Andrea Nozza
Department of Medical
Oncology and Hematology, Humanitas Cancer Center, Humanitas Clinical
and Research Hospital IRCCS, Rozzano, Milan, Italy
Published: September 1, 2017
Received: May 26, 2017
Accepted: August 5, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017051 DOI
10.4084/MJHID.2017.051
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
POEMS
syndrome is a rare, chronic and disabling condition. The causes of this
condition remain unknown; however, chronic overproduction of
proinflammatory cytokines appears to be a major contributor. Early
diagnosis is essential to start treatment before the clinical state of
the patient becomes compromised.
A complete evaluation of the
disease at its onset is critical to the treatment decision. In
localized disease, curative doses of radiation (50 Gy) are the
recommended therapy. On the other hand, patients with disseminated
disease should be given systemic therapy. Treatment-related morbidity
can be minimized by an efficient induction therapy that modifies the
cytokine status, improving clinical condition and control disease
severity before mobilization and transplantation. Patients not suitable
for hematopoietic stem cell transplantation (HSCT) are usually treated
with alkylator-based therapy. Novel agents may also offer benefits to
patients with a poor performance status or renal dysfunction, and
induce transplantation eligibility. Given the biological
characteristics of POEMS, immunomodulatory effects and the absence of
neurotoxicity, lenalidomide appears to be an effective therapy for the
treatment of POEMS, both as short induction therapy before PBSCT and in
non-transplant eligible patients, as it showed high response rate and
durable responses.
At present, however, guidelines for the diagnosis and treatment of POEMS are not available and appear advocated.
|
Introduction
POEMS
syndrome is a multisystemic disease secondary to a plasma cell
dyscrasia. POEMS is an acronym for a range of distinct features
[peripheral neuropathy (P), organomegaly (O), endocrinopathy (E)
monoclonal plasma-cells proliferative disorder (M) and skin changes
(S)], even if the diagnosis does not require that all these symptoms
are present. Furthermore, many others clinical signs are not included
in the definition of POEMS, such as sclerotic bone lesions,
papilledema, edema ascites and effusions, pulmonary hypertension,
Castleman' disease (CD), thrombocytosis and erythrocytosis, and
increased of serum vascular endothelial growth factor (VEGF).[1,2]
POEMS SYNDROME has also been called osteosclerotic myeloma, Crow-Fukase
syndrome, PEP syndrome (plasma cell dyscrasia, endocrinopathy,
polyneuropathy), or Takatsuki syndrome. POEMS syndrome is a rare
disease, but it is often under-recognized.
The primary clinical
feature of this syndrome is a progressive polyneuropathy with a
predominant motor disability. The disease is potentially fatal, and
patient’s quality of life deteriorates due to a progressive neuropathy,
massive peripheral edema, pleural effusion, and ascites. Serious
complications such as multiorgan failure due to capillary leak
syndrome, restrictive lung disease, and pulmonary hypertension result
in an adverse prognosis.
Despite its seriousness, at present no
guidelines or standardized criteria for the diagnosis and treatment of
POEMS syndrome are available. This narrative review provides an update
of the current evidence on this condition. Literature research was last
updated in May 2017.
Pathogenesis
Although
significant progress has achieved in the diagnosis, management, and
treatment of POEMS syndrome, its physiopathology remains unknown.
Up-regulation of various pro-inflammatory cytokines and growth factors
(tumor necrosis factor-alpha (TNF-alpha), interleukin-1 (IL1),
interleukin-6 (IL6) and above all vascular endothelial growth factor
(VEGF) play a crucial role in the pathogenesis of the POEMS syndrome,
contributing to vascular leak and polyneuropathy.[3-5]
In particular, VEGF is markedly elevated in POEMS patients and
correlates with the activity of the disease. Unfortunately, VEGF
inhibition with specific inhibitors (e.g., bevacizumab) did not result
in an effective treatment thus suggesting that VEGF may be only one
component of a much more complex cytokine network.[6,7]
It has also been proposed that clonal B/plasma cells with genetic
mutations of the V-region of the Ig lambda gene could produce excess
cytokines (primarily, VEGF) through an yet undiscovered mechanism,
leading to the clinical manifestations of POEMS.[8]
Diagnostic Criteria
POEMS
syndrome is a rare disease, with diverse clinical manifestations, which
lead the patient on a diagnostic “Odyssey” across multiple specialists,
without a correct and definitive diagnosis. Moreover, the clinical
features of POEMS widely differ from patient to patient, and all
symptoms are not always present. Therefore, patients may experience a
delay in the initiation of appropriate treatment.
Early
diagnosis is critical to reduce the morbidity rate and increase
survival. However, the median time from onset of diagnosis is 13-18
months.[9]
Diagnostic criteria for POEMS were first proposed in 2003[10] and were revised in 2007 after the diagnostic relevance of VEGF level was confirmed.[11]
In more details, clinical features have been divided into mandatory
criteria, major criteria, and minor criteria, in line with the
indications of The International Myeloma Working Group (Table 1).[12]
The presence of both "mandatory criteria", at least one major criteria
and at least one minor criteria are needed for the diagnosis of POEMS
syndrome.
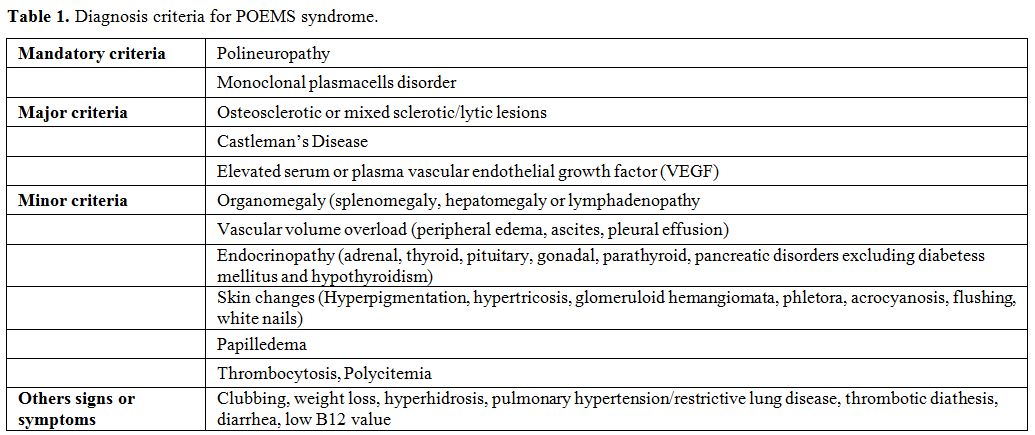 |
Table 1. Diagnosis criteria for POEMS syndrome |
Mandatory Criteria
Polyneuropathy.
Patients in the early stage of POEMS are frequently misdiagnosed with
CIDP (chronic inflammatory demyelinating polyneuropathy), as both
conditions involve the peripheral nerves and may present with
albumin-cytologic dissociation in the cerebrospinal fluid.[13]
Moreover, nerve conduction studies and electrophysiological examination
can be used to distinguish POEMS from other polyneuropathies with more
prominent sign of axonal degeneration and more neurogenic injury in
lower limbs muscles.[14,15]
Symptoms of
peripheral neuropathy are usually particularly evident, and consist in
tingling, paresthesia, and coldness; motor involvement follows sensory
symptoms. Cranial nerves are not involved except for papilledema.
Severe weakness is frequently reported, and patients experience an
inability to climb stairs, rise from a chair or to hold a firm grip.
Over time, muscle weakness becomes more marked than the sensory loss.
Peripheral
neuropathy is due to endothelial injury, caused directly or indirectly
by abnormal activation of endothelial cells by VEGF expressed in the
nerves.[5]
Monoclonal plasma cells proliferative disorder.
All patients have a monoclonal protein (M-protein, lambda-type chain),
which can be detected either in serum and/or urine with immunofixation
tests.[8] The concentration of this protein is modest (median 1,0 mg/dl). Bence Jones proteinuria is uncommon.[16]
Serum protein electrophoresis is normal in 25% of patients, while in
the remaining patients it presents a polyclonal gammopathy patterns: in
