Annita Kolnagou1,2, Christina N Kontoghiorghe1 and George J Kontoghiorghes1
1 Postgraduate Research Institute of Science, Technology, Environment and Medicine Limassol, Cyprus.
2 Thalassaemia Unit, Paphos General Hospital, Paphos, Cyprus.
Corresponding
author: George
J. Kontoghiorghes, Postgraduate Research Institute of Science,
Technology, Environment and Medicine, 3 Ammochostou Street, Limassol
3021, Cyprus. Tel: +35726272076; Fax: +35726272076. E-mail:
kontoghiorghes.g.j@pri.ac.cy
Published: October 18, 2017
Received: July 19, 2017
Accepted: October 8, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017060 DOI
10.4084/MJHID.2017.060
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
We
report two separate episodes of transfusion-related acute lung injury
(TRALI) in two thalassaemia patients who received red blood cell
transfusions from the same multiparous donor. Both cases had the same
symptomatology and occurred within 60 minutes of transfusion. The
patients presented dyspnoea, sweating, fatigue, dizziness, fever, and
sense of losing consciousness. The chest x-ray showed a pulmonary
oedema-like picture with both lungs filled with fluid. The patients
were treated in the intensive therapy unit. They were weaned off the
ventilator and discharged following hospitalization 7 and 9 days
respectively. The TRALI syndrome was diagnosed to be associated with
HLA-specific donor antibodies against mismatched HLA-antigens of the
transfused patients. Haemovigilance improvements are essential for
reducing the morbidity and mortality in transfused patients. Blood from
multiparous donors should be tested for the presence of IgG HLA-Class I
and -Class II antibodies before being transfused in thalassaemia and
other chronically transfused patients.
|
Introduction
The
major adverse events caused by transfusions are mainly related to the
transfer of non matched blood components, acute and delayed reactions,
transmission of viral or bacterial infections, transfusion-related
acute lung injury (TRALI) etc.[1-5]
To minimise
the risk of adverse events, the donated blood is thoroughly screened
for antibodies, viruses and other risk factors before storage and
transfusion.
In the case of TRALI which occurs in 0.04-0.16 % of
transfusions, almost all types of blood products have been associated
with adverse events, such as packed red blood cells (RBC), fresh frozen
plasma and platelets.[1,2] TRALI has been estimated to
be the third cause of transfusion-related mortality with current
mortality rates ranging in the patients affected between 5 to 25%.[4,6]
About
two-thirds of the TRALI incidences are thought to be immune-mediated
and involve mainly the passive transfusion of leucocyte antibodies in
blood products.[7] Antibody-mediated TRALI is an
important cause of transfusion-associated morbidity and is the leading
cause of transfusion-related mortality.[7]
Human leukocyte antigen (HLA)-Class I, HLA-Class II or
neutrophil-specific antibodies, particularly HNA-3a have been
implicated in most of the reported cases of TRALI.[3,8]
The
mechanism for TRALI in thalassaemia involves mainly the transfer of
blood donor antibodies through contamination of the transfused RBC and
reaction with the anti-HLA antigens of lymphocytes of the recipient,
affecting mainly the lung endothelium of the recipient causing
pulmonary oedema. Blood donor antibodies are particularly prevalent in
some categories of blood donors such as multiparous women, where
antibodies are formed in response to sensitisation from foetal blood
infiltration during multiple pregnancies.[3]
Case Report
Approval
of the report of the cases was obtained from the committee of clinical
studies of the Ministry of Health and the Bioethics Committee of
Cyprus. The patients gave their informed consent for reporting the
study.
Two male thalassaemia major patients of 28 (A) and 31 (B)
years had separate episodes of the TRALI syndrome in 2004 and 2011
respectively, which were caused as a result of the transfusion of
packed RBC from the same multiparous woman blood donor. During the
period of TRALI, patient A was splenectomised with a mean rate of RBC
transfusions of 186 ml/kg/year while patient B had splenomegaly
(19x11cm) and was hypertransfused with a mean rate of RBC transfusions
