-6A and HHV-6B also cause opportunistic
infections in immunocompromised individuals, including systemic
infections and organ disorders, particularly encephalitis, hepatitis,
colitis, spinal cord insufficiency, pneumonia, interstitial
pneumonitis.[20] It can also favor insurgency of
acute lymphoblastic or non-lymphoblastic leukemias, cutaneous T-cell
lymphoma, immunoblastic lymphoma, acute lymphoid leukemia[21] and Hodgkin's lymphoma.[19,22] Recently, HHV-6 has been described to be associated with Drug Rash with Eosinophilia and Systemic Symptoms.[23]
The literature reported that HHV-6 and HIV-1 would act in concert by
infecting and causing CD4+ T cell lysis, thereby accentuating
immunosuppression and progression towards AIDS by accelerating the
death of infected CD4+ T cells.[24-26] However, HHV-6
detection in the blood decreases with AIDS progression, since virus
replication target cells, the CD4+ T cells, are reduced.[27] In HIV infected patients, HHV-6 reactivation are associated with encephalitis,[28] pneumonia[29] or retinitis.[30,31]
It is currently unknown whether HHV-6 acts simply as an opportunistic
pathogen or in synergy with HIV on the disease progression. Some of our
previous studies have resulted in the detection of EBV, HHV-6, and CMV
among blood donors[32,33] and HIV-positive mothers.[34]
These studies also made possible to determine the molecular
epidemiology of these herpes viruses in the subpopulations concerned by
the latter studies. To date, there are no studies that revealed
information on the subtypes of circulating EBV and HHV-6 in Burkina
Faso. Thus, the present study not only targets the characterization of
EBV and HHV-6 subtypes; but also the impact of infection of both
viruses on CD4 T cell count, HIV-1 viral load, and treatment in people
living with HIV-1.
Materials and Methods
Study setting.
This prospective study was carried out in Ouagadougou, Burkina Faso
from May 2016 to March 2017. The samples were collected at the Saint
Camille Hospital of Ouagadougou (HOSCO), and the molecular analyses
were carried out at the Molecular Biology and Genetics Laboratory
(LABIOGENE) of the University Ouaga I Prof. Joseph KI-ZERBO and the
Pietro Annigoni Biomolecular Research Center (CERBA).
Sampling.
The study included 238 HIV-1 positive patients recruited during their
routine visit at HOSCO. The samples consisted of 3 mL of venous blood
collected in EDTA tubes. The whole blood was aliquoted and stored at
-20°C until DNA extraction for molecular analysis. Sociodemographic
characteristics, CD4 T cell count and HIV-1 viral load results were
collected in patient follow-up registries with their free and informed
consent.
DNA extraction and qualitative diagnosis of HHV-6 and EBV by real-time PCR. Genomic DNA was extracted from whole blood using the standard salting-out method as previously described by Miller et al.[35]
EBV and HHV-6 subtypes identification was carried out by real-time PCR
using specific primers and probes previously described by Kwok et al.
for EBV and Yavarian et al. for HHV-6.[36,37]
The
amplification for two viruses subtyping was carried out at 95°C for 5
minutes corresponding to initial denaturation, followed by 45 cycles of
95°C for 15 seconds and 55°C for 30 seconds.
Statistical analysis. A database was compiled on Microsoft Excel 2013 and then analyzed using the software Epi InfoTM
7 and Statistical Package for Social Sciences (SPSS) 21.0 (IBM, Armonk,
NY, USA). The results were analyzed according to socio-demographic
characteristics, clinical parameters, CD4 T cell count and HIV-1
plasmatic viral load. The chi-square test was used for the comparisons,
and the difference was considered statistically significant for P value
≤ 0.05.
Ethical considerations.
Our study was approved by the Ethics Committee on Health Research
(CERS) of Burkina Faso (Ref: DELIBERATION N° 2014-9-113). Written
informed consent was obtained from all the participants, and the
results confidentiality was respected.
Results
Socio-demographic characteristics.
Our study involved 238 people living with HIV-1 (PLHIV-1). The study