these cases, M-proteins could be overlooked if immunofixation is not
performed. In addition, although the immunoglobulin free light chains
are elevated in 90% of POEMS patients, the ratio is abnormal in only
18% of cases,[17] thus making this test of limited value.
Information on plasma cells in POEMS is scant. In more than 95% of cases, they are lambda-light restricted.[18] The V-region of the Ig lambda gene interested was limited to the V lambda 1 subfamily (IGLV1).[8,19]
Kang et al. reported in 20 newly diagnosed POEMS cytogenetic
aberrations similar to other plasma cell dyscrasias, but with a
different incidence.[20] In particular, 14q32 (IGH)
translocation was observed in 45% of the cases and included the t(4;14)
and t(11;14) translocation (15% and 25% of the cases, respectively). In
addition, 25% of the patients presented deletions of 13q14 and 20% had
an amplification of 1q21. No significant correlation between clinical
features and cytogenetic abnormalities was observed, although patients
with IGH translocations were more likely to exhibit papilledema.
Major Criteria
Bone lesions. Osteosclerotic lesions are reported approximately in 95% of patients[11] even if several reports from China showed a lower rate of bone lesions (27-41%) which may suggest ethnic differences.[9,21]
Bone
lesions could be sclerotic, lytic with sclerotic rims or mixed
sclerotic/lytic lesions with soup-bubble appearance. Lytic lesions
without sclerotic rims are uncommon. In about half of patients, a
single bone lesion is found, while in the others lesions are multiple.
The pelvis, spine, ribs and proximal extremity are the most common
sites of bone lesions. Hypercalcemia is not usually reported at
diagnosis; bone pain and fractures are sporadic.
Imaging
approaches used for evaluation of bone involvement in POEMS are simple
skeletal radiograph and computed tomography of bone. (Figure 1) Bone uptake in bone scintigraphy has been described, although false negatives are possible.[22,23] Lesions have variable FDG uptake, but positron emission tomography (PET) scan usually does not identify all lesions.[24] PET scan can, however, be useful in monitoring response to therapy in patients with high baseline FDG uptake.[25,26]
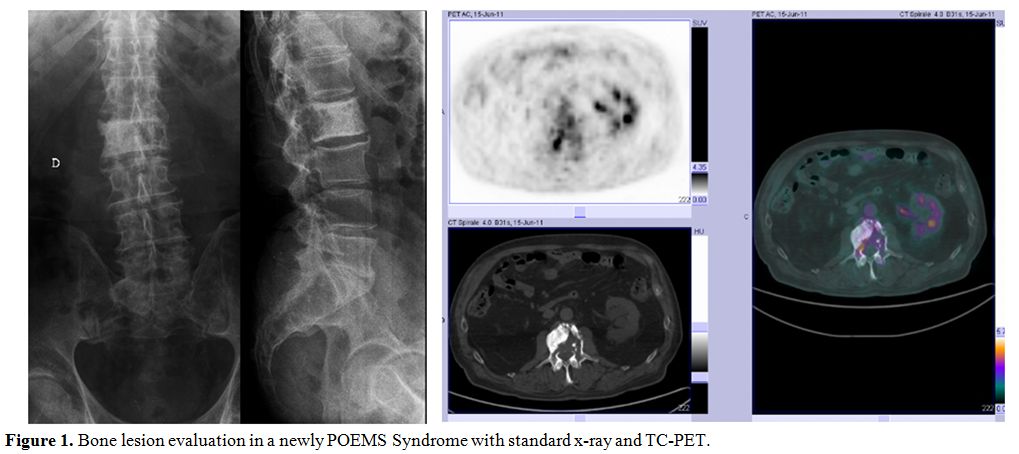 |
Figure 1. Bone lesion evaluation in a newly POEMS Syndrome with standard x-ray and TC-PET. |
Castleman’s disease.
CD is a rare lymphoproliferative disorder with many different
presentations, ranging from asymptomatic single lymph node to
multifocal disease with a plethora of symptoms. CD and POEMS are
frequently associated, and approximately 15-24% of patients with POEMS
syndrome also have CD, the majority of them had hyaline vascular type.[10,16,27]
However, this proportion may be an underestimation since many patients
do not undergo lymph node biopsy. Multicentric CD with and without
peripheral neuropathy tend to be different; those patients with
peripheral neuropathy are more likely to have edema and impaired
peripheral circulation, and they are also more prone to have a
monoclonal lambda protein in their serum and/or urine. In these
patients, neuropathy is more often sensory and subtle; these patients
show high levels of VEGF and IL6, and a higher frequency of
thrombocytosis.[28]
VEGF serum levels.
VEGF is expressed by osteoblasts, bone tissue, macrophages, tumor
cells, plasma cells, and megakaryocytes; both IL1 and IL6 have been
shown to stimulate VEGF production.
VEGF normally targets
endothelial cells and induces a rapid and reversible increase in
vascular permeability. Increased VEGF could account for some clinical
characteristics of POEMS, such as organomegaly, edema, skin changes and
neuropathy, increasing microvascular permeability of the blood vessels
with endoneurial edema.[29,30] Serum VEGF levels tend
to be 5-10 fold higher in POEMS syndrome compared with healthy controls
or patients with other neuropathic disorders.[31]
Many
studies confirm that VEGF levels could be used as a biomarker to
monitor disease activity and differentiate POEMS syndrome from
amyloidosis, monoclonal gammopathy of undetermined significance (MGUS),
multiple myeloma (MM) and CIDP.[29,31,32]
It is still unclear the best approach for VEGF evaluation, whether in
serum or plasma. Serum VEGF levels are affected by the release of
platelet-derived VEGF because of ex vivo platelet activation during the
clotting process or because of the presence of thrombocytosis in some
patients.[33] Furthermore, the normal and pathologic
reference ranges for VEGF (in serum or plasma) are not well defined to
date, even if a VEGF value more than 1000 pg/ml is considered
pathological.
Minor Criteria
Organomegaly. Hepatomegaly, splenomegaly, and lymphadenopathy at the onset of POEMS syndrome have been reported in 50-78% of patients.[10,34]
When present, organomegaly is mild, and bulky disease is unusual.
Lymphadenopathy could be related to concomitant CD, although lymph node
biopsy is performed in a minority of patients.[16]
Extravascular volume overload.
Extravascular volume overload (peripheral edema, ascites, pleural
effusion, pericardial effusion) is reported in 80% of POEMS patients.[35]
Peripheral edema and ascites are more common than pleural or
pericardial effusion. Cytological and biochemical analysis of ascites
document the characteristics of exudate. Vascular injury change of the
peritoneal surface and/or permeability of the capillaries in visceral
peritoneum are considered a mechanism of extravascular volume overload.[36] Extravascular volume overload can cause important morbidity in POEMS, and it is associated with shorter survival.[11]
Endocrinopathy.
Endocrinopathy is a crucial but poorly understood feature of POEMS
syndrome. The majority of patients have evidence of multiple
endocrinopathies in the four principal axes (gonadal, thyroid, glucose
and adrenal), such as hypogonadism, diabetes mellitus, hypothyroidism,
hyperprolactinemia, adrenal insufficiency, gynecomastia and
hypoparathyroidism.[1] The etiology of endocrinopathy is unknown, even if VEGF could be a major contributor.
Most
information regarding these abnormalities and POEMS comes from case
reports or small series. In a retrospective evaluation on 170 POEMS
cases, 84% of patients documented at least one endocrine abnormality at
presentation or during the disease.[37]
Hypogonadism
is the most common endocrine abnormality: this condition, with lower
levels of testosterone and erectile dysfunction, is reported in over
70% of males.[38] Moreover, gynecomastia in men and irregular menses in woman have been described.[34]
Given the high prevalence of diabetes mellitus and hypothyroidism in
the general population, these endocrine abnormalities are not
considered among the criteria for the diagnosis of POEMS syndrome.
Skin changes. Skin changes were described in more than 75% of POEMS patients at diagnosis.[10,34]
Major dermatological findings consist in hyperpigmentation and
hemangiomas, present as multiple red-purple lesions especially on the
trunk and proximal limbs. Others skin changes are hypertrichosis
(especially of extremities or face, present in one-fourth of cases),
acrocyanosis, white nails, hyperemia, erythema, flushing, rubor and
clubbing (Figure 2).[38] A high prevalence of acquired facial lipoatrophy preceding POEMS diagnosis was also described.[39]