of 366 ml/kg/year. Patient A was non-iron loaded with serum ferritin
246 μg/l, magnetic resonance imaging (MRI) T2* of the heart 23.4 ms and
liver 17.0 ms and other clinical complications included osteopenia,
hypogonadism and cholelithiasis. Patient B was iron loaded with serum
ferritin 2790 μg/l, MRI T2* of the heart 40.9 ms and liver 5.9 ms and
in addition to cholecystectomy and splenomegaly, clinical complications
included osteopenia and hypothyroidism. Patient A was receiving
deferiprone and patient B deferoxamine for the treatment of iron
overload, as well as other drugs for the treatment of other clinical
complications of the underlying disease.
The diagnosis of the
TRALI syndrome in each case was generally difficult because of the
rarity of the complication, number of symptoms and the timing of the
event. Despite the diagnostic difficulties, the symptoms in both cases
were the same (Table 1). A
difference in the timing of the initiation of the clinical symptoms due
to TRALI was observed between the two patients. In the patient A, the
acute respiratory distress symptoms began in about 10-15 minutes after
the initiation of the transfusion involving 15-20 ml from one unit of
320 ml of packed RBC from one blood donor. In the patient B, the
symptoms began at home at about 60 minutes following the transfusion of
two RBC units of 300 ml and 280 ml from two different blood donors
respectively.
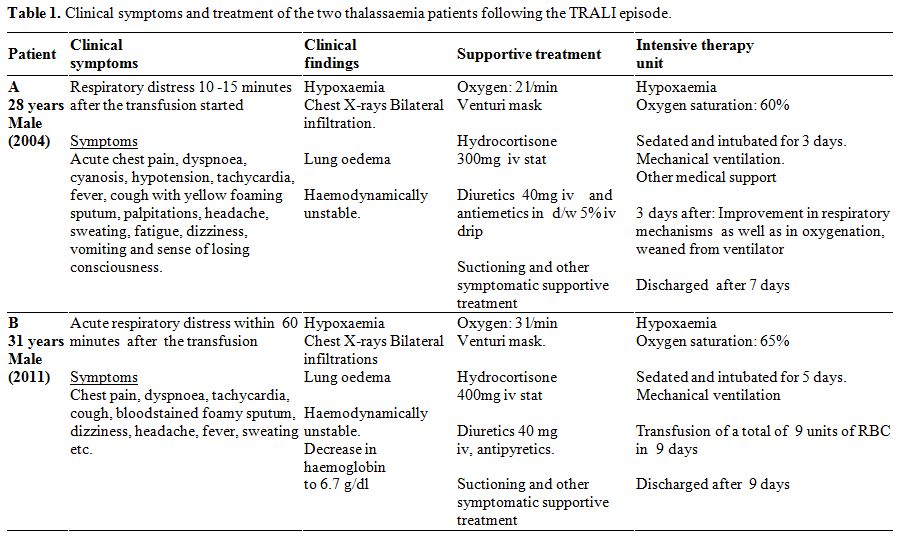 |
Table
1. Clinical symptoms and treatment of the two thalassaemia patients following the TRALI episode. |
In
both cases, the patients presented a number of symptoms including
dyspnoea, sweating, fatigue, dizziness, fever, and sense of losing
consciousness (Table 1).
Clinical and laboratory investigations indicated the presence of
hypoxaemia with oxygen saturation in patients A and B of less than
60% and 65% respectively, increased respiratory rate, low blood
pressure and increased pulse rate. The chest x-ray showed pulmonary
oedema with both lungs filled with fluid (Table 1, Figure 1).
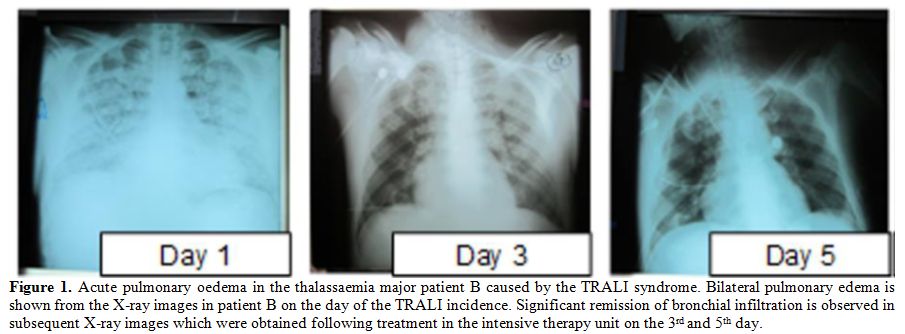 |
Figure 1. Acute pulmonary oedema in the
thalassaemia major patient B caused by the TRALI syndrome. Bilateral
pulmonary edema is shown from the X-ray images in patient B on the day
of the TRALI incidence. Significant remission of bronchial infiltration
is observed in subsequent X-ray images which were obtained following
treatment in the intensive therapy unit on the 3rd and 5th day. |
Supportive
treatment included the administration of oxygen, adrenaline, cortisone,
diuretics, suctioning and other symptomatic treatment before admission
to the intensive therapy unit, where further deterioration of lung
function was observed which led to sedation and intubation on a
mechanical ventilator (Table 1).
Improvement in the respiratory parameters including oxygenation,
remission of the lung oedema, as well as RBC transfusions and other
medical support allowed the patients to be weaned off the ventilator
and discharged from the intensive therapy unit following the
hospitalisation of a total of 7 days and 9 days for patients A and B
respectively.
In both cases, the laboratory findings suggested
the cause of an allergic reaction as a result of the transfusion. In
the patient A, high lymphocyte count (7.83%) was detected in the
transfused packed RBC. Furthermore, on the first day of the TRALI
episode the T cell marker count of CD5 and CD7 increased to 48% and 54%
respectively, the anti-RBC antibodies to 48% and the anti-HLA
antibodies to 20%. On the second day following the episode the CD5 and
CD7 counts decreased to 25% and 28% respectively, the anti-RBC
antibodies to 20% and the anti-HLA antibodies remained unchanged at
20%.
In the case of patient B, HLA-typing was performed on both
the patient’s and the blood donors’ samples, as well as HLA-specific
antibody testing on the donor's serums. A multiparous woman blood donor
was found to be positive for the presence of IgG HLA-Class I and -Class
II antibodies against the following mismatched antigens of patient B:
HLA -A30, -A33, -B8, -DR4, and -DR17. The complement cytotoxicity
cross-match against the T and B patient lymphocytes was also positive.
The
TRALI syndrome was diagnosed in both cases as a result of the presence
of residual plasma and leucocytes in the RBC transfusion, which was
