Anna Candoni1, Federico De Marchi1, Fabio Vescini2, Sara Mauro1, Cristina Rinaldi3, Marco Piemonte4, Nicholas Rabassi1, Maria Vittoria Dubbini1 and Renato Fanin1
1 Division of Hematology, University Hospital-Santa Maria Misericordia, Udine, Italy.
2 Division of Endocrinology, University Hospital of Udine.
3 Therapeutic
Apheresis Unit and Stem Cell Manipulation Laboratory, Department of
Transfusion Medicine, University Hospital of Udine.
4 Otorhinolaryngology Unit, University Hospital of Udine.
Published: October 16, 2017
Received: July 8, 2017
Accepted: October 8, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017058 DOI
10.4084/MJHID.2017.058
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Antithyroid
drugs can be a rare cause of agranulocytosis (0.5% of treated
patients). Suspension of these drugs is mandatory in these patients and
may result in worsening hyperthyroidism. We report the case of a
27-year-old woman who is 3 months post-partum, breastfeeding, and
suffering with Graves’ disease hyperthyroidism treated first with
methimazole and then with propylthiouracil due to a methimazole
allergy. She was admitted for urosepsis and agranulocytosis. The
patient was diagnosed with propylthiouracil related agranulocytosis,
diffuse toxic goiter and thyro-gastric syndrome. Antithyroid drug
therapy was stopped resulting in a worsening of thyrotoxicosis.
Agranulocytosis was treated with 8 doses of G-CSF with full recovery.
To rapidly restore euthyroidism and to perform a thyroidectomy, the
patient received 6 therapeutic plasma exchange (TPE) procedures, to
clear thyroid hormones and anti-TSH receptor antibodies from blood,
resulting in a pre-surgical euthyroid state without antithyroid drug
therapy. Two years after thyroidectomy, the patient is well under
thyroid hormone replacement therapy with a normal granulocyte count.
|
Introduction
Graves’
disease is an autoimmune thyroid syndrome characterized by the presence
of anti-thyroid antibodies, hyperthyroidism, goiter, ophthalmopathy and
rarely by dermopathy (pretibial myxedema). Thyrostatic drugs that
inhibit thyroid hormone productions, such as methimazole or
propylthiouracil, are commonly used to treat this disease.[1-6] In the majority of cases these agents are well tolerated and allergic reactions are rare.[1-6]
Skin reactions (mainly urticaria), gastro-intestinal disturbances,
arthralgia and hepatotoxicity are reported as possible side effects
while vasculitis and neutropenia or agranulocytosis are very rare.[1-6]
Here
we report a rare case of a patient with hyperthyroidism and Graves’
disease, allergic to methimazole, treated with propylthiouracil, who
developed a propylthiouracil-related agranulocytosis. The patient
required granulocyte colony-stimulating factor (G-CSF) administration
and received therapeutic plasma exchange (TPE) procedures in order to
remove thyroid hormones and anti-TSH receptor antibodies from blood,
achieving a mandatory euthyroid state to perform a subsequent safe and
curative total thyroidectomy.
Case Report
A
27-year-old breastfeeding woman was admitted to our Department of
Hematology for the detection of agranulocytosis complicated with
uroseptic fever (blood and urine culture positives for Escherichia
Coli). Her medical history revealed Graves’ disease hyperthyroidism
diagnosed in 2007. She was treated with methimazole with repeated
episodes of extensive erythroderma causing drug discontinuation and
then with propylthiouracil 300 mg/day for 11 months.
On physical
examination the patient was tachycardic (105 bpm) and febrile (38.5
°C). A diffuse enlargement of thyroid gland and a mild ophthalmopathy
were reported. Antibiotic treatment with piperacillin tazobactam (4.5 g
3 times a day) was started and the following tests were performed:
-Differential Complete Blood Count:
hemoglobin 9.8 g/dl (normal range 12-16), MCV 90 fl, platelets
129,000/mmc (normal range 150,000 to 400,000), leukocytes 1000/mmc
(normal range 4000-11000) with only 2% granulocytes (20/mmc).
-Laboratory tests:
lactate dehydrogenase 826 IU/L, haptoglobin <10 mg/dL, total