Of note, a rapid improvement in hemangiomas, hyperpigmentation,
hypertrichosis, and vascular skin changes is often associated with
treatments for POEMS.
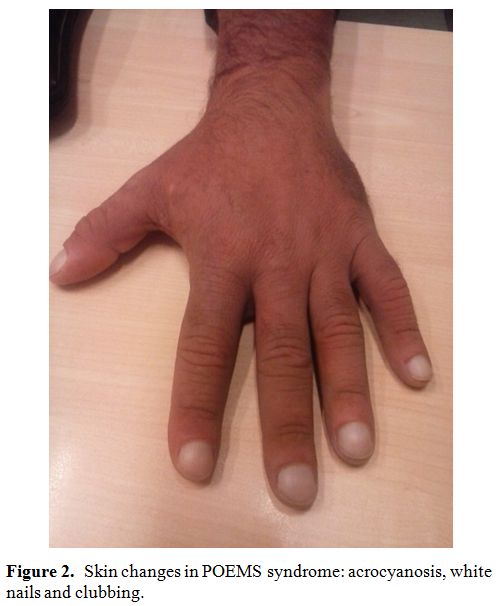 |
Figure 2. Skin changes in POEMS syndrome: acrocyanosis, white nails and clubbing.. |
Papilledema.
Papilledema, usually bilateral, is an early sign of POEMS syndrome.
This finding is reported in 29-64% of patients and correlates with poor
prognosis.[40] An association between papilledema and plasma VEGF levels was also described.[41]
In a recent study, serum VEGF concentrations were significantly
different in patients with papilledema, and those without; the serum
levels of this cytokine decreased, and papilledema alleviated after
treatment.[42] Cerebrospinal fluid protein levels in
POEMS are increased in all patients, with level >100 mg/dL in more
than half of patients showing average total cell count.[43]
Hematological alterations. In POEMS patients, thrombocytosis is common (50%), and polyglobulia may be observed (15%).[11]
Patients with thrombocytosis and erythrocytosis are often diagnosed as
a chronic myeloproliferative disease before considering the diagnosis
of POEMS syndrome. In these patients, JAK2 evaluation is always
negative. Anemia is rare unless the patient present concomitant CD.
Others Signs and Symptoms
Pulmonary manifestation.
These findings include pulmonary hypertension, restrictive lung
disease, reduced muscular function, impaired diffusion lung CO (DLCO).
Pulmonary hypertension is reported in 27-48% of patients.[44,45]
Pulmonary hypertension is reversible after successful treatment of
POEMS syndrome; survival of these patients is worse than in those
without pulmonary hypertension.[44]Patients
with pulmonary hypertension are more likely to present extravascular
volume overload. Whether the digital clubbing seen in POEMS is a
reflection of underlying pulmonary hypertension and/or parenchymal
disease is not clear yet.Bone marrow Histopathology.
A recently published study identified several and distinctive
histopathologic features in bone marrow of POEMS Syndrome patients.[20]
Monoclonal plasma cells (majority λ light chain restricted), are
usually less than 10%. Lymphoid aggregates were found in more than 40%
of patients, rimmed by polyclonal plasma cells. Megakaryocyte
hyperplasia is present in one-half of bone marrow of POEMS, with
megakaryocytic clustering and cytologic atypia, thus mimicking a
myeloproliferative neoplasm. However, JAK2 mutation is always negative.
This histopathological evidence is highly suggestive of POEMS.
Differential Diagnosis
The
median time from onset of symptoms to diagnosis of POEMS ranges from 13
to 18 months; indeed, many patients are initially misdiagnosed as
having other disorders, such as CIDP. Also, some conditions are
associated with a plasma cell disorder and polyneuropathy, with or
without o bone lesions. Many studies confirm that VEGF levels could be
useful for differentiating POEMS syndrome from amyloidosis, MGUS, MM
and CIDP: In these diseases, VEGF levels are usually low.[29,31,32] Features contributing to the differential diagnosis are reported below.MGUS.
MGUS is characterized by an M-protein in serum, without other systemic
findings. It should be noted that polyneuropathy may be seen in
patients with MGUS.[46]Multiple myeloma.
A polyneuropathy is a rare event in MM patients, and it is related to
concomitant amyloidosis. Bone lesions in MM are not sclerotic, but
normally osteolytic; moreover, the presence of anemia, hypercalcemia,
renal insufficiency, and a high proportion of bone marrow plasma cells,
which are frequently present in MM, are not characteristics of POEMS.Solitary plasmacytoma of bone.
Patients with solitary plasmacytoma of bone usually show only a single
osteolytic bone lesion, whereas in POEMS syndrome the bone lesions are
osteosclerotic. Systemic signs and symptoms, such as anemia,
hypercalcemia, and renal insufficiency are absent in solitary
plasmacytoma.Amyloidosis.
Amyloidosis is often associated with monoclonal gammopathy, skin
lesions, and polyneuropathy. Biopsy of involved tissues (fat aspirate,
bone marrow, kidney, heart, sural nerve) allows making a differential
diagnosis with POEMS, showing typical amyloid fibrils.Chronic inflammatory demyelinating polyneuropathy (CIDP).
Both CIDP and POEMS are characterized by a subacute motor-dominant
demyelinating polyradiculoneuropathy. Nerve conduction study and
electromyography can adequately distinguish POEMS syndrome from CIDP.[15,47]
Compared with CIDP, POEMS patients demonstrate greater axonal loss
(reduction of motor amplitudes and increased fibrillation potentials),
more considerable slowing of the intermediate nerve segments, less
frequently temporal dispersion and conduction block, and absent sural
sparing.
Treatment
The
clinical course of POEMS syndrome is usually chronic and disabling,
with a progressive worsening of the clinical condition and quality of
life. The median survival of these patients is about 14 years, during
which patients received some different therapies.[10,48]
However, due to its rarity and the difficulties encountered in its
diagnosis, only one randomized controlled study has been completed in
POEMS.[49] To date, evidence on POEMS treatment has been collected only in retrospective studies, often with small sample size. Treatment for newly-diagnosed POEMS syndrome depends on the extension of the disease (Figure 3)
In patients with isolated bone lesion without bone marrow clonal plasma
cells involvement, curative doses of radiation (40-50 Gy) is the
recommended therapy. In patients with a disseminated disease (more bone
lesions and/or bone marrow plasmacytosis), systemic therapy is
recommended.[50]
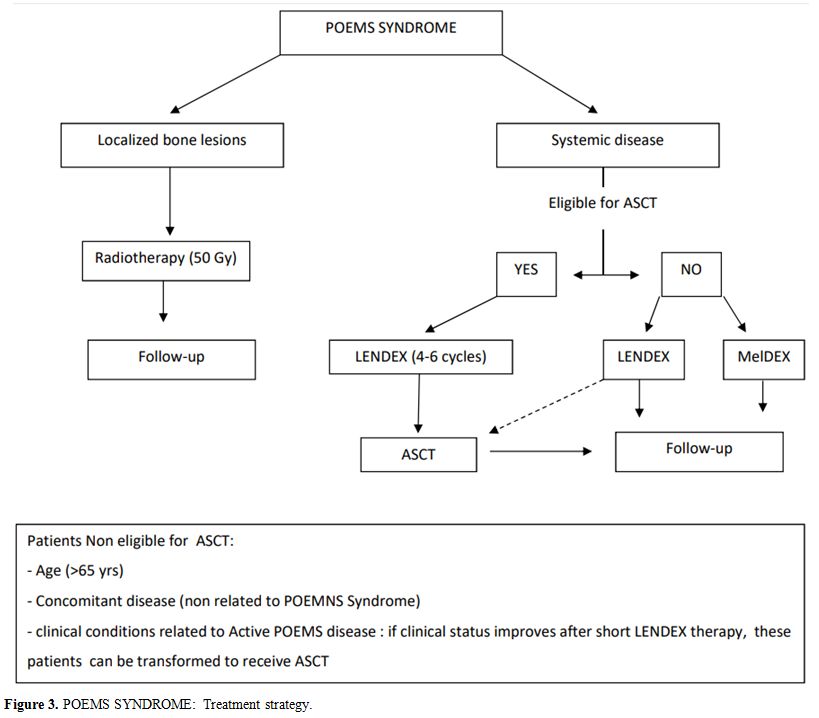 |
Figure 3. POEMS SYNDROME:
Treatment strategy. |
Radiotherapy.
Approximately 26% of newly-diagnosed POEMS patients present localized
bone lesions. In this setting, radiotherapy (RT) improves symptoms and
can also be curative.[51] The rate of clinical and hematological response ranges from 47 to 75%, and from 45 to 50%, respectively.[51,52]
In a series of 35 POEMS patients, RT resulted in a 4-year overall
survival (OS) rate of 97% and a 4-year failure-free survival rate of
52%.[52] More than 50% of patients treated with RT
show a substantial improvement of neuropathy, although this effect it
is not evident for at least six months in some subjects.[10]
The maximal response could be not attained until 2–3 years since the
first evidence of effect. Other features like anasarca, papilledema,
pulmonary hypertension and skin changes may show an earlier
improvement, usually seen few months after the end of radiotherapy.[53,54]If
the bone lesion is large, RT could be considered as primary therapy
despite bone marrow infiltration. Systemic treatments may be added
according to clinical response.Systemic treatments. In patients with a disseminated disease, systemic therapy is recommended.[50]
Treatments used in immune-mediated neuropathies (plasmapheresis, IV Ig)
are almost invariably ineffective in POEMS. Corticosteroids, either
given orally or intravenously, are also rarely effective and usually
only attenuate symptoms for a short time, without affecting
