Marzia Varettoni1, Irene Defrancesco2, Luca Diamanti3, Enrico Marchioni3, Lisa Maria Farina4 and Anna Pichiecchio4
1 Department of Hematology Oncology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
2 Department of Molecular Medicine, University of Pavia, Pavia, Italy
3 Neuroscience Consortium, University of Pavia, Monza Policlinico and Pavia Mondino, Pavia, Italy
4 IRCCS, 'C. Mondino' National Neurological Institute, Pavia, Italy
Corresponding
author: Marzia Varettoni. Department of Hematology Oncology Fondazione IRCCS Policlinico San Matteo, Pavia, Italy. E-mail:
m.varettoni@smatteo.pv.it
Published: October 18, 2017
Received: July 19, 2017
Accepted: September 17, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017061 DOI
10.4084/MJHID.2017.061
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
The
Bing-Neel syndrome is a rare neurological complication of Waldenström’s
Macroglobulinemia which results from a direct involvement of central
nervous system by malignant lymphoplasmacytic cells. The clinical
suspicion of Bing-Neel syndrome may be overlooked because neurologic
symptoms are heterogeneous, nonspecific and sometimes underhand. A
definitive diagnosis of Bing-Neel syndrome can be confidently made
using brain and spinal cord magnetic resonance imaging as well as
histopathology and/or cerebrospinal fluid analysis to confirm the
neoplastic infiltration of central nervous system. The detection in the
cerebrospinal fluid of patients with Bing-Neel syndrome of the MYD88
(L265P) somatic mutation, which is highly recurrent in Waldenström’s
Macroglobulinemia, proved useful for the diagnosis and monitoring of
central nervous system involvement. Despite recommendations recently
published, there is still no clear consensus on treatment of Bing-Neel
syndrome, which includes systemic immunochemotherapy, intrathecal
chemotherapy and brain irradiation as possible options. Ibrutinib, a
Bruton kinase inhibitor approved for Waldenström’s Macroglobulinemia,
has been recently added to the therapeutic armamentarium of Bing-Neel
syndrome due to its ability to pass the blood-brain barrier. However,
prospective clinical trials are eagerly awaited with the aim to define
the optimal treatment strategy.
Here we describe four
illustrative cases of Bing-Neel syndrome diagnosed and treated at our
Institution and review the literature on this topic.
|
Introduction
Waldenström’s
Macroglobulinemia (WM) is a rare B-cell lymphoproliferative disorder
characterized by the presence of a serum IgM paraprotein associated
with bone marrow infiltration by lymphoplasmacytic lymphoma (LPL).[1]
Although WM is primarily localized in the bone marrow, up to 15-20% of
patients has an extramedullary disease with lymphadenopathies and/or
splenomegaly, while extranodal involvement is uncommon.[2]
The
Bing-Neel syndrome (BNS) is a rare neurological complication of WM
resulting from a direct infiltration of central nervous system (CNS) by
lymphoplasmacytic cells that may occur at any time during the course of
the disease.[3] BNS was first described in 1936 by
Jens Bing and Axel Valdemar Neel who observed two women with
neurological symptoms in the setting of hyperglobulinemia, in whom no
evidence of myeloma was found at autopsy.[4]
Limited
information about the incidence, clinical presentation, prognosis and
treatment of BNS is currently available in the literature. Since the
first description, approximately 50 patients with BNS have been
reported as case reports, while in the last few years two retrospective
series including 44 and 34 patients respectively have been published.[5,6]
During
the 8th International Workshop of WM held in London in 2014 a task
force on BNS was established with the aim to produce practical
guidelines for the diagnosis and treatment of BNS.[7]
Here
we present four cases of BNS diagnosed and treated at a single
institution between 2012 and 2016, and review the literature on this
rare complication of WM.
Description of Cases
Patient 1. This case was partially reported in a previous publication[8]
and here updated with a longer follow-up. A 64-year-old man was
admitted to hospital for confusion, progressive cognitive decline,
slurred speech, and ataxia. Cerebro-spinal fluid (CSF) analysis showed
a high white blood cell (WBC) count (196/mm3),
an elevated protein level (67 mg/dl) with normal glucose values (73
mg/dl). Flow cytometric analysis showed a clonal B lymphocyte
population CD19+, CD20+, CD22+, SIg+, CD5+, CD23-, CD10-, FMC7+, CD79b+
representing 73% of WBC. Brain and spinal MRI showed communicating
normal pressure hydrocephalus and both subtentorial and hemispheric
leptomeningeal enhancement after gadolinium (Figure 1A).
An IgM kappa monoclonal (M) protein was found in the serum (3 g/L).