associated with HLA-specific antibodies of the multiparous woman blood
donor against mismatched HLA-antigens of the transfused patients A and
B.
The multiparous blood donor related to the TRALI incidences was
a mother of four children and had in total six pregnancies. Following
the first incidence of TRALI, she was advised to terminate blood
donations. However, seven years later she ignored the earlier advice
and donated blood which resulted in the second TRALI incidence. Discussion
To
our knowledge, these are the first cases of thalassaemia patients who
had a TRALI episode originating from the same blood donor. It appears
that despite significant efforts to eliminate or minimise the risks of
toxicity associated with transfusions, the immunological toxic side
effects are evident, especially in chronically transfused patients.
Further improvements in haemovigilance are essential for reducing the
morbidity and mortality associated with transfusions.
In the
case of thalassaemia major, chronic transfusions cause alloantibody
formation in response to the transfused RBC. In one study it was
estimated that 22% of the thalassaemia patients have alloantibodies,
with a higher rate in splenectomised (36%) compared to
non-splenectomised (13%) patients.[9,10] Similarly,
autoantibodies (Coombs +ve) are found in about 25% and autoantibodies
with alloantibodies in 44%, which is higher in splenectomised
thalassaemia patients (56%).[9,10] The presence of
autoantibodies and alloantibodies appear to increase with age due to
exposure to more antigens from different blood donors as a result of
the increase in the number of transfusions. It is mainly observed in
older thalassaemia patients, especially when considering that with the
recent introduction of improved treatments life expectancy has
increased and thalassaemia patients are living longer and approaching
normal lifespan.[11]
Allergic and
febrile reactions are common side effects of RBC transfusions in
thalassaemia patients. Similarly, other common side effects include
delayed haemolytic reactions and bacterial or viral (Hepatitis B and C)
infections.[12]
Despite that TRALI is a
rare syndrome, the symptoms are rapid and life-threatening. The
patients affected by TRALI should be urgently diagnosed and receive the
appropriate emergency treatment. Recipient predisposition and number of
other factors appear to affect the aetiology and prognosis of
individual patients with TRALI.[3,13,14]
In
relation to haemovigilance and TRALI, the findings of this study
signify and suggest that additional steps should be taken about the
TRALI prevention measures which are commonly adopted, i.e. the
prevention from plasma donation of female donors with previous
pregnancies or any other donors who had received transfusions. In
particular, in the light of the present findings, the permission for
RBC donation of individuals from the above groups should be
reconsidered since it may prove ineffective from preventing TRALI in
similar cases.[2-4,13,14]
Within
the context of hemovigilance, there is also a need for the preparation
of further guidelines at the national and international level to
minimise the incidence of TRALI. For example, a male-only strategy for
RBC or other blood product donation for transfusions could decrease the
incidence of TRALI in thalassaemia and other similar categories of
patients.[14] Similarly, RBC and other blood
component samples from multiparous women donors should be thoroughly
tested for the presence of IgG HLA-Class I and -Class II antibodies and
excluded from blood donation in thalassaemia or other high-risk
categories of patients.[3,4,13,14]
Acknowledgements
This
work was supported by internal funds of the Postgraduate Research
Institute of Science, Technology, Environment and Medicine. We would
like to thank Drs A. Varnavidou-Nicolaidou and D. Voniatis of the
Nicosia General Hospital for the immunological studies and other staff
of the Paphos General Hospital involved in the medical care of the two
patients.
Dedication
This paper is dedicated to Evdoxia Mantilari Kontoghiorghe, who passed away in February 2017.
References
- Webert KE, Blajchman MA. Transfusion-related acute lung injury. Transfus Med Rev. 2003;17:252-262. https://doi.org/10.1016/S0887-7963(03)00039-7