progression.[55] Therapies for POEMS are borrowed
from other plasma cell dyscrasias and include autologous stem cells
transplantation (ASCT), alkylating agents such as melphalan or
cyclophosphamide, or “new drugs” (thalidomide, lenalidomide,
Bortezomib).Autologous stem cell transplantation.
ASCT is considered the preferred initial therapy for young patients. It
is associated with a durable response, even if some patients may
experience relapse.[26] A recent multicenter
retrospective analysis suggested the effectiveness of ASCT when
incorporated into the clinical management of POEMS.[56]
Complete response was reported in 48.5%, partial response in 20.8%,
less than a partial response in 30.7% of patients. With a median follow
up of 48 months, 90% of patients were alive, and 16.5% of patients had
progressed. The 1-year non-relapse mortality was 3.3%. The likelihood
of PFS and OS were 84% and 94% at three years, 74% and 89% at five
years, respectively. Tandem transplant has been applied in a case
report, but no further information is available.[57]However,
clinical conditions related to the disease (effusion, pulmonary
hypertension, renal impairment) and severe end-organ dysfunction make
some young patients ineligible for upfront ASCT. Kanai et al. reported
an overproduction of IL12 and VEGF in untreated POEMS patients.[58]
In these patients induction therapy (cyclophosphamide or lenalidomide,
thalidomide or bortezomib in combination with high-dose dexamethasone)
modifies the hypercytokinemia status, improving clinical condition and
control disease severity making more patients eligible for ASCT. The
use of induction therapy before ASCT also reduces the incidence of
peritransplant complications.[59,60]The
optimal regimen for peripheral blood stem cell (PBSC) collection is
still controversial; PBSCs could be collected using rather high-dose
cyclophosphamide plus G-CSF of G-CSF alone.[61,62] Factors associated with inadequate mobilization are hepatomegaly, splenomegaly, ascites and renal failure:[60,62]
all these symptoms are related to disease severity, indicating that
relieving the disease activity before mobilization is crucial for
mobilization. Induction therapy before mobilization reduces the level
of various cytokines and could also limit the risk of an inadequate
mobilization and adverse events.The efficacy of plerixafor with G-CSF has been reported in two poor mobilizers.[63]
Indeed, although ASCT has a high activity in POEMS, it is a potentially
lethal procedure associated with significant morbidities, such as
engraftment syndrome. This complication is reported in 23-47% of
transplants and is characterized by fever, rash, diarrhea, weight gain,
as well as respiratory symptoms and signs that occur 7-15 days since
stem cell infusion.[64] Normalization of cytokine milieu with pre-transplant induction therapy reduces the incidence of this complication.[60]Alkylator-base therapy.
In patient nonsuitable for ASCT, alkylator-based therapy with melphalan
or cyclophosphamide plus corticosteroids could be the treatment of
choice. These treatments are associated with clinical and neurological
response in approximately 40-50% of patients and a 2-year OS rate of
78%.[10] However, limiting exposure to alkylating
agents is important because secondary hematological neoplasia
(leukemia, myelodysplasia) could occur. In
the first prospective clinical trial performed in POEMS syndrome, 31
newly-diagnosed patients were treated with 12 cycles of melphalan and
dexamethasone.[65] After a median follow-up of 21
months, 81% of the patients showed an hematologic response, 100% had a
reduction of serum VEGF levels, and 100% experienced improvements in
neurological symptoms. However, only scant data on the long term effect
of this therapy is available.[65]Thalidomide.
Given its antiangiogenic, anti-inflammatory, and immunomodulating
properties, thalidomide has been tested in POEMS syndrome. It showed
evidence of clinical efficacy, but its neurotoxicity makes thalidomide
unsuitable for patients with this condition, due to preexisting severe
neuropathy.[66-68] The results of a multicenter,
randomized, double-blind study comparing thalidomide plus dexamethasone
versus dexamethasone alone showed a reduction of VEGF serum level with
thalidomide, but also an increase of side effects without hematological
response.[69]Lenalidomide.
In POEMS patients, several cytokines other than VEGF are elevated.
Lenalidomide, a thalidomide-derived immunomodulatory analog, blocks the
increased secretion of IL6, TNF-alpha, and VEGF. These molecules
stimulate T cell proliferation and the production of IL2, IL10, and
IFN-gamma, while they inhibit IL1beta and IL6 and modulate IL12
production. Therefore, lenalidomide appears the most promising drug for
the treatment of POEMS syndrome. Lenalidomide
efficacy in the pre-treated setting has been reported in several case
reports or small series of patients, with long-lasting responses and
good tolerability.[70-74] In a retrospective pooled
analysis of 51 subjects, the 12 newly diagnosed patients treated with
lenalidomide showed a 12-month PFS rate of 93% and a 24-month rate of
47%.[75]More recently, the efficacy of lenalidomide as frontline therapy has been further confirmed.[76,77]
Overall, the efficacy of lenalidomide was promptly evident, with
stabilization or improvement of symptoms already after first cycles,
rapid resolution of vascular volume overload, skin changes and
pulmonary hypertension. Importantly, a sufficient number of CD34+ cells
was harvested after lenalidomide treatment.In
an unpublished series from our Center, we treated with lenalidomide 18
subjects, 13 pretreated and 5 with new diagnosed not eligible for HSCT
(Nozza et al., manuscript submitted). With a median follow-up of 39
months, progression-free survival (PFS) at three years was 59%, and
overall survival (OS) was 100%. After six months of therapy, 83% of the
patients had improved clinical and neurological conditions,
particularly regarding regression of edema and ascites, amelioration of
skin lesions and regression of adenopathies. A rapid neurological
improvement was documented in all but one patient, and correlated with
a statistically significant improvement in neurophysiologic parameters.
In addition, we reported a reduction of VEGF already after one cycle of
lenalidomide. On
these bases, lenalidomide, as an induction therapy before the
transplant, can improve the patient clinical status and decrease
transplant-related morbidity. Furthermore, it can be used as a salvage
therapy after relapse.Bortezomib.
Bortezomib, alone or in combination, has been used in newly-diagnosed
and in relapsed patients, with highly satisfactory responses on
neuropathy, serum VEGF level, and extravascular overload.[78-81]
However, the potential risk of progression of existing neuropathy
associated with the use of bortezomib may limit its usage in patients
with POEMS syndrome.Bevacizumab.
Since most patients with POEMS syndrome present increased serum VEGF
levels, therapy with anti-VEGF factors is considered an appealing
strategy. Badros et al. first reported the efficacy of anti-VEGF
therapy in patients with POEMS.[6] In a study of 17
patients treated with bevacizumab (only one in monotherapy),
bevacizumab based-therapy resulted in a rapid decrease in the serum