Bone marrow biopsy showed infiltration by LPL (30% of cellularity). The
MYD88 (L265P) mutation was detectable by allele-specific PCR on bone
marrow CD19+ mononuclear cells. Total body computed tomography (CT)
scan revealed multiple bone lesions in the pelvis, and
18-Fluorodeoxyglucose Positron Emission Tomography (18-FDG-PET) showed
an abnormally high uptake in the pelvis, with a maximum standardized
uptake value (SUV) of 7.6. The biopsy of the largest bone lesion showed
an infiltration by lymphoplasmacytic lymphoma. The final diagnosis was
WM with BNS as presenting symptom. The patient was initially treated
with immunochemotherapy with R-HyperC-VAD (rituximab plus high-dose
methotrexate and cytarabine alternating with hyperfractionated
cyclophosphamide, vincristine, doxorubicin, and dexamethasone) but
treatment was withheld after the first dose of methotrexate due to
acute renal failure and worsening of patient’s clinical conditions.
Three weekly intrathecal administrations of methotrexate 15 mg were
given. The patient was considered ineligible to further intensive
chemotherapy and then switched to Rituximab plus Bendamustine (28-day
cycles with Rituximab 375 mg/m2 day 1 and Bendamustine 90 mg/m2
days 1-2) associated with intrathecal Methotrexate 15 mg on day 1 of
each cycle. At the end of treatment, the patient’s clinical conditions
were markedly improved. Serum paraprotein after therapy was 0.5 g/L.
MRI of the brain showed the persistence of communicating hydrocephalus
and almost complete disappearance of the leptomeningeal enhancement (Figure 1B).
Flow cytometry on CSF was normal. Bone marrow biopsy showed the
complete regression of lymphoplasmacytic infiltration, and MYD88
(L265P) mutation was undetectable on bone marrow CD19+ mononuclear
cells. MRI of the pelvis showed a reduction of bone lesions, with a
normal uptake at 18-FDG-PET. After 6 months, the patient underwent
autologous stem cells transplantation. The patient is in complete
remission, with undetectable monoclonal protein in serum and urine 21
months after transplant.
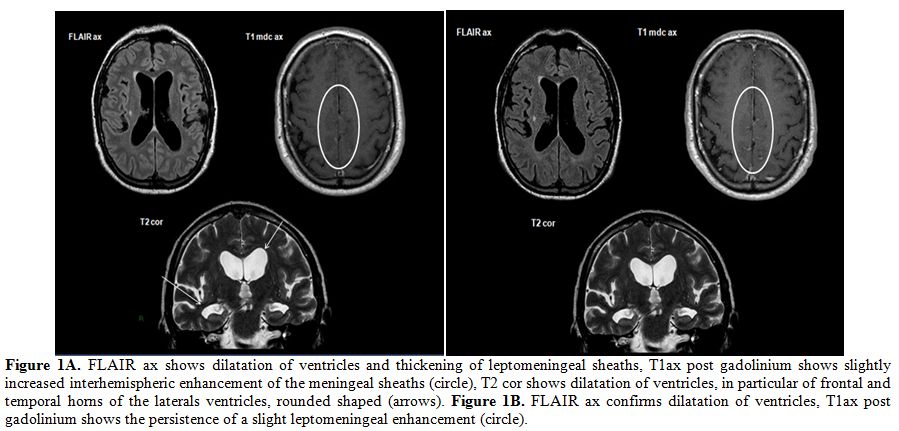 |
Figure 1A. FLAIR ax shows dilatation of
ventricles and thickening of leptomeningeal sheaths, T1ax post
gadolinium shows slightly increased interhemispheric enhancement of the
meningeal sheaths (circle), T2 cor shows dilatation of ventricles, in
particular of frontal and temporal horns of the laterals ventricles,
rounded shaped (arrows). Figure 1B. FLAIR ax confirms dilatation of
ventricles, T1ax post gadolinium shows the persistence of a slight
leptomeningeal enhancement (circle). |
Patient 2.
A 60-year-old woman was admitted to hospital because of ataxia and a
distal sensitive-motor deficit to the four limbs. Electroneurography
(ENG) and electromyography (EMG) showed a severe sensory-motor
demyelinating polyneuropathy. Blood analyses revealed the presence of a
small serum IgM kappa M protein (7.3 g/L) and presence of anti-Myelin
Associated Glycoprotein (MAG) antibodies (title 1:193000). Brain and
spinal MRI revealed thickening and contrast enhancement of spinal
leptomeninges and roots of cauda equina, shaded enhancement of pia
mater and ependyma and of bilateral internal auditory meatus (Figure 2A and 2B).
The CSF analysis showed an increased WBC count (105/mmc) and a high
protein level (121 mg/dl) reflecting blood-brain barrier disruption.