bilirubin 2.70 mg/dL, direct bilirubin 0.64 mg/dL, Coombs test
negative; CRP 37 mg/L, erythrocyte sedimentation rate 40 mm/h, B12
Vitamin 171 ng/L (normal values 211-911); renal, hepatic and
coagulation tests, folic acid, electrolytes and immunoglobulins were in
range. TSH value was less than 0.01 microUI/mL (normal values 0.35 to
5), fT3 6.2 pg/mL (2.3 to 4.2 normal values), fT4 24 pg/mL (8.9 to
17.6). The following auto-antibodies were positive: anti-Thyroglobulin
(140 IU/ml- normal values<60), anti-thyroid peroxidase (> 1000
IU/ml-normal value<60), anti-myeloperoxidase (155 AU/ml, normal
value <20), anti-TSH receptor (3.84 IU/L; normal value <0.4),
anti-intrinsic factor 35 U/mL (normal value 0-5), anti-neutrophil
cytoplasm antibodies (ANCA-1:620, perinuclear pattern). Rheumatoid
factor, anti-ds DNA, anti-ENA and anti-transglutaminase antibodies were
negative.
-Bone Marrow Aspirate and Biopsy
were performed and showed a normocellular hematopoietic parenchyma with
marked selective reduction of granulopoiesis, without blastic cells or
lymphoid infiltrates. A mild and probably secondary hematopoietic
dysplasia with excess of erythroid precursors was reported. Bone marrow
karyotype test was normal.
-Electrocardiogram and cardiac examination documented sinus tachycardia without any signs of heart failure.
-Abdominal Ultrasound
documented a slight increase in spleen size. No abdominal organ had
parenchymal abnormalities and there were no deep enlarged lymph nodes.
According
to the above investigations the patient was diagnosed with
"Agranulocytosis related to propylthiouracil therapy and complicated by
uroseptic fever, thyro-gastric syndrome (intrinsic factor antibodies
positivity, vitamin B12 deficiency, anemia, hemolysis and mild
thrombocytopenia) in patient with diffuse toxic goiter and
thyrotoxicosis".
The treatment plan was shared with
endocrinologists, cardiologists and surgeons. Propylthiouracil was
stopped while beta blocker therapy (propranolol, 120 mg/day) was
maintained. For the treatment of agranulocytosis, the patient received
8 doses of G-CSF, subcutaneously, at a dose of 300 µg/daily. Therapy
with piperacillin-tazobactam and steroids was given for 16 days (fever
>38°C for 11 days). Thyro-gastric syndrome required supplementation
with vitamin B12, 5000 IU/day IV for 8 days.
The patient was then
evaluated for surgical treatment and a total thyroidectomy was
scheduled. To perform this procedure, normal peripheral blood counts
and a euthyroid state were required to avoid thyroid storm during
surgery. To reach this goal without antithyroid drugs, 6 therapeutic
plasma exchange (TPE) procedures were performed over 12 days to remove
the circulating thyroid hormones and the TSH receptor antibodies. In
detail, TPE was carried out to exchange 1–1.5 plasma volumes every two
days using Spectra Optia apheresis machine (Manufacturer TERUMO BCT).
Albumin 5% and normal saline solution were used as the replacement
products; heparin and acid citrate dextrose (ACD-A) 350-400 ml were
used as anticoagulants at a 12:1 anticoagulant ratio. Patient underwent
TPE using a 16 G peripheral access needle in an antecubital fossa vein;
a 20 G venous cannula was placed in the opposite arm for the return
line. In order to avoid severe hypocalcemia and acid ACD toxicity
during the procedure the patient received an intravenous infusion of
10% calcium gluconate, providing up to 850 mg of calcium. Vital signs
were monitored at the beginning, and end of each procedure and patient
was monitored for adverse events. Pre and post procedural hematological
and renal parameters were evaluated.
Figure 1 shows the decrease of thyroid hormones and anti TSH receptor antibodies during TPE procedures. Figure 2 shows granulocytic recovery (PMN recovery) during and after G-CSF therapy.
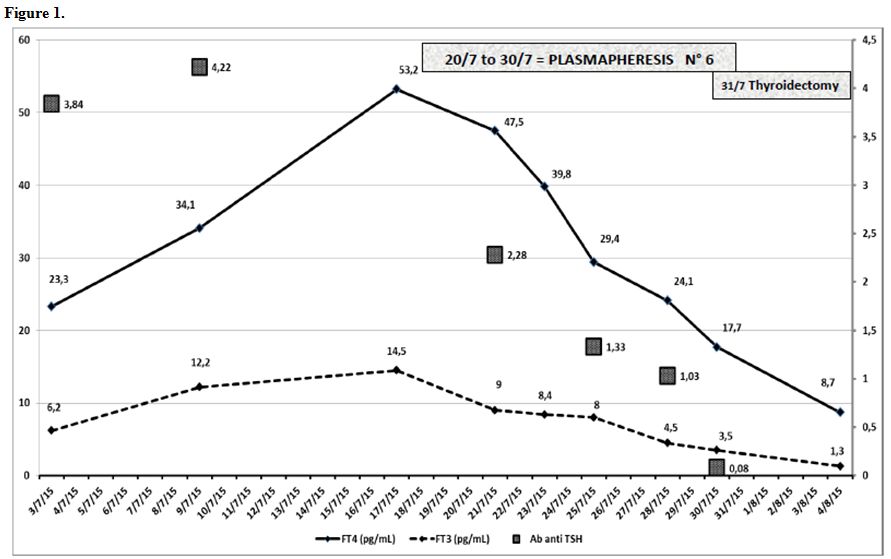 |
Figure 1.
|
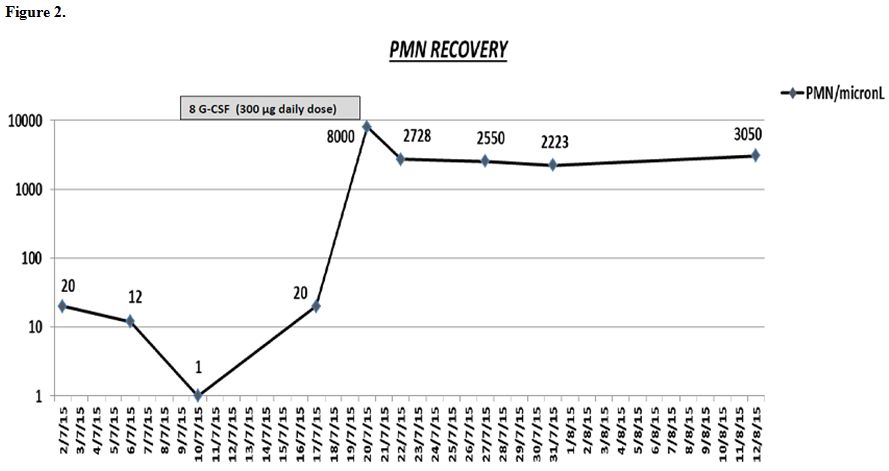 |
Figure 2. |
After
the administration of 8 daily doses of G-CSF and 6 TPE procedures the
patient reached a euthyroid state and a total thyroidectomy was