VEGF levels, which, however, was not necessarily associated with
clinical improvement.[82] Moreover, some patients
showed improved neuropathy and systemic symptoms, but this effects
could also be related to the association of bevacizumab with other
cytotoxic drugs. Six out of 17 treated patients died without showing
any response. The reduced effectiveness of bevacizumab may be linked to
the fact that several cytokines (IL-6, IL-12, TNF-α) other than VEGF
are elevated in POEMS syndrome. Therefore, inhibition of VEGF alone is
not sufficient to suppress disease activity. It has also been suggested
that sudden VEGF removal with bevacizumab therapy may cause a collapse
of newly-formed fragile vessels, since VEGF is a major angiotrophic
factor, and may lead to an increase capillary leakage.[83]
Response Evaluation
At
present, there are neither guidelines nor standard criteria for the
assessment of the response in POEMS patients. The reduction of
M-Protein, the modification of the VEGF value and the improvement of
clinical or neurological parameters/symptoms present at the onset are
often used in clinical practice to assess the response to treatment. In
POEMS syndrome, M-protein size is typically small, thus making standard
MM response criteria inapplicable in most cases. Moreover, patients can
obtain clinical benefit even without hematologic response.[65] Free
light chains ratio is in the range in the majority of patients,
therefore making this test not especially useful for response
evaluation in POEMS.[17] There are also challenges using vascular
endothelial growth factor (VEGF) as a response criterion, also because
VEGF assays are not standardized. There is even disagreement about
which measurement – either serum or plasma - should be preferred. From
a clinical standpoint, there are no criteria able to define the
responses or improvements for most of the clinical parameters in POEMS:
often measurements are relegated to the vague “improvement” or
undefined “response” category. For evaluating the most important
symptom of POEMS syndrome (peripheral neuropathy), the Overall
Neuropathy Limitations Scale (ONLS), a simple tool, has been used;
however, it does not distinguish between sensory, motor, or painful
variants of neuropathy.The
issue of response evaluation in POEMS has recently been raised by
Angela Dispenzieri,[84] who stressed the need to use common and plain
language.In
our Institute, we decided to assess patients at diagnosis, during
treatment periods and follow-up by the following tools: (i) three
neurological scales (ONLS: to assess limitations and disability caused
by peripheral neuropathy, Medical Research Council (MRC) scale to
evaluate muscle strength, Inflammatory Neuropathy Cause and Treatment
(INCAT) disability score to evaluate sensations in the arms and legs,
and nerve conduction studies (EMG). (ii) a specifically-developed
clinical scale (Clinical Response Evaluation Scale, CRES), which takes
account the modification of ten clinical features [monoclonal protein,
blood alteration, organomegaly (liver and spleen), lymphadenopathy,
endocrinopathy, skin alteration, peripheral edema, effusions (pleural,
ascites), impaired lung function (by spirometry), bone lesions]; (iii)
serum VEGF levels, in order to evaluate improvement of motor conduction
velocity, distal latency and distal CMAP amplitude. The variation of
the score of these scales during treatment could estimate the magnitude
of clinical response.
Prognosis and Relapse
The
course of POEMS syndrome is chronic and progressive, but ASCT and novel
agents may prolong survival. At present, there is no standard risk
stratification for POEMS syndrome, but some features such as
extravascular volume overload, pulmonary hypertension, fingernail
clubbing, renal dysfunction, have been associated with shorter OS.[9,62] However, the level of serum VEGF and the number of clinical features at onset do not appear to affect OS.[11]Recently,
Whang et al. retrospectively analyzed 362 newly diagnosed POEMS
patients, identifying four baseline clinical variables associated with
poorer OS (age >50 years, pulmonary hypertension, pleural effusion
and estimated glomerular filtration rate <30 ml/min).[85]
These clinical variables were included in a nomogram, which could
predict the 5- and 10-year OS. This nomogram is not ready for routine
practice, but these four variables should be taken into consideration
for counseling patients. The
incidence of relapse or progression have been reported in some studies,
but they included only a limited number of patients with a short
follow-up.[26,52,73] A retrospective analysis of 262 relapsed patients with POEMS syndrome has been recently published.[86]
The Authors reported that 4% of patients with POEMS had a primary
refractory disease, 20% showed a progression of disease within five
years and an additional 10% after five years. Low albumin at onset and
failure to achieve a complete hematological response with induction
therapy were independent risk factors for PFS in relapsed patients.Systemic
treatment should be initiated in the case of clinical/symptomatic
relapse, and observation is reasonable in patients with isolated
hematological relapsed or with VEGF elevation. The majority of relapsed
patients could be salvaged with second-line therapy, showing prolonged
PFS in more than 90% of cases.[73] RT be also
considered in relapsed patients with 1 or 2 bone lesions on PET, with a
long-lasting disease control. However, at present, it is not possible
to define the best salvage therapy in POEMS, due to the lack of
randomized trials and the small sample size and methodological
limitations of available studies. Overall, lenalidomide, alone or in
combinations with high-dose dexamethasone, seems the most promising
molecule in this setting of patients, with remarkable results even with
prolonged treatments and a good tolerability profile.[73,75] Conclusions
POEMS
syndrome is a rare disease, often unrecognized. Its etiology is
uncertain, although VEGF appears a major contributor to the onset of
many symptoms. Differential diagnosis and the rapid recognition of
POEMS remain key issues since treatment should be started as early as
possible to prevent the worsening of the clinical condition of
patients.
In
localized disease, RT is the treatment of choice. Lenalidomide may also
be considered as initial short-term therapy (4-6 months) in young
patients with POEMS syndrome eligible for high-dose therapy and HSCT,
as well as in those patients whose clinical conditions could be
exclusion criteria, in order to induce a rapid improvement and
transform transplantation eligibility. In addition, lenalidomide might
represent a suitable long-term therapy in patients who are not
candidate for transplant, or who relapsed after high-dose systemic
therapy.
Acknowledgments
Editorial
assistance for the preparation of this manuscript was provided by Luca
Giacomelli, Ph.D.; this assistance was supported by internal funds.
References
- Bardwick PA, Zvaifler NJ, Gill GN, et al. Plasma
cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M
protein, and skin changes: the POEMS syndrome. Report on two cases and
a review of the literature. Medicine (Baltimore). 1980;59:311-22. https://doi.org/10.1097/00005792-198007000-00006