Cytofluorimetric analysis of CSF identified the presence of monotypic
CD20+ CD5+, CD23-, CD10- lymphocytes with kappa chain monoclonal
restriction, representing 84% of WBC. The bone marrow biopsy showed a
lymphoplasmacytic infiltration (60-70% of cellularity) consistent with
an LPL. MYD88 and CXCR4 mutation status were evaluated on
CD19+-selected bone marrow mononuclear cells using allele-specific PCR
and Sanger sequencing respectively. The patient was found to be
MYD88-mutated and CXCR4-wild type.
Six 28-day cycles of Rituximab (375 mg/m2 day 1) and Bendamustine (90 mg/m2
days 1-2) associated with six intrathecal injections of methotrexate
(15 mg day 1) were administered. At the end of therapy, chemistry and
cytofluorimetry on CSF were normal. Neurological symptoms remained
stable while post-treatment MRI showed the absence of contrast
enhancement in the spinal cord and cauda equina. Bone marrow biopsy was
normal. These findings, taken together, were consistent with a partial
response according to current guidelines.[7] After three months neurological symptoms worsened. Brain and spinal MRI (Figure 2C and 2D)
showed thickening of roots of cauda equina and shaded contrast
enhancement of medullary cone and leptomeninges in the posterior
cranial fossa. Cytofluorimetric CSF analysis detected a clonal B
lymphocyte population, accounting for 44% of WBC, indicating CNS
progression of the disease. Since Ibrutinib was shown to pass the
blood-brain-barrier and to be active in BNS,[9,10] treatment was started in March 2017.
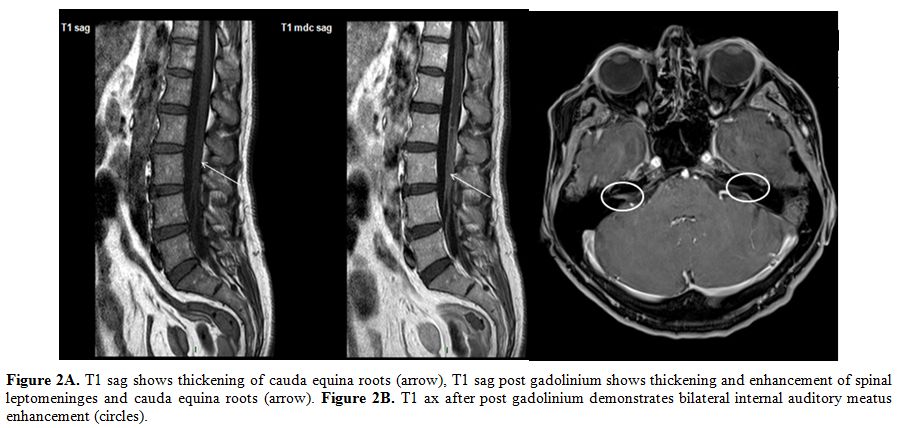 |
Figure 2A. T1 sag shows thickening of
cauda equina roots (arrow), T1 sag post gadolinium shows thickening and
enhancement of spinal leptomeninges and cauda equina roots (arrow).
Figure 2B. T1 ax after post gadolinium demonstrates bilateral internal
auditory meatus enhancement (circles). |
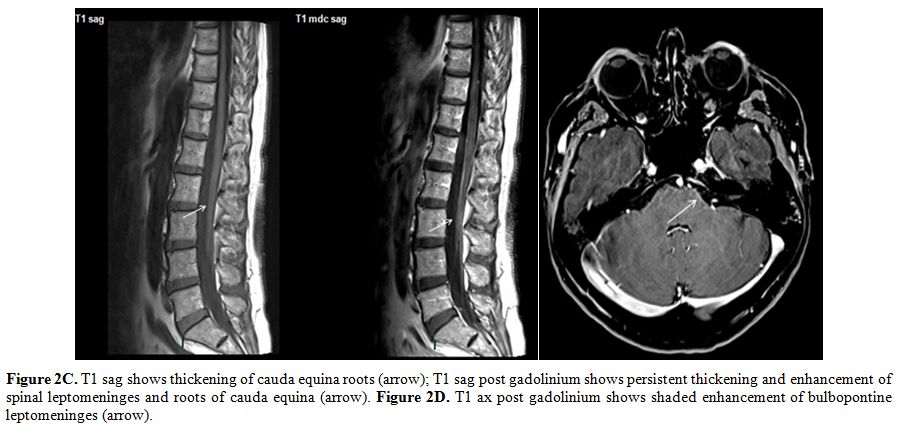 |
Figure 2C. T1 sag shows thickening of
cauda equina roots (arrow); T1 sag post gadolinium shows persistent
thickening and enhancement of spinal leptomeninges and roots of cauda
equina (arrow). Figure 2D. T1 ax post gadolinium shows shaded
enhancement of bulbopontine leptomeninges (arrow). |
Patient 3.