population consisted of 66.8% of women and 33.2% of men. Children under
five years of age accounted for 9.7% of the study population while
90.3% of the individuals were over 15 years of age. The median age was
24.7 ± 18.9 years.
Prevalence of Herpes Virus Infections.
Of the 238 patients tested in this study, 13.0% (31/238) were positive
for at least one of the viruses (EBV or HHV-6). The prevalence of EBV,
EBV-1 and EBV-2 was 6.7% (16/238); 3.9% (9/238) and 4.6% (11/238)
respectively. EBV-1/EBV-2 co-infection was observed in 2.1% (5/238)
patients of the study population. HHV-6 infections were detected in
7.1% (17/238) of the individuals with prevalence of 6.3% (15/238) and
5.0% (12/238) respectively for HHV- 6A and HHV-6B. HHV-6A/HHV-6B
co-infection was observed in 10 patients or a prevalence of 4.2%
(10/238). Two (2/238 or 0.8%) patients were co-infected with EBV/HHV-6.
According to age, herpes infection was more observed in patients more
than fifteen (15) years, except HHV-6B which was more observed in the
group of more than 15 years old (Table 1 and Table 2). However, it should be noted that these results were not statistically significant.
Analysis
of the results by sex showed that infections were more common in men
than in women, except for the higher prevalence of HHV-6A in males
compared to females (Table 1 and 2).
The difference between EBV prevalence in men and woman was slightly significant (P = 0.05).
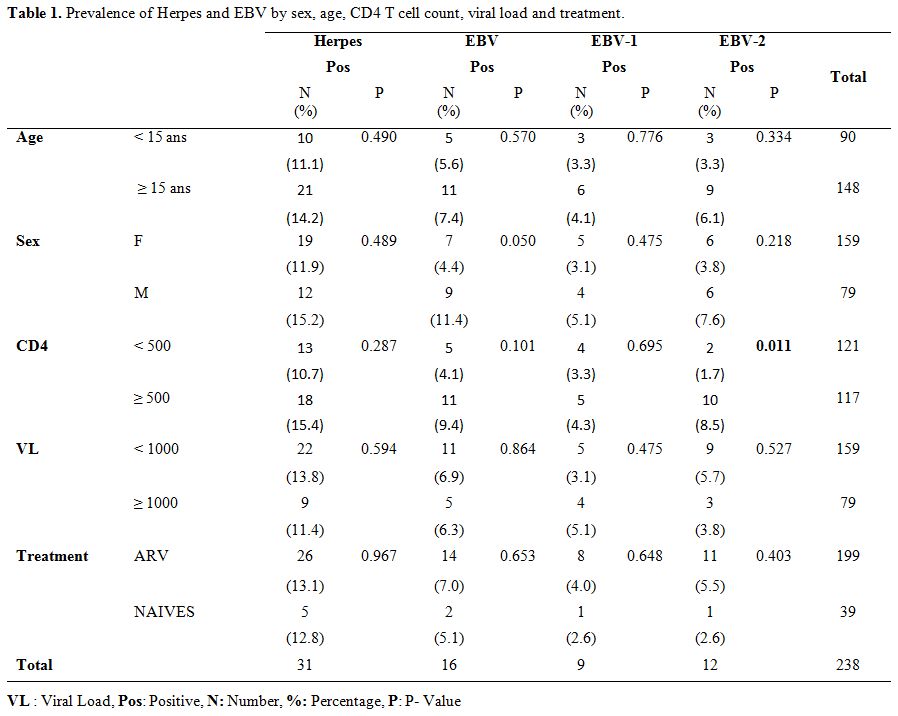 |
Table 1.
Prevalence of Herpes and EBV by sex, age, CD4 T cell count, viral load and treatment. |
 |
Table 2. Prevalence and HHV-6 by sex, age, CD4 T cell count, viral load and treatment. |
Herpes virus infections and CD4 cell count.
Depending on the CD4 T cell count, our results show that patients with
high CD4 T cell counts (CD4 T cell count ≥ 500/mL) are the most
infected with herpes virus, EBV, EBV-1, and EBV-2. (Table 1).
This difference was very significant for EBV-2 (P = 0.011) but was not
significant for the others. HHV-6, HHV-6A and HHV-6B infections were
more common in patients with low CD4 counts (CD4 T cell count <
500/mL) or those with high CD4 counts (CD4 T cell count ≥ 500 / mL) (Table 2). It should be noted that this difference was not statistically significant.
Herpes viral infections and HIV viral load.
Our results were also analyzed according to the patients HIV-1 plasma
viral loads. This analysis showed that patients with a low viral load
(VL < 1000 copies/mL) were the most infected except for EBV-1 and
HHV-6A which were more common in patients with high viral loads (VL
> 1000 copies/mL) (Table 1 and 2). However, these differences were not statistically significant.
Herpes virus infections and ARV treatment. The analysis of the results according to ARV treatment (Table 1 and 2),
showed that patients on ARV treatment were the most infected with
herpes viruses, EBV, EBV-1, EBV-2, and HHV-6A. Meanwhile, the
individuals, naïve to the treatment were more infected by HHV-6A and
HHV-6B (Note that these patients were all co-infected with
HHV-6A/HHV-6B). These results were not statistically significant.
Discussion
The
purpose of this study was to characterize EBV and HHV-6 subtypes and to
assess their infections impact on CD4 T cell count, HIV-1 viral load
and treatment in people living with HIV-1.
Our study focused on
238 PL-HIV and showed that the prevalence of EBV and HHV-6 was 6.7% and
7.1% respectively in our study population. These results are similar to
those reported by Tao et al. with a prevalence of 5.4% for EBV among
blood donors in Burkina Faso.[32] Our results are
also consistent with those reported by Traoré et al. (5.1% and 6.0%
respectively for EBV and HHV-6) in blood donors and Ouedraogo et al.
(6.0% and 6.1% respectively for EBV and HHV-6) in pregnant women in
Burkina Faso.[33,34] These findings suggest that the
prevalence of herpes viruses determined by qualitative real-time PCR
method are much lower compared to their seroprevalence. These results
confirm that most people infected with herpes virus in the course of