performed. No peri or post-operative complications were reported. Pre
surgery differential blood cell count was as follows: hemoglobin 10.2
g/dl, platelets 218,000/mmc, leukocytes 3,900/mmc with granulocytes 57%
(2223/mmc).
Thyroid histological examination showed a
hyperfunctioning, hyperplastic adenomatous parenchyma, consistent with
Graves’ disease.
Two year after thyroidectomy the patient is well,
undergoing thyroid hormone replacement therapy and maintaining a normal
granulocyte count.
Discussion
Hematologic
complications during thyroid disease therapy are rare and often require
a multidisciplinary approach. This case report shows the complexity of
these situations, in which multiple and rare hematological and
non-hematological complications are often present together. In this
particular case we found:-
Propylthiouracil-related agranulocytosis in a patient allergic to
methimazole and therefore without any available drug therapy for her
hyperthyroidism.-
A thyro-gastric syndrome with evidence of antibodies against intrinsic
factor and proven B12 vitamin deficiency resulting in a secondary
hematopoietic dysplasia with anemia and mild thrombocytopenia,
successfully treated with B12 vitamin supplementation.-
Hyperthyroidism with signs and symptoms of thyrotoxicosis with the need
to remove, without any antithyroid drug available, circulating thyroid
hormones and anti-TSH antibodies in order to perform a safe
thyroidectomy (to obtain a definitive surgical hyperthyroidism
resolution).Agranulocytosis
(defined as an absolute granulocyte count less than 100 cells/mmc) is a
rare complication during antithyroid drug therapy with a frequency of
less than 0.5% both in methimazole and propylthiouracil treated cases.[1-3] In particular, propylthiouracil-induced agranulocytosis occurs more frequently in the first 3 months of treatment.[1-3]
This condition represents an absolute indication for propylthiouracil
discontinuation and may be complicated by severe life-threatening
infections. Rather than being a direct toxic drug effect,
agranulocytosis seems to be related to an immunological mechanism with
production of drug-induced anti-granulocytes antibodies.[1-7] The presence of these antibodies (ANCA) has also been documented in our case report.Only
rare cases of thyrotoxicosis successfully treated with TPE, without any
related complications, has been described in the literature.[8-12]
There were also some data regarding the beneficial effects of intensive
TPE followed by immunosuppressive therapy in severe progressive Graves’
ophthalmopathy with most marked effects on soft tissue involvement,
proptosis, intraocular pressure and visual acuity.[13]
Recently, TPE has been successfully used also in a case of extreme
iatrogenic thyrotoxicosis due to excessive thyroid replacement hormones
ingestion showing a rapid decline of total and free T3 and symptoms
resolution.[14]In
autoimmune based thyroid diseases the positive effect of TPE and its
ability to restore a euthyroid state is probably not only linked to
direct thyroid hormones removal, but it might also be explained by the
clearance of anti-TSH and anti-thyroid antibodies (which maintained the
hyperthyroidism), as we showed in Figure 1.[8-13] Our
case confirms the efficacy and the optimal tolerability of TPE
procedure and its benefit in the cases where a pharmacological approach
is contraindicated or not available. However, the effect of TPE is
gradual and only transient, lasting less than 3-4 days. For this reason
close and repeated plasma exchange sessions (in our case 6 over 12
days) were necessary to obtain a result and, therefore, this
therapeutic option cannot be considered as the only curative treatment
in these situations.[8-14] Nevertheless, TPE can be a lifesaving
procedure to obtain a transitional euthyroid state before complete
thyroidectomy with minimal risk of thyroid storm or surgical bleeding
induced by thyroid tissue hypervascularization. References
- Curtis BR, Drug-induced immune neutropenia/agranulocytosis. Immunohematology 2014;30(2): 95-101. PMid:25247619