A 68-year-old man was admitted to hospital for fatigue, weight loss,
pain and motor deficit to the lower limbs. Blood cell counts were
normal. Serum electrophoresis revealed the presence of an IgM kappa M
protein of 17.5 g/L. Bone marrow biopsy demonstrated an LPL with a bone
marrow infiltration of 60% associated with interstitial and
perivascular deposits of amyloid. MYD88 L265P mutation was found by
allele-specific PCR on bone marrow CD19+ mononuclear cells. Fat pad
biopsy was also positive for amyloid deposits. Brain and spinal MRI
detected leptomeningeal disease infiltration of the spinal cord and
cauda equina (Figure 3). The CSF analysis revealed WBC count of 11/mm3
and an elevated protein level of 382 mg/dl reflecting severe disruption
of the blood-brain barrier. Flow cytometric analysis of CSF showed
infiltration by clonal B lymphocytes CD19+, CD20+, CD22+, CD5-, CD10-,
CD23-. EMG and ENG showed demyelinating sensitive-motory polyneuropathy
to upper and lower limbs. The final diagnosis was WM complicated by AL
amyloidosis with initial cardiac involvement, BNS, and peripheral
neuropathy.
Six cycles of Rituximab (375 mg/m2 day 1) and Bendamustine (90 mg/m2
days 1-2) with six intrathecal injections of Methotrexate (15 mg day 1)
were administered. At the end of therapy, we observed a reduction
>50% of M protein and bone marrow infiltration and resolution of
lymphadenopathies. Spinal cord MRI showed the absence of contrast
enhancement in the spinal cord and cauda equina. CSF analysis showed
elevated protein level without malignant cells by flow cytometry. These
findings were consistent with a complete response of BNS according to
current guidelines.
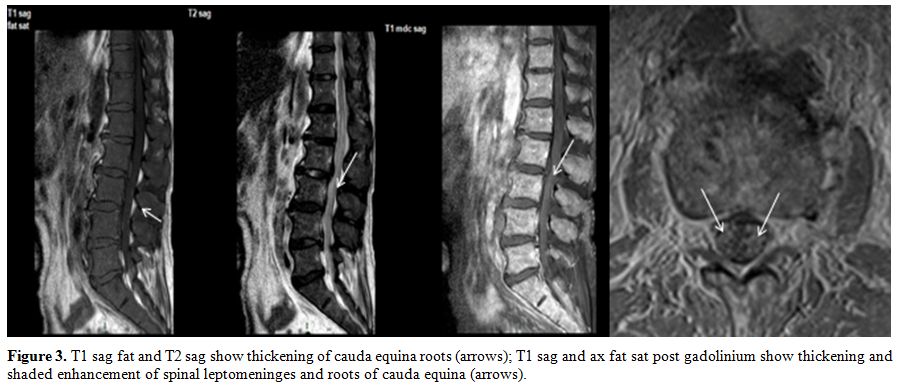 |
Figure 3. T1 sag fat and T2 sag show
thickening of cauda equina roots (arrows); T1 sag and ax fat sat post
gadolinium show thickening and shaded enhancement of spinal
leptomeninges and roots of cauda equina (arrows). |
Patient 4.
A 38-year-old man was diagnosed with LPL associated with a serum IgG
kappa M protein in 2012. At the time of diagnosis, the patient had
systemic symptoms, multiple adenopathies and a bone marrow infiltration
of 70%. The patient was refractory to first-line treatment with six
21-day cycles of R-CHOP and developed Rituximab intolerance after the
third cycle. During salvage therapy with DHAP (Cisplatin, high dose
cytarabine, and dexamethasone), the patient had a focal seizure crisis
with secondary generalization. CSF analysis revealed an elevated
protein level with no detectable lymphoid cells. Brain MRI showed a
cortical-subcortical right temporal area with enhanced contrast,
consistent with CNS parenchymal localization of lymphoma (Figure 4A),
while the CT scan demonstrated progression of adenopathies. A biopsy of
the brain lesion was not feasible. The patient was refractory to
treatment with ICE chemotherapy (Ifosfamide, carboplatin, etoposide)
and hyper-CVAD and was then treated with six 28-day cycles of
Bendamustine (90 mg/m2 days 1-2) associated with six doses of intrathecal Methotrexate (day 1). At the end of treatment, the brain MRI was normal (Figure 4B),
and CT scan showed regression of lymphadenopathies. Bone marrow biopsy
was negative, and no M-protein was detectable in serum or urine. In
conclusion, patient obtained complete remission of LPL and BNS. After
15 months the patient had an isolated CNS relapse. He was treated with
high-dose cytarabine without response and then with total brain
irradiation (24 Gy) which induced a clinical improvement and
significant reduction of hemispheric lesions at MRI.