their lives are more likely to have a latent infection without viremia
than a cleared infection.
This study has allowed us to
characterize for the first time EBV and HHV-6 subtypes circulating in
Burkina Faso. We found that EBV-1 (3.9%) and EBV-2 (4.6%) subtypes
predominate with approximately the same proportions. Our results
corroborate data from the literature showing that EBV-1 subtype is more
prevalent in Europe, North America, and Asia; while a predominance of
both subtypes is observed in Africa and New Guinea.[38-43] EBV-2 is also found in HIV-infected Europeans and Australians,[44-46] and approximately 20% of the healthy population in North America is co-infected with both subtypes.[47]
The
subtyping of HHV-6 shows that HHV-6A and HHV-6B subtypes are both
present in our study population with similar proportions of 6.3% and
5.0% respectively for HHV-6A and HHV-6B. The literature suggests that
HHV-6B is found throughout the world while HHV-6A is less common in
Asia, North America and Europe.[16] Primary HHV-6 infection is due to 86-100% of cases in subtype A (HHV-6A) in African children.[19]
Our results are similar to those reported in the literature. Thus, in
their study, Baillargeon et al. found a lower rates of HHV-6 shedding
in the genital tract of pregnant, 7/297 [2.0%]; non-pregnant, 8/214
[3.7%] women; of 14 samples subtyped, four (29%) were subtype A.[48] whereas our study shows a slight prevalence of HHV-6A compared to HHV-6B even if these differences are not significant.
The
analysis of our data according to the different age groups did not give
any significant results for neither EBV, HHV-6 nor for the
corresponding sub-types. However, the highest prevalence of overall
infection with these two viruses was observed in the group of
individuals who were less than five years old.
According to sex,
men were more infected with both viruses as well as the corresponding
subtypes. This result is especially significant for EBV infection.
Analysis
of our data by CD4 T cell count and HIV-1 viral load shows that herpes
infection is more common in patients with high CD4 T cell counts and
those with a low viral load compared to patients with low CD4 T cell
count and a high HIV viral load. This result is particularly
significant for EBV. We also found that the prevalence of herpes
viruses were high in HAART patients compared with those who were not
under treatment. Our results corroborate those of Piriou et al. who
have shown that although HAART treatment improves CD4 T cell
restoration while contributing to lower HIV viral load, it did not
affect EBV infection.[49] Thus, it is possible that
herpes infection is more likely to be found in HAART patients with high
CD4 T cell counts. This hypothesis is also supported by several studies
which have shown that herpes/HIV co-infection further enhances CD4 T
cell proliferation and thus broadens the types of target cells
susceptible to HIV infection,[24,49-52] resulting in a high HIV viral load. Our results corroborate those of Erwan Piourou et al.,[49]
Who also concluded that even in the long term HAART treatment does not
reduce the herpes viral load, but above all, it makes it possible to
fight against reactivation cases.
Conclusions
This
study made it possible to characterize the subtypes of EBV and HHV-6.
It showed the subtypes EBV-1, EBV-2, HHV-6A, and HHV-6B all circulating
in Burkina Faso with almost identical proportions. We also support the
hypothesis that HIV HAART treatment would not act on herpes virus
infection but could prevent reactivation of these viruses.
Acknowledgements
The
authors would like to thank Saint Camille Hospital of Ouagadougou
(HOSCO) and Biomolecular Research Center Pietro Annigoni (CERBA). They
express their gratitude to the Italian Episcopal Conference (CEI) and
the West African Economic and Monetary Union (WAEMU) through the PACER2
program.
References
- Damania B. Oncogenic gamma-herpesviruses:
comparison of viral proteins involved in tumorigenesis. Nat Rev
Microbiol. 2004; 28:656-668. https://doi.org/10.1038/nrmicro958 PMid:15263900