- Teofili
L, Bianchi M, Zanfini BA, Catarci S, Sicuranza R, Spartano S et al.
Acute lung injury complicating blood transfusion in post-partum
hemorrhage: incidence and risk factors. Mediterr J Hematol Infect Dis.
2014;6:e2014069. https://doi.org/10.4084/mjhid.2014.069 PMid:25408855 PMCid:PMC4235434

- Peters
AL, Van Stein D, Vlaar AP. Antibody-mediated transfusion-related acute
lung injury; from discovery to prevention. Br J Haematol.
2015;170:597-614. https://doi.org/10.1111/bjh.13459 PMid:25921271

- Moalic
V, Vaillant C, Ferec C. Transfusion related acute lung injury (TRALI):
an unrecognised pathology. Pathol Biol (Paris). 2005;53:111-115. https://doi.org/10.1016/j.patbio.2004.06.001 PMid:15708656

- Bulanov AIu. Transfusion-associated lung injury (TRALI): obvious and incomprehensible. Anesteziol Reanimatol. 2009;5:48-52
 .
.
- Porretti
L, Cattaneo A, Coluccio E, Mantione E, Colombo F, Mariani M.
Implementation and outcomes of a transfusion-related acute lung injury
surveillance programme and study of HLA/HNA alloimmunisation in blood
donors. Blood Transfus. 2012;10:351-359. PMid:22395353 PMCid:PMC3417735

- Muller
MC, Porcelijn L, Vlaar AP. Prevention of immune-mediated
transfusion-related acute lung injury; from bloodbank to patient. Curr
Pharm Des. 2012; 18: 3241-3248. https://doi.org/10.2174/1381612811209023241 PMid:22621273

- Keller-Stanislawski
B, Reil A, Günay S, Funk MB. Frequency and severity of
transfusion-related acute lung injury- German haemovigilance data
(2006-2007). Vox Sang. 2010;98:70-77. https://doi.org/10.1111/j.1423-0410.2009.01232.x PMid:19671122

- Singer
ST, Wu V, Mignacca R, Kuypers FA, Morel P, Vichinsky EP.
Alloimmunization and erythrocyte autoimmunization in
transfusion-dependent thalassemia patients of predominantly asian
descent. Blood. 2000;96:3369-3373. PMid:11071629

- Chaudhari CN. Red Cell Alloantibodies in multiple transfused thalassaemia patients. MJAFI 2011; 67: 34-37. https://doi.org/10.1016/S0377-1237(11)80008-0

- Kolnagou
A, Kontoghiorghe CN, Kontoghiorghes GJ. Transition of Thalassaemia and
Friedreich ataxia from fatal to chronic diseases. World J Methodol.
2014;4:197-218. https://doi.org/10.5662/wjm.v4.i4.197 PMid:25541601 PMCid:PMC4274580

- Prati
D. Benefits and complications of regular blood transfusion in patients
with beta-thalassaemia major. Vox Sang.2000;79:129-137. https://doi.org/10.1046/j.1423-0410.2000.7930129.x PMid:11111230

- Silliman
CC, Boshkov LK, Mehdizadehkashi Z, Elzi DJ, Dickey WO, Podlosky L et
al. Transfusion-related acute lung injury: epidemiology and a
prospective analysis of etiologic factors. Blood. 2003;101: 454-462. https://doi.org/10.1182/blood-2002-03-0958 PMid:12393667

- Lucas
G, Win N, Calvert A, Green A, Griffin E, Bendukidze N, et
al.Reducing the incidence of TRALI in the UK: the results of screening
for donor leucocyte antibodies and the development of national
guidelines.Vox Sang. 2012;103:10-17. https://doi.org/10.1111/j.1423-0410.2011.01570.x PMid:22150747

[TOP]







 .
. 