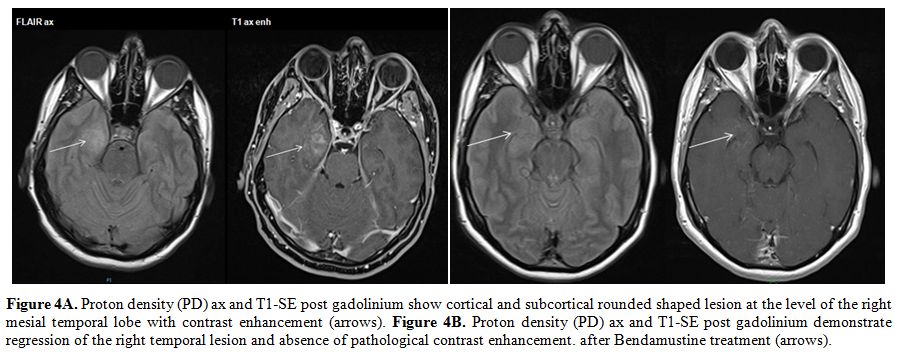 |
Figure 4A. Proton density (PD) ax and
T1-SE post gadolinium show cortical and subcortical rounded shaped
lesion at the level of the right mesial temporal lobe with contrast
enhancement (arrows). Figure 4B. Proton density (PD) ax and T1-SE post
gadolinium demonstrate regression of the right temporal lesion and
absence of pathological contrast enhancement. after Bendamustine
treatment (arrows). |
Discussion of Cases and Review of the Literature
What is the Incidence of BNS?
The exact incidence of BNS is unknown. The incidence of BNS in
retrospective studies is likely to be underestimated because the
awareness of this potential complication of WM has only recently
increased, as witnessed by the publication of two retrospective series
in the last few years.[5,6] Besides the four cases
reported here, two more cases had been previously diagnosed at our
Institution. Therefore, 6 cases were diagnosed since 2003 (when current
diagnostic criteria of WM were established) to 2016 in a series of 186
WM patients, corresponding to a prevalence of 3.2 %. Anyway,
prospective observational studies are needed to address this issue.
When does BNS Occur During the Disease Course? BNS may occur at any time during the course of the disease.[3]
In three of four cases here reported BNS was the first presenting
symptom in patients without a previous history of WM, whereas the last
patient developed CNS involvement eight months after the diagnosis of
WM. In the international multicentric retrospective study conducted by
Castillo, the diagnosis of BNS was concomitant with the diagnosis of WM
in one-third of cases and subsequent in two thirds. In the latter
scenario, the median time interval between diagnosis of WM and the
diagnosis of BNS was 8.9 years.[6]
BNS may occur
independently of a systemic progression of WM and may also present when
patients are receiving WM-directed therapy, even in patients in
complete remission. As CNS is a well-known “sanctuary site”, not
reached by most drugs used to treat WM, the occurrence of isolated CNS
progression is not unexpected.
What Are the Symptoms of BNS? Clinical
presentation of BNS is extremely heterogeneous without any specific
sign or symptom: the most frequent neurological manifestations are
balance disorders with ataxia (48%) or cranial nerve involvement (36%,
mainly facial and oculomotor nerve). Other symptoms include headache,
cognitive impairment with frontal syndrome, memory loss or dementia
(27%), paresis and motor symptoms, sensory symptoms (25%) such as
dysesthesia, paresthesia, psychiatric symptoms, headache (18%), cauda
equina syndrome (14%), motor deficits (14%), blurred vision.[5]
Convulsions, hemiparesis or aphasia may occur in the tumoral form.
Symptoms are gradually progressive, generally developing in weeks or
months.[7] Since symptoms are often nonspecific,
clinical suspicion of BNS is essential. The presence of a concomitant
peripheral neuropathy, as in two of the four cases here described, may
be misleading and delay the diagnosis of BNS. Of note, the median time
between onset of neurological symptoms and the diagnosis of BNS in the
French study was 4 months (range 0-36) and more than 1 year in 20% of
cases.[5]
When Should BNS be Suspected?
Clinical suspicion of BNS is based on the presence of neurological
symptoms in patients with an already established diagnosis of WM or
with an IgM monoclonal protein in the serum.
The differential
diagnosis of BNS mainly includes hyperviscosity syndrome (HVS) and
levels of IgM along with an evaluation of serum viscosity may be useful
to distinguish HVS from BNS.[11] Some neurological
symptoms of BNS may mimic those of peripheral neuropathies with
anti-MAG antibodies which may occur in WM and other IgM-related
disorders.[5] Patients with anti-MAG antibodies mostly
present with a sensory ataxia and distal muscle weakness which slowly
develops over the years.[12] Since symptoms of BNS
and those of peripheral neuropathy may be overlapping and the two
conditions may coexist in the same patient, WM patients with a
peripheral neuropathy should be carefully evaluated by an expert
neurologist to exclude a concomitant involvement of CNS, in particular,
an infiltration of cauda equina.