- Balfour
HH, Jr., Odumade OA, Schmeling DO, Mullan BD, Ed JA, Knight JA, Vezina
HE, Thomas W, Hogquist KA. Behavioral, virologic, and immunologic
factors associated with acquisition and severity of primary
Epstein-Barr virus infection in university students. J Infect Dis.
2013; 2071:80-88. https://doi.org/10.1093/infdis/jis646 PMid:23100562 PMCid:PMC3523797

- Bagan
L, Ocete-Monchon MD, Leopoldo-Rodado M, Murillo-Cortes J,
Diaz-Fernandez JM, Medina-Gonzalez R, Gimeno-Cardona C, Bagan JV.
Prevalence of salivary Epstein-Barr virus in potentially malignant oral
disorders and oral squamous cell carcinoma. Med Oral Patol Oral Cir
Bucal. 2016; 212:e157-160. https://doi.org/10.4317/medoral.20785 PMCid:PMC4788793

- Macsween KF, Crawford DH. Epstein-Barr virus-recent advances. Lancet Infect Dis. 2003; 33:131-140. https://doi.org/10.1016/S1473-3099(03)00543-7

- Godshall
SE, Kirchner JT. Infectious mononucleosis. Complexities of a common
syndrome. Postgrad Med. 2000; 1077:175-179, 183-174, 186.