- Andersohn
F, Konzen C, Garbe E. Systematic review: agranulocytosis induced by
nonchemotherapy drugs. Ann Intern Med 2007; 146: 657-65. https://doi.org/10.7326/0003-4819-146-9-200705010-00009 PMid:17470834

- Copper DS. The side effects of antithyroid drugs. Endocrinologist 1999; 9: 457-67. https://doi.org/10.1097/00019616-199911000-00008

- Khaliq
W, Ponor L, Cheripalli P, Tangella K, Chaudry Z. Agranulocytosis
secondary to propylthiouracil. QJ Med 2012; 105: 1109-1111. https://doi.org/10.1093/qjmed/hcr168 PMid:21920992

- Cho,
Sang Shon, Dae Yoon, Management of a pregnant patient with Graves'
Disease complicated by Propylthiouracil induced agranulocytois. The
Korean J of Internal Med 2005; 20: 335-338. https://doi.org/10.3904/kjim.2005.20.4.335 PMid:16491833 PMCid:PMC3891081

- Finucane
FM, O'Connell J, Kinsley BT. Propylthiouracil induced C-ANCA positive
agranulocytosis complicating Graves' thyrotoxicosis in pregnancy. Ir J
Med Sci 2008; 177(1):69-71. https://doi.org/10.1007/s11845-007-0055-5 PMid:17611791

- Waldhauser
L, Uetrecht J. Oxidation of propylthiouracil to reactive metabolites by
activated neutrophils. Implications for agranulocytosis. Drug Metab
Dispos 1991; 19: 354-9. PMid:1676636

- Winters
JL. Plasma exchange: concepts, mechanisms, and overview of the American
Society of Apheresis guidelines. Hematology 2012:7-12.
PMid:23233554

- Bilir
BE, Atile NS, Kirkizlar O, Kömürcü Y, Akpinar S, Sezer A, et al.
Effectiveness of preoperative plasmapheresis in a pregnancy complicated
by hyperthyroidism and anti-thyroid drug-associated angioedema. Gynecol
Endocrinol. 2013; 29(5):508-10. https://doi.org/10.3109/09513590.2012.754871 PMid:23383744

- Ezer
A, Caliskan K, Parlakgumus A, Belli S, Kozanoglu I, Yidirim S.
Preoperative therapeutic plasma exchange in patients with
thyrotoxicosis. J Clin Apher 2009; 24: 111-114. https://doi.org/10.1002/jca.20200 PMid:19484727

- Vyas
AA, Vyas P, Fillipon NL, Vijayakrishnan R, Trivedi N. Successful
treatment of thyroid storm with plasmapheresis in a patient with
methimazole-induced agranulocytosis. Endocr Pract. 2010; 16(4):673-6. https://doi.org/10.4158/EP09265.CR PMid:20439250

- Guvenc
B, Unsal C, Gurkan E, Dincer S. Plasmapheresis in the treatment of
hyperthyroidism associated with agranulocytosis: a case report. J Clin
Apher 2004; 19(3): 148-50. https://doi.org/10.1002/jca.20014 PMid:15493048

- Glinoer
D1, Etienne-Decerf J, Schrooyen M, Sand G, Hoyoux P, Mahieu P, Winand
R. Beneficial effects of intensive plasma exchange followed by
immunosuppressive therapy in severe Graves' ophthalmopathy.Metab
Pediatr Syst Ophthalmol 1988;11(3):133-40.

- Shah
KK, Mbughuni MM, Burgstaler EA, Block DR, Winters JL. Iatrogenic
thyrotoxicosis and the role of therapeutic plasma exchange. J Clin
Apher 2017 [in press]. https://doi.org/10.1002/jca.21536 PMid:28319287

[TOP]

