How many Forms of BNS Do Exist?
CNS involvement may occur in two forms: the majority of BNS patients
(75% in the French study, more than 90% in the series reported by
Castillo) present a diffuse form with leptomeningeal enhancement on
imaging. The tumoral form is less common and is characterized by the
presence of one or more parenchymal lesions, and in these cases,
patients usually present with focal neurologic deficits.[13]
It is more challenging to diagnose because biopsy is not easily
feasible in most cases. In the fourth case here reported the
parenchymal lesion could not be biopsied, but the regression of CNS
lesion as well as of lymphadenopathies after treatment with
Bendamustine confirmed ex-post the diagnosis of BNS. In this patient,
CNS involvement could be consistent with BNS resulting from a direct
infiltration of lymphoplasmacytic cells, even though the serum M
protein was not an IgM and therefore the diagnosis was
lymphoplasmacytic lymphoma rather than WM.
Which Tests Are Necessary for the Diagnosis of BNS?
MRI of the brain and spinal cord is an essential test for the diagnosis
of CNS involvement by lymphoma, and it is also recommended in case of
suspected BNS due to its high sensitivity for the detection of
malignant infiltration.[7] In BNS, brain and spinal
cord MRI is abnormal in 78% of cases generally showing enhancement
and/or thickening of meningeal sheets, abnormal enhancement of cranial
and spinal nerves, thickening and enhancement of cauda equina. Imaging
alterations described above are supportive but not sufficient for the
diagnosis of BNS,[7] while the absence of MRI findings should not exclude BNS.[14]
However, the diagnosis of BNS in the absence of radiological
abnormalities should be made with caution and only after a
multidisciplinary discussion of the case.[5]
CSF
analysis should be performed after MRI to avoid endocranial
hypertension and non-specific meningeal enhancement that occurs after
CSF sampling. CSF analysis may show an elevated opening pressure,
elevated total protein (>100 mg/dl) reflecting the disruption of
blood-brain barrier, normal or decreased glucose and increased WBC
count (between 100 and 500 cells/mm3).[3,7]
In order to confirm the neoplastic infiltration of CSF and to exclude
inflammatory or infective causes, flow-cytometric analysis of CSF is
mandatory to demonstrate the presence of clonal B-cells with the same
immunophenotypic features as those in bone marrow BM. Of note, while a
positive test substantiates the diagnosis, negative results do not
exclude BNS considering the low sensitivity of cytological testing due
to the low number of neoplastic cells.[3] The presence
of an IgM monoclonal protein in the CSF per se does not indicate a
neoplastic infiltration of CNS, because if a blood-brain-barrier
disruption is present, the leakage of M-proteins from the blood into
CSF may occur due to increased permeability of the barrier. Although
not specific for the BNS, IgM-index calculation[15] could be used to identify a proper IgM production beyond the blood-brain barrier.
Involvement of the eye is rare[16,17] however it is recommended to consult a neuro-ophthalmologist in patients with visus or ocular motility impairment.
According
to criteria recently proposed, a definite diagnosis of BNS requires a
histological biopsy of cerebrum or meninges or the demonstration of a
clonal B cell population with the same with the typical phenotype of WM
in the CSF. Immunohistochemistry usually shows a malignant population
expressing the same antigens of WM cells, i.e. pan-B antigens (CD19,
CD20, CD79a, CD79b), in most cases also B-cell memory markers (CD27,
CD52), plasma cells markers (CD138 and IgM), while CD5 and CD3 are
expressed in a minority of cases.[1]
Differential
diagnosis has to take into account primary central nervous system
lymphoma (PCNSL) but also other indolent lymphomas or transformation to
high-grade lymphomas which involve the CNS.[7]
Are molecular tests essential for the diagnosis of BNS? Immunoglobulin
gene rearrangement analysis represents an essential tool able to
establish the clonal nature of the lymphoid B-cell population and the
clonal relationship between CNS and BM B lymphocytes, strongly
supporting the diagnosis of BNS.[7] In 2012, a somatic
mutation in the MYD88 gene leading to the substitution of a leucine
with a proline at position 265 (MYD88 L265P) was found to be highly
prevalent in WM patients.[18] Poulain et al.[19]
recently reported for the first time the diagnostic value of MYD88
L265P mutation detection in BNS patients. They identified a MYD88 L265P
mutation in the CSF and BM of all BNS cases using quantitative-PCR
(q-PCR) and Sanger sequencing. Molecular testing in CNS biopsy and CSF
might support the diagnosis of BNS and has recently been added to the
diagnostic armamentarium. Moreover, the disappearance of MYD88 L265P
mutation correlates with clinical response, suggesting a potential for
monitoring response to therapy and minimal residual disease.[19]
However, the MYD88 L265P mutation in the CNS biopsy or CSF samples is
not specific for BNS and has also been detected in one-third of
patients with primary central nervous system lymphoma (PCNSL).[20]
What is the Prognosis of BNS? There are no recognized prognostic factors for BNS. Simon L et al.[5]
in a retrospective series of 44 patients reported an overall survival
rate of 71% at 5 years and 59% at 10 years after the diagnosis of BNS,
while the median overall survival from the diagnosis of WM was 17.1
years.