- Wang
X, Yang K, Wei C, Huang Y, Zhao D. Coinfection with EBV/CMV and other
respiratory agents in children with suspected infectious mononucleosis.
Virol J. 2010; 7:247. https://doi.org/10.1186/1743-422X-7-247 PMid:20858235 PMCid:PMC2949848

- Burkitt D. A sarcoma involving the jaws in African children. Br J Surg. 1958; 46197:218-223. https://doi.org/10.1002/bjs.18004619704

- Flavell KJ, Murray PG. Hodgkin's disease and the Epstein-Barr virus. Mol Pathol. 2000; 535:262-269. https://doi.org/10.1136/mp.53.5.262

- Adjei
AA, Armah HB, Gbagbo F, Boamah I, Adu-Gyamfi C, Asare I. Seroprevalence
of HHV-8, CMV, and EBV among the general population in Ghana, West
Africa. BMC Infect Dis. 2008; 8:111. https://doi.org/10.1186/1471-2334-8-111 PMid:18706107 PMCid:PMC2528010

- Iizasa
H, Nanbo A, Nishikawa J, Jinushi M, Yoshiyama H. Epstein-Barr Virus
(EBV)-associated gastric carcinoma. Viruses. 2012; 412:3420-3439. https://doi.org/10.3390/v4123420 PMCid:PMC3528272

- Webster-Cyriaque
J, Middeldorp J, Raab-Traub N. Hairy leukoplakia: an unusual
combination of transforming and permissive Epstein-Barr virus
infections. J Virol. 2000; 7416:7610-7618. https://doi.org/10.1128/JVI.74.16.7610-7618.2000

- Munz
C, Lunemann JD, Getts MT, Miller SD. Antiviral immune responses:
triggers of or triggered by autoimmunity? Nat Rev Immunol. 2009;
94:246-258. https://doi.org/10.1038/nri2527 PMid:19319143 PMCid:PMC2854652

- Ferlay
J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW,
Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in
Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;
496:1374-1403. https://doi.org/10.1016/j.ejca.2012.12.027 PMid:23485231

- van
Baarle D, Hovenkamp E, Callan MF, Wolthers KC, Kostense S, Tan LC,
Niesters HG, Osterhaus AD, McMichael AJ, van Oers MH, Miedema F.
Dysfunctional Epstein-Barr virus (EBV)-specific CD8(+) T lymphocytes
and increased EBV load in HIV-1 infected individuals progressing to
AIDS-related non-Hodgkin lymphoma. Blood. 2001; 981:146-155. https://doi.org/10.1182/blood.V98.1.146

- Mazeron
M-C, Amiel C, Agut H. Mesure et interprétation des charges virales dans
les infections à herpèsvirus humains (cytomégalovirus, virus
Epstein-Barr, herpèsvirus humains 6 et 8). Revue Francophone des
Laboratoires. 2016; 2016487:47-54. https://doi.org/10.1016/S1773-035X(16)30371-9

- Ablashi
D, Agut H, Alvarez-Lafuente R, Clark DA, Dewhurst S, DiLuca D, Flamand
L, Frenkel N, Gallo R, Gompels UA, Hollsberg P, Jacobson S, Luppi M,
Lusso P, Malnati M, Medveczky P, Mori Y, Pellett PE, Pritchett JC,
Yamanishi K, Yoshikawa T. Classification of HHV-6A and HHV-6B as
distinct viruses. Arch Virol. 2014; 1595:863-870. https://doi.org/10.1007/s00705-013-1902-5 PMid:24193951 PMCid:PMC4750402

- De
Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology,
clinical features, and therapy. Clin Microbiol Rev. 2005; 181:217-245. https://doi.org/10.1128/CMR.18.1.217-245.2005 PMid:15653828 PMCid:PMC544175

- Thader-Voigt
A, Jacobs E, Lehmann W, Bandt D. Development of a microwell adapted
immunoblot system with recombinant antigens for distinguishing human
herpesvirus (HHV)6A and HHV6B and detection of human cytomegalovirus.
Clin Chem Lab Med. 2011; 4911:1891-1898. https://doi.org/10.1515/cclm.2011.666

- Bates
M, Monze M, Bima H, Kapambwe M, Clark D, Kasolo FC, Gompels UA.
Predominant human herpesvirus 6 variant A infant infections in an HIV-1
endemic region of Sub-Saharan Africa. J Med Virol. 2009; 815:779-789. https://doi.org/10.1002/jmv.21455 PMid:19319952