- Takatsuki
K, Sanada I. Plasma cell dyscrasia with polyneuropathy and endocrine
disorder: clinical and laboratory features of 109 reported cases. Jpn J
Clin Oncol. 1983;13:543–55. PMid:6315993

- Gherardi
RK, Bélec L, Soubrier M, et al. Overproduction of proinflammatory
cytokines imbalanced by their antagonists in POEMS syndrome. Blood.
1996;87:1458. PMid:8608236

- Lesprit
P, Godeau B, Authier FJ, et al. Pulmonary hypertension in POEMS
syndrome: a new feature mediated by cytokines. Am J Respir Crit Care
Med.0 1998;157:907.

- Scarlato
M, Previtali SC, Carpo M, et al. Polyneuropathy in POEMS syndrome: role
of angiogenic factors in the pathogenesis. Brain. 2005;128:1911. https://doi.org/10.1093/brain/awh519 PMid:15975949

- Badros A, Porter N, Zimrin A. Bevacizumab therapy for POEMS syndrome. Blood. 2005; 106:1135. https://doi.org/10.1182/blood-2005-03-0910 PMid:16033956

- Straume O, Bergheim J, Ernst P. Bevacizumab therapy for POEMS syndrome. Blood. 2006; 107:4972–3. https://doi.org/10.1182/blood-2005-12-5045 PMid:16754779

- Abe
D, Nakaseko C, Takeuchi M, et al. Restrictive usage of monoclonal
immunoglobulin lambda light chain germline in POEMS syndrome. Blood.
2008;112(3):836–9 https://doi.org/10.1182/blood-2007-12-126979 PMid:18497319

- Li
J, Zhou DB, Huang Z, et al. Clinical characteristics and long-term
outcome of patients with POEMS syndrome in China. Ann Hematol.
2011;90:819-26. https://doi.org/10.1007/s00277-010-1149-0 PMid:21221584

- Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome Blood. 2003;101:2496-506. https://doi.org/10.1182/blood-2002-07-2299 PMid:12456500

- Dispenzieri A. POEMS SYNDROME. Blood Rev. 2007;21:285-99. https://doi.org/10.1016/j.blre.2007.07.004 PMid:17850941

- Rajkumar
SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working
Group updated criteria for the diagnosis of multiple myeloma. Lancet
Oncol 2014;15:e538. https://doi.org/10.1016/S1470-2045(14)70442-5

- Saperstein
DS, Kats JS, Amato AA et al. Clinical spectrum of chronic acquired
demyelinating polyneurophaly. Muscle nerve 2001;24:311-24. https://doi.org/10.1002/1097-4598(200103)24:3<311::AID-MUS1001>3.0.CO;2-A

- Guo
X, Qin X, Zhang Y. Electrophysiological features of POEMS syndrome and
chronic inflammatory demyelinating polyneuropathy. J Clin Neurosci.
2014;21:587-90. https://doi.org/10.1016/j.jocn.2013.05.023 PMid:24268501

- Nasu
S, Misawa S, Sekiguchi Y, et al. Different neurological and
physiological profiles in POEMS syndrome and chronic inflammatory
demyelinating polyneuropathy.J Neurol Neurosurg Psychiatry.
2012;83:476-99. https://doi.org/10.1136/jnnp-2011-301706 PMid:22338030

- Nakanishi T, Sobue I, Toyokura Y. The Crow-Fukase syndrome: a study of 102 cases in Japan. Neurology. 1984;34:712-20. https://doi.org/10.1212/WNL.34.6.712 PMid:6539431

- Stankowski-Drengler
T, Gertz MA, Katzmann JA, et al. Serum immunoglobulin free light chain
measurements and heavy chain isotype usage provide insight into disease
biology in patients with POEMS syndrome. Am J Hematol. 2010;85:431-4. https://doi.org/10.1002/ajh.21707

- Dao
LN, Hanson CA, Dispenzieri A, et al. Bone marrow histopathology in
POEMS syndrome: a distinctive combination of plasma cell, lymphoid, and
myeloid findings in 87 patients. Blood. 2011;117:6438-44. https://doi.org/10.1182/blood-2010-11-316935 PMid:21385854 PMCid:PMC3123015

- Nakaseko
C, Abe D, Takeuci M, et al. Restricted oligo-clonal usage of monoclonal
immunoglobuline (Lambda) light chains germline in POEMS Syndrome. ASH
annual meeting Abstract 2007; 110: 2483
- Kang
WY, Shen KN, Duan MH, et al. 14q32 translocations and 13q14 deletions
are common cytogenetic abnormalities in POEMS syndrome. Eur J Haematol.
2013;91:490-6. https://doi.org/10.1111/ejh.12189 PMid:23957213

- Shibuya K, Misawa S, Horikoshi T, et al. Detection of bone lesions by CT in POEMS syndrome Intern Med. 2011;50:1393-6. https://doi.org/10.2169/internalmedicine.50.5263 PMid:21720058

- Shi X, Hu S, Luo X, et al. CT characteristics in 24 patients with POEMS syndrome. Acta Radiol. 2016;57:51-7. https://doi.org/10.1177/0284185114564614 PMid:25571895

- Narváez
JA, Majós C, Narváez J, et al. POEMS syndrome: unusual radiographic,
scintigraphic and CT features. Eur Radiol. 1998;8:134-6. https://doi.org/10.1007/s003300050353 PMid:9442145

- Albertí
MA, Martinez-Yélamos S, Fernandez a, et al. 18F-FDG PET/CT in the
evaluation of POEMS syndrome. Eur J Radiol. 2010;76:180-2. https://doi.org/10.1016/j.ejrad.2009.06.004 PMid:19581061

- Pan Q, Li J, Li F, et al. Characterizing POEMS Syndrome with 18F-FDG PET/CT. J Nucl Med. 2015;56:1334-7. https://doi.org/10.2967/jnumed.115.160507 PMid:26182964

- D'Souza
A, Lacy M, Gertz M, et al. Long-term outcomes after autologous stem
cell transplantation for patients with POEMS syndrome (osteosclerotic
myeloma): a single-center experience. Blood. 2012;120:56-62. https://doi.org/10.1182/blood-2012-04-423178 PMid:22611150

- Bitter
MA, Komaiko W, Franklin WA, et al. Giant lymph node hyperplasia with
osteoblastic bone lesions and the POEMS (Takatsuki's) syndrome. Cancer.
1985;56(1):188-94. https://doi.org/10.1002/1097-0142(19850701)56:1<188::AID-CNCR2820560132>3.0.CO;2-Z

- Li J, Zhou DB. New advances in the diagnosis and treatment of POEMS SYNDROME, Br J Haematol. 2013;161:303-15. https://doi.org/10.1111/bjh.12236 PMid:23398538

- Watanabe
O, Maruyama I, Arimura K et al. Overproduction of vascular endothelial
growth factor/vascular permeability factor is causative in Crow-Fukase
(POEMS) syndrome. Muscle Nerve. 1998;21:1390-7. https://doi.org/10.1002/(SICI)1097-4598(199811)21:11<1390::AID-MUS5>3.0.CO;2-4

- Arimura
K. Increased vascular endothelial growth factor (VEGF) is causative in
Crow-Fukase syndrome Rinsho Shinkeigaku. 1999;39:84-5. PMid:10377815