In the series of Castillo et al.[6] the
estimated 3-year overall survival (OS) rate was 59%. Age >65 years,
previous treatment for WM and platelet count <100x109/L
were identified as adverse prognostic factors for survival in the
univariate analysis. These findings potentially suggest that BNS
occurring during the disease course may have a worse outcome compared
to BNS occurring at the time of diagnosis of WM.
What Are Treatment Approaches?
Treatment approaches are not uniform, reflecting the lack of
standardization for this rare entity. The choice of therapy should be
based on patient condition, medical history, preference and experience
of a physician.[7] In the recent retrospective surveys
of Simon and Castillo, the overall response rate (ORR) was 70% to
first-line therapy, and no differences could be made according to
treatment type. Remission has been reported either with intrathecal
injection and/or systemic chemotherapies, including high-dose
Methotrexate or Cytarabine which are able to penetrate the blood-brain
barrier. Intrathecal treatment should be combined with systemic
treatment since monotherapy with intrathecal drugs rarely induces
durable responses.[7]
Nucleoside analogs have
been demonstrated to pass the blood-brain barrier. Several previous
reports suggested that fludarabine was effective in Chronic Lymphocytic
Leukemia with the involvement of CNS.[21,22] Vos et al. recently reported the efficacy of Fludarabine for the treatment of BNS,[23]
confirming its usefulness as a therapeutic option. In our experience,
treatment with Rituximab-Bendamustine associated with intrathecal
Methotrexate was well tolerated and effective, representing a suitable
treatment option for BNS patients, especially for those who are not
eligible for intensive treatment.[8] Rituximab has
been used in largest series mostly associated with chemotherapy;
monotherapy is not advised due to its presumed low blood-brain barrier
penetration.
Ibrutinib, a BTK inhibitor, has recently introduced in the treatment of WM due to its efficacy in WM.[2]
Recent reports suggest that Ibrutinib either at the dose of 420mg or
560 mg is active and able to penetrate the blood-brain barrier[24,10] and pharmacodynamic studies show CSF diffusion with a good neuromeningeal distribution.[9]
BNS
is sensitive to radiotherapy (RT). Localized RT to affected regions
(20-40 Gy) is preferred to whole brain irradiation and may be used
alone or in combination with chemotherapy. Enhanced neurotoxicity has
been reported mainly in the elderly,[25] and cognitive impairment has been reported to occur after whole brain irradiation.[26]
Therefore, RT should not be considered a first-line therapy but should
be reserved for patients failing other treatment options.[7]
Although
there is no clear consensus about the role of autologous stem cell
transplantation in patients with BNS, frontline intensification seems
to be associated with long-term remissions.[5,10]
However, toxic deaths are described for autologous stem cell
transplantation so that transplantation should be considered only for
suitable patients.[5]
What is the Goal of Treatment in Patients with BNS? Treatment
should be considered in symptomatic patients with a definitive
diagnosis of BNS. The goal of treatment should be to reverse clinical
symptoms and increase overall survival, though a complete eradication
of all malignant cells is not always possible. In fact, in some cases,
the disease is still detectable on post-treatment CSF analysis, while
patients become asymptomatic. Radiologic lesions may persist after
successful treatment, but they do not necessarily constitute persisting
disease. Therefore treatment should be guided by the clearance of
patient’s symptoms.[7] Neurological sequelae could
determine the persistence of symptoms, due to the low regenerative
ability of CNS and PNS: they must not be interpreted as treatment
failure, but treatment should be continued until the best clinical
result is achieved.
How Should the Neurological Response be Evaluated after Treatment?
CSF response can be monitored during and after treatment: normalization
of CSF analysis indicates an adequate response. Detection of MYD88
L265P mutation using qPCR on CSF represents a promising useful
molecular tool to monitor response to chemotherapy[19] sequentially. Response criteria proposed in the recently published guidelines[7] are reported in Table 1.