- Caserta MT, Mock DJ, Dewhurst S. Human herpesvirus 6. Clin Infect Dis. 2001; 336:829-833. https://doi.org/10.1086/322691 PMid:11512088

- Salahuddin
SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, Kaplan M,
Halligan G, Biberfeld P, Wong-Staal F, Kramarsky B, et al. Isolation of
a new virus, HBLV, in patients with lymphoproliferative disorders.
Science. 1986; 2344776:596-601. https://doi.org/10.1126/science.2876520

- Torelli
G, Marasca R, Luppi M, Selleri L, Ferrari S, Narni F, Mariano MT,
Federico M, Ceccherini-Nelli L, Bendinelli M, et al. Human
herpesvirus-6 in human lymphomas: identification of specific sequences
in Hodgkin's lymphomas by polymerase chain reaction. Blood. 1991;
7710:2251-2258
 .
.
- Descamps
V, Valance A, Edlinger C, Fillet AM, Grossin M, Lebrun-Vignes B,
Belaich S, Crickx B. Association of human herpesvirus 6 infection with
drug reaction with eosinophilia and systemic symptoms. Arch Dermatol.
2001; 1373:301-304.

- Lusso
P, Ensoli B, Markham PD, Ablashi DV, Salahuddin SZ, Tschachler E,
Wong-Staal F, Gallo RC. Productive dual infection of human CD4+ T
lymphocytes by HIV-1 and HHV-6. Nature. 1989; 3376205:370-373. https://doi.org/10.1038/337370a0 PMid:2463490

- Kositanont
U, Wasi C, Wanprapar N, Bowonkiratikachorn P, Chokephaibulkit K,
Chearskul S, Chimabutra K, Sutthent R, Foongladda S, Inagi R, Kurata T,
Yamanishi K. Primary infection of human herpesvirus 6 in children with
vertical infection of human immunodeficiency virus type 1. J Infect
Dis. 1999; 1801:50-55. https://doi.org/10.1086/314826 PMid:10353860

- Emery
VC, Atkins MC, Bowen EF, Clark DA, Johnson MA, Kidd IM, McLaughlin JE,
Phillips AN, Strappe PM, Griffiths PD. Interactions between
beta-herpesviruses and human immunodeficiency virus in vivo: evidence
for increased human immunodeficiency viral load in the presence of
human herpesvirus 6. J Med Virol. 1999; 573:278-282. https://doi.org/10.1002/(SICI)1096-9071(199903)57:3<278::AID-JMV11>3.0.CO;2-3

- Fairfax
MR, Schacker T, Cone RW, Collier AC, Corey L. Human herpesvirus 6 DNA
in blood cells of human immunodeficiency virus-infected men:
correlation of high levels with high CD4 cell counts. J Infect Dis.
1994; 1696:1342-1345. https://doi.org/10.1093/infdis/169.6.1342

- Knox
KK, Harrington DP, Carrigan DR. Fulminant human herpesvirus six
encephalitis in a human immunodeficiency virus-infected infant. J Med
Virol. 1995; 453:288-292. https://doi.org/10.1002/jmv.1890450309

- Nigro
G, Luzi G, Krzysztofiak A, D'Orio F, Aiuti F. Detection of IgM
antibodies to human herpesvirus 6 in Romanian children with
nonprogressive human immunodeficiency virus disease. Pediatr Infect Dis
J. 1995; 1410:891-894. https://doi.org/10.1097/00006454-199510000-00014

- Qavi
HB, Green MT, Lewis DE, Hollinger FB, Pearson G, Ablashi DV. HIV-1 and
HHV-6 antigens and transcripts in retinas of patients with AIDS in the
absence of human cytomegalovirus. Invest Ophthalmol Vis Sci. 1995;
3610:2040-2047.