- Nobile-Orazio
E, Terenghi Fm, Giannotta C, et al. serum VEGF levels in POEMS syndrome
and in immune mediated neuropaties. Neurology. 2009;72:1024-6. https://doi.org/10.1212/01.wnl.0000344569.13496.ff PMid:19289745

- Briani
C, Fabrizi GM, Ruggero S, et al. Vascular endothelial growth factor
helps differentiate neuropathies in rare plasma cell dyscrasias. Muscle
Nerve. 2011;43:164-7. https://doi.org/10.1002/mus.21872 PMid:21254078

- Webb
NJ, Bottomley MJ, Watson CJ, Brenchley PE. Vascular endothelial growth
factor (VEGF) is released from platelets during blood clotting:
implications for measurement of circulating VEGF levels in clinical
disease. Clin Sci (Lond) 1998;94:395. https://doi.org/10.1042/cs0940395

- Soubrier
MJ, Dubost JJ, Sauvezie BJ. POEMS syndrome: a study of 25 cases and a
review of the literature. French Study Group on POEMS Syndrome Am J
Med. 1994;97:543-53. https://doi.org/10.1016/0002-9343(94)90350-6

- Cui RT, Yu SY, Huang XS, et al The characteristics of ascites in patients with POEMS syndrome. Ann Hematol. 2013;92:1661-4. https://doi.org/10.1007/s00277-013-1829-7 PMid:23811954

- Higuchi
M, Kamijo H, Koyama T, et al. POEMS syndrome caused refractory ascites
in a polycystic disease patient undergoing hemodialysis. Clin Exp
Nephrol. 2003;7(4):301-5. https://doi.org/10.1007/s10157-003-0247-x PMid:14712361

- Gandhi GY, Basu R, Dispenzieri A, Endocrinopathy in POEMS syndrome: the Mayo Clinic experience Mayo Clin Proc. 2007;82:836-42. https://doi.org/10.4065/82.7.836 PMid:17605964

- Miest
RY, Comfere NI, Dispenzieri A, et al. Cutaneous manifestations in
patients with POEMS syndrome. Int J Dermatol. 2013;52:1349. https://doi.org/10.1111/j.1365-4632.2012.05648.x PMid:23557151

- Barete
S, Mouawad R, Choquet S, et al. Skin manifestations and vascular
endothelial growth factor levels in POEMS syndrome: impact of
autologous hematopoietic stem cell transplantation Arch Dermatol.
2010;146:615-23. https://doi.org/10.1001/archdermatol.2010.100 PMid:20566924

- Cui R, Yu S, Huang X. Papilloedema is an independent prognostic factor for POEMS syndrome J Neurol. 2014;261:60-5. https://doi.org/10.1007/s00415-013-7143-4 PMid:24141729

- Kaushik
M, Pulido JS, Abreu R, et al. Ocular findings in patients with
polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy,
and skin changes syndrome. Ophthalmology. 2011;118:778-82. https://doi.org/10.1016/j.ophtha.2010.08.013 PMid:21035860

- Zhang
X, Cai QQ, Huang XF, et al. Ocular manifestations and treatment
outcomes in chinese patients with poems syndrome. Retina. 2016; Epub
ahead of print. https://doi.org/10.1097/IAE.0000000000001409

- Kelly JJ Jr, Kyle RA, Miles JM, Dyck PJ. Osteosclerotic myeloma and peripheral neuropathy. Neurology. 1983; 33:202. https://doi.org/10.1212/WNL.33.2.202

- Li J, Tian Z, Zheng HY, et al. Pulmonary hypertension in POEMS syndrome. Haematologica. 2013;98:393-8. https://doi.org/10.3324/haematol.2012.073031 PMid:22983590 PMCid:PMC3659947

- Chandrashekaran
S Dispenzieri A, Cha SS, et al. Pulmonary morbidity improves after
autologous stem cell transplantation in POEMS syndrome Respir Med.
2015;109:122-30. https://doi.org/10.1016/j.rmed.2014.11.005 PMid:25433952 PMCid:PMC4758677

- Nobile-Orazio E. Neuropathy and monoclonal gammopathy. Handb Clin Neurol. 2013;115:443-59. https://doi.org/10.1016/B978-0-444-52902-2.00025-4 PMid:23931795

- Mauermann
ML, Sorenson EJ, Dispenzieri A, et al. Uniform demyelination and more
severe axonal loss distinguish POEMS syndrome from CIDP. J Neurol
Neurosurg Psychiatry. 2012;83:480. https://doi.org/10.1136/jnnp-2011-301472 PMid:22396441

- Allam
JS, Kennedy CC, Aksamit TR, et al. Pulmonary manifestations in patients
with POEMS syndrome: a retrospective review of 137 patients. Chest.
2008;133:969–74. https://doi.org/10.1378/chest.07-1800 PMid:18198255

- Misawa
S, Sato Y, Katayama K, et al. Safety and efficacy of thalidomide in
patients with POEMS syndrome: a multicentre, randomised, double-blind,
placebo-controlled trial. Lancet Neurol. 2016;15:1129-37. https://doi.org/10.1016/S1474-4422(16)30157-0

- Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119:5650-8. https://doi.org/10.1182/blood-2012-03-378992 PMid:22547581 PMCid:PMC3425020

- Suh YG, Kim YS, Uh CO, et al. The role of radiotherapy in the management of POEMS syndrome Radiat Oncol. 2014;9:265. https://doi.org/10.1186/s13014-014-0265-8 PMid:25431020 PMCid:PMC4253631

- Humeniuk
MS, Gertz MA, Lacy MO, et al. Outcomes of patients with POEMS syndrome
treated initially with radiation. Blood. 2013:122:68-73. https://doi.org/10.1182/blood-2013-03-487025 PMid:23699599 PMCid:PMC4067496

- Brewis
MJ, Church AC, Peacock AJ, et al. Pulmonary hypetension in POEMS
syndrome: resolution following radiotherapy. Pulm Circ. 2014;4:732-5. https://doi.org/10.1086/678553 PMid:25610609 PMCid:PMC4278633

- Dispenzieri A. POEMS syndrome: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014;89:214-23. https://doi.org/10.1002/ajh.23644 PMid:24532337

- Dispenzieri A. POEMS syndrome: update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90:951-62. https://doi.org/10.1002/ajh.24171 PMid:26331353

- Cook
G, Iacobelli S, van Biezen E, et al. High-dose therapy and autologous
stem cell transplantation in patients with POEMS syndrome: a
retrospective study of the Plasma Cell Disorder sub-committee of the
Chronic Malignancy Working Party of the European Society for Blood
& Marrow Transplantation. Haematologica. 2017;102:160-7. https://doi.org/10.3324/haematol.2016.148460 PMid:27634201 PMCid:PMC5210246

- Kojima
H, Katsuoka Y, Katsura Y, et al. Successful treatment of a patient with
POEMS syndrome by tandem high-dose chemotherapy with autologous CD34+
purged stem cell rescue. Int J Hematol. 2006;84:182–5. https://doi.org/10.1532/IJH97.06067 PMid:16926143

- Kanai
K, Sawai S, Sogawa K, et al. Markedly upregulated serum interleukin-12
as a novel biomarker in POEMS syndrome. Neurology. 2012;79:575-82. https://doi.org/10.1212/WNL.0b013e318263c42b PMid:22843279

- Jimenez-Zepeda
VH, Trudel S, Reece DE. Cyclophosphamide and prednisone induction
followed by cyclophosphamide mobilization effectively decreases the
incidence of engraftment syndrome in patients with POEMS syndrome who
undergo stem cell transplantation. Am J Hematol. 2011;86:873-5. https://doi.org/10.1002/ajh.22115 PMid:21815185