- Reux
I, Fillet AM, Agut H, Katlama C, Hauw JJ, LeHoang P. In situ detection
of human herpesvirus 6 in retinitis associated with acquired
immunodeficiency syndrome. Am J Ophthalmol. 1992; 1143:375-377. https://doi.org/10.1016/S0002-9394(14)71814-8

- Tao
I, Bisseye C, Nagalo BM, Sanou M, Kiba A, Surat G, Compaore TR, Traore
L, Nikiema JB, Pietra V, Zongo JD, Simpore J. Screening of Hepatitis G
and Epstein-Barr Viruses Among Voluntary non Remunerated Blood Donors
(VNRBD) in Burkina Faso, West Africa. Mediterr J Hematol Infect Dis.
2013; 51:e2013053. https://doi.org/10.4084/mjhid.2013.053 PMid:24106603 PMCid:PMC3787664

- Traore
L, Tao I, Bisseye C, Diarra B, Compaore TR, Nebie Y, Assih M, Ouedraogo
A, Zohoncon T, Djigma F, Ouermi D, Barro N, Sanou M, Ouedraogo RT,
Simpore J. Molecular diagnostic of cytomegalovirus, Epstein Barr virus
and Herpes virus 6 infections among blood donors by multiplex real-time
PCR in Ouagadougou, Burkina Faso. Pan Afr Med J. 2016; 24:298. https://doi.org/10.11604/pamj.2016.24.298.6578 PMid:28154653 PMCid:PMC5267872

- Ouedraogo
AR, Kabre M, Bisseye C, Zohoncon TM, Asshi M, Soubeiga ST, Diarra B,
Traore L, Djigma FW, Ouermi D, Pietra V, Barro N, Simpore J. [Molecular
tests in diagnosis of Cytomegalovirus (CMV), human herpesvirus 6
(HHV-6) and Epstein-Barr virus (EBV) using real-time PCR in HIV
positive and HIV-negative pregnant women in Ouagadougou, Burkina Faso].
Pan Afr Med J. 2016; 24:223. https://doi.org/10.11604/pamj.2016.24.223.9406 PMid:27800078 PMCid:PMC5075482

- Miller
SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting
DNA from human nucleated cells. Nucleic Acids Res. 1988; 163:1215. https://doi.org/10.1093/nar/16.3.1215

- Kwok
H, Chan KW, Chan KH, Chiang AK. Distribution, persistence and
interchange of Epstein-Barr virus strains among PBMC, plasma and saliva
of primary infection subjects. PLoS One. 2015; 103:e0120710. https://doi.org/10.1371/journal.pone.0120710 PMid:25807555 PMCid:PMC4373854

- Yavarian
J, Shatizadeh Malekshahi S, Yavarian R, Yazdani S, Janani L, Shafiei
Jandaghi NZ, Kiani SJ, Ahamadkhaniha H. Type specific Real time PCR for
detection of human herpes virus 6 in schizophrenia and bipolar
patients: a case control study. BMC Psychiatry. 2015; 15:296. https://doi.org/10.1186/s12888-015-0662-z PMid:26584549 PMCid:PMC4653940

- Abdel-Hamid
M, Chen JJ, Constantine N, Massoud M, Raab-Traub N. EBV strain
variation: geographical distribution and relation to disease state.
Virology. 1992; 1901:168-175. https://doi.org/10.1016/0042-6822(92)91202-6

- Chang
CM, Yu KJ, Mbulaiteye SM, Hildesheim A, Bhatia K. The extent of genetic
diversity of Epstein-Barr virus and its geographic and disease
patterns: a need for reappraisal. Virus Res. 2009; 1432:209-221. https://doi.org/10.1016/j.virusres.2009.07.005 PMid:19596032 PMCid:PMC2731007

- Correa
RM, Fellner MD, Alonio LV, Durand K, Teyssie AR, Picconi MA.
Epstein-barr virus (EBV) in healthy carriers: Distribution of genotypes
and 30 bp deletion in latent membrane protein-1 (LMP-1) oncogene. J Med
Virol. 2004; 734:583-588. https://doi.org/10.1002/jmv.20129 PMid:15221903

- Falk
KI, Zou JZ, Lucht E, Linde A, Ernberg I. Direct identification by PCR
of EBV types and variants in clinical samples. J Med Virol. 1997;
514:355-363. https://doi.org/10.1002/(SICI)1096-9071(199704)51:4<355::AID-JMV15>3.0.CO;2-H

- Kunimoto
M, Tamura S, Tabata T, Yoshie O. One-step typing of Epstein-Barr virus
by polymerase chain reaction: predominance of type 1 virus in Japan. J
Gen Virol. 1992; 73 (Pt 2):455-461. https://doi.org/10.1099/0022-1317-73-2-455 PMid:1311368

- Sixbey
JW, Shirley P, Chesney PJ, Buntin DM, Resnick L. Detection of a second
widespread strain of Epstein-Barr virus. Lancet. 1989; 28666:761-765. https://doi.org/10.1016/S0140-6736(89)90829-5

- Sculley
TB, Apolloni A, Hurren L, Moss DJ, Cooper DA. Coinfection with A- and
B-type Epstein-Barr virus in human immunodeficiency virus-positive
subjects. J Infect Dis. 1990; 1623:643-648. https://doi.org/10.1093/infdis/162.3.642

- van
Baarle D, Hovenkamp E, Kersten MJ, Klein MR, Miedema F, van Oers MH.
Direct Epstein-Barr virus (EBV) typing on peripheral blood mononuclear
cells: no association between EBV type 2 infection or superinfection
and the development of acquired immunodeficiency syndrome-related
non-Hodgkin's lymphoma. Blood. 1999; 9311:3949-3955.

- Yao
QY, Tierney RJ, Croom-Carter D, Dukers D, Cooper GM, Ellis CJ, Rowe M,
Rickinson AB. Frequency of multiple Epstein-Barr virus infections in
T-cell-immunocompromised individuals. J Virol. 1996; 708:4884-4894.

- Walling
DM, Brown AL, Etienne W, Keitel WA, Ling PD. Multiple Epstein-Barr
virus infections in healthy individuals. J Virol. 2003; 7711:6546-6550.
https://doi.org/10.1128/JVI.77.11.6546-6550.2003 PMCid:PMC155020

- Baillargeon
J, Piper J, Leach CT. Epidemiology of human herpesvirus 6 (HHV-6)
infection in pregnant and nonpregnant women. J Clin Virol. 2000;
163:149-157. https://doi.org/10.1016/S1386-6532(99)00086-4

- Piriou
E, Jansen CA, van Dort K, De Cuyper I, Nanlohy NM, Lange JM, van Oers
MH, Miedema F, van Baarle D. Reconstitution of EBV latent but not lytic
antigen-specific CD4+ and CD8+ T cells after HIV treatment with highly
active antiretroviral therapy. J Immunol. 2005; 1753:2010-2017. https://doi.org/10.4049/jimmunol.175.3.2010

- Ensoli
B, Lusso P, Schachter F, Josephs SF, Rappaport J, Negro F, Gallo RC,
Wong-Staal F. Human herpes virus-6 increases HIV-1 expression in
co-infected T cells via nuclear factors binding to the HIV-1 enhancer.
EMBO J. 1989; 810:3019-3027.

- Lusso
P, De Maria A, Malnati M, Lori F, DeRocco SE, Baseler M, Gallo RC.
Induction of CD4 and susceptibility to HIV-1 infection in human CD8+ T
lymphocytes by human herpesvirus 6. Nature. 1991; 3496309:533-535. https://doi.org/10.1038/349533a0 PMid:1846951

- Lusso
P, Malnati MS, Garzino-Demo A, Crowley RW, Long EO, Gallo RC. Infection
of natural killer cells by human herpesvirus 6. Nature. 1993;
3626419:458-462. https://doi.org/10.1038/362458a0 PMid:7681936

[TOP]

























 .
.




