- Li
J, Zhang W, Duan MH. PBSC mobilization in newly diagnosed patients with
POEMS syndrome: outcomes and prognostic factors. Bone Marrow
Transplant. 2013;48:233-7. https://doi.org/10.1038/bmt.2012.138 PMid:22825426

- Muto
T, Ohwada C, Takaishi K et al. Safety and Efficacy of Granulocyte
Colony-Stimulating Factor Monotherapy for Peripheral Blood Stem Cell
Collection in POEMS Syndrome. Biol Blood Marrow Transplant.
2017;23:361-3. https://doi.org/10.1016/j.bbmt.2016.10.024 PMid:27840209

- Shimizu
N, Nakaseko C, Sakaida E, et al. Factors associated with the efficiency
of PBSC collection in POEMS syndrome patients undergoing autologous
PBSC transplantation. Bone Marrow Transplant. 2012;47:1010-2. https://doi.org/10.1038/bmt.2011.211 PMid:22041850

- Shimizu
N, Sakaida E, Ohwada C, et al. Mobilization of PBSCs in poor mobilizers
with POEMS syndrome using G-CSF with plerixafor. Bone Marrow
Transplant. 2012;47:1587-8. https://doi.org/10.1038/bmt.2012.80 PMid:22609884

- Dispenzieri
A, Lacy MQ, Hayman SR, et al. Peripheral blood stem cell transplant for
POEMS syndrome is associated with high rates of engraftment syndrome.
Eur J Haematol. 2008;80:397–406. https://doi.org/10.1111/j.1600-0609.2008.01037.x PMid:18221391 PMCid:PMC2327207

- Li
J, Zhang W, Jiao L, et al. Combination of melphalan and dexamethasone
for patients with newly diagnosed POEMS syndrome. Blood.
2011;117:6445-9. https://doi.org/10.1182/blood-2010-12-328112 PMid:21393478 PMCid:PMC3123016

- Sinisalo M, Hietaharju A, Sauranen J, Wirta O. Thalidomide in POEMS syndrome: case report. Am J Hematol 2004;76:66-8. https://doi.org/10.1002/ajh.20051 PMid:15114600

- Kim SY, Lee SA, Ryoo HM, et al. Thalidomide for POEMS syndrome. Ann Hematol. 2006;85:545-6. https://doi.org/10.1007/s00277-006-0119-z PMid:16718498

- Kuwabara
S, Misawa S, Kanai K, et al. Thalidomide reduces serum VEGF levels and
improves peripheral neuropathy in POEMS syndrome. J Neurol Neurosurg
Psychiatry. 2008;79:1255-7. https://doi.org/10.1136/jnnp.2008.150177 PMid:18469028

- Misawa
S, Sato Y, Katayama K, et al; Japanese POEMS Syndrome for Thalidomide
(J-POST) Trial Study Group. Safety and efficacy of thalidomide in
patients with POEMS syndrome: a multicentre, randomised, double-blind,
placebo-controlled trial. Lancet Neurol. 2016;15:1129-37. https://doi.org/10.1016/S1474-4422(16)30157-0

- Dispenzieri A, Klein CJ, Mauermann ML. Lenalidomide therapy in a patient with POEMS Syndrome. Blood. 2007;110:1075-6. https://doi.org/10.1182/blood-2007-03-082354 PMid:17644745

- Vannata
B, Laurenti L, Chiusolo P, et al. Efficacy of lenalidomide plus
dexamethasone for POEMS syndrome relapsed after autologous peripheral
stem-cell transplantation. Am J Hematol. 2012;87:641-2. https://doi.org/10.1002/ajh.23195 PMid:22488443

- Suyani
E, Yagci M, Sucak GT. Complete remission with a combination of
lenalidomide, cyclophosphamide and prednisolone in a patient with
incomplete POEMS syndrome. Acta Haematol. 2011;126:199-201. https://doi.org/10.1159/000329896 PMid:21849771

- Cai
QQ, Wang C, Cao XX, et al. Efficacy and safety of low-dose lenalidomide
plus dexamethasone in patients with relapsed or refractory POEMS
syndrome. Eur J Haematol. 2015;95:325-30. https://doi.org/10.1111/ejh.12492 PMid:25401269

- Royer
B, Merlusca L, Abraham J, et al. Efficacy of lenalidomide in POEMS
syndrome: a retrospective study of 20 patients. Am J Hematol.
2013;88:207-12. https://doi.org/10.1002/ajh.23374 PMid:23335406

- Zagouri
F, Kastritis E, Gavriatopoulou M, et al. Lenalidomide in patients with
POEMS syndrome: a systematic review and pooled analysis. Leuk Lymphoma.
2014;55:2018-23. https://doi.org/10.3109/10428194.2013.869329 PMid:24295131

- Lestang
E, Caristan A, Néel A, et al. Lenalidomide as frontline therapy in
polyneuropathy, organomegaly, endocrinopathy, monoclonal protein and
skin changes syndrome: a retrospective case series of eight patients.
Leuk Lymphoma. 2015;56:1895-6. https://doi.org/10.3109/10428194.2014.974595 PMid:25347429

- Yang
H, Huang X, Cai Q. Improvement of sexual function in POEMS syndrome
after combination therapy of Lenalidomide and dexamethasone. Orphanet J
Rare Dis. 2016;11:80. https://doi.org/10.1186/s13023-016-0461-8 PMid:27317315 PMCid:PMC4912786

- Tang X, Shi X, Sun A,et al. Successful bortezomib-based treatment in POEMS syndrome. Eur J Haematol. 2009;83:609-10. https://doi.org/10.1111/j.1600-0609.2009.01330.x PMid:19674063

- Kaygusuz I, Tezcan H, Cetiner M, et al. Bortezomib: a new therapeutic option for POEMS syndrome. Eur J Haematol. 2010;84:175-7. https://doi.org/10.1111/j.1600-0609.2009.01341.x PMid:19732138

- Ohguchi
H, Ohba R, Onishi Y, et al. Successful treatment with bortezomib and
thalidomide for POEMS syndrome. Ann Hematol. 2011;90:1113-4. https://doi.org/10.1007/s00277-010-1133-8 PMid:21153416

- Warsame
R, Kohut IE, Dispenzieri A. Successful use of cyclophosphamide,
bortezomib, and dexamethasone to treat a case of relapsed POEMS Eur J
Haematol. 2012;88:549. https://doi.org/10.1111/j.1600-0609.2012.01780.x PMid:22416898

- Sekiguchi
Y, Misawa S, Shibuya K, et al. Ambiguous effects of anti-VEGF
monoclonal antibody (bevacizumab) for POEMS syndrome. J Neurol
Neurosurg Psychiatry. 2013;84:1346–8. https://doi.org/10.1136/jnnp-2012-304874 PMid:23463868

- Straume O, Bergheim J, Ernst P, et al. Bevacizumab therapy for POEMS syndrome. Blood. 2006;107:4972–3. https://doi.org/10.1182/blood-2005-12-5045 PMid:16754779

- Dispenzieri A. Ushering in a new era for POEMS. Blood. 2011;117:6405-6. https://doi.org/10.1182/blood-2011-03-342675 PMid:21680803

- C
Wang, X-F Huang, Q-Q Cai, et al. Prognostic study for overall survival
in patients with newly diagnosed POEMS syndrome. Leukemia.
2017;31:100–6. https://doi.org/10.1038/leu.2016.168 PMid:27338259

- Kourelis
TV, Buardi FK, GertzMA, et al. Risk factors for and outcomes of
patients with POEMS syndrome who experience progression after
first-line treatment. Leukemia. 2016; 30, 1079-85. https://doi.org/10.1038/leu.2015.344 PMid:26669974

[TOP]


























































