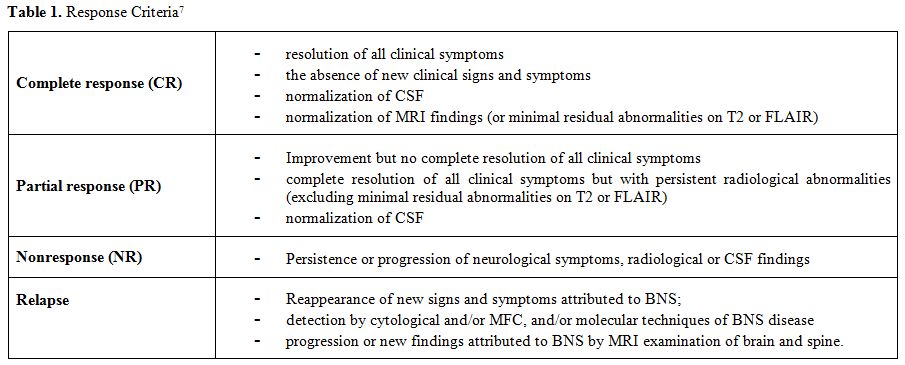 |
Table 1. Response Criteria[7] |
Conclusions and Open Issues
BNS
is a rare and probably under-recognized complication of WM which can
occur at any time during the course of the disease, even in patients
who are responding to systemic therapy. BNS should be suspected early
in patients with WM who develop unexplained neurological signs and
symptoms. Patients with an established diagnosis of WM, manifesting any
neurological symptom (including symptoms which could be consistent with
peripheral neuropathy) should be promptly evaluated by a
multidisciplinary team, in order to run the appropriate neurological
investigations for BNS. This attitude could shorten the time for the
diagnosis of BNS, potentially ameliorating outcome. MRI and CSF
analysis are essential for the diagnosis. The diagnostic accuracy of
BNS could be improved by the detection of MYD88 L265P mutation in CSF.
However further investigations are necessary to assess the utility of
this test for the diagnosis and evaluation of response. Treatment
remains challenging because of lack of standardization and information
about prognosis is still scanty. Therefore prospective studies are
eagerly awaited with the aim of better defining treatment strategies
and outcome, significantly improving our knowledge about this rare
complication of WM.
References
- Owen RG, Treon SP, Al-Katib A, et al.
Clinicopathological definition of Waldenstrom's Macroglobulinemia:
consensus panel recommendations from the Second International Workshop
on Waldenstrom's Macroglobuliemia. Semin Oncol, 2003; 30(2):110-115 https://doi.org/10.1053/sonc.2003.50082 PMid:12720118

- Treon SP. How I treat Waldenstrom Macroglobulinemia. Blood 2015; 126: 721-732 https://doi.org/10.1182/blood-2015-01-553974 PMid:26002963

- Malkani
RG, Tallman M, Gottardi-Littel N, et al. Bing Neel syndrome: an
illustrative case and a comprehensive review of the published
literature. J Neuroncol 2010, 96(3): 301-312 https://doi.org/10.1007/s11060-009-9968-3 PMid:19618118

- Bing
J, von Neel A. Two cases of hyperglobulinaemia with affection of the
central nerv-ous system on a toxi-infectious basis. Acta Med Scand
1936; 88: 492-506 https://doi.org/10.1111/j.0954-6820.1936.tb12571.x

- Simon
L, Fitsiori A, Lemal R et al, Bing-Neel syndrome, a rare complication
of Walden-ström's Macroglobulinemia: analysis of 44 cases and review of
literature. A study on be-half of the French Innovative Leukemia
Organization (FILO). Haematologica 2015; 100 (12): 1587-1594 https://doi.org/10.3324/haematol.2015.133744 PMid:26385211 PMCid:PMC4666335

- Castillo
JJ, D'Sa S, Lunn MP, et al. Central nervous system involvement by
Waldenstrom macroglobulinaemia (Bing-Neel syndrome): a
multi-institutional retrospective study. Br J Haematol 2016; 172:
709–715 https://doi.org/10.1111/bjh.13883 PMid:26686858 PMCid:PMC5480405

- Minnema
MC, Kimby E, D'Sa S et al, Guideline for the diagnosis, treatment and
response criteria for Bing-Neel syndrome. Haematologica, 2017;
102(1):43-51 https://doi.org/10.3324/haematol.2016.147728 PMid:27758817 PMCid:PMC5210231

- Varettoni
M, Marchioni E, Bonfichi M, et al. Successful treatment with Rituximab
and Bendamustine in a patient with newly diagnosed Waldenstrom's
Macroglobulinemia complicated by Bing-Neel syndrome. Am J Hematol 2015;
90: E152-E153 PMID:25975811

- Cabannes-Hamy
A, Lemal R, Goldwirt L, et al. Efficacy of Ibrutinib in the treatment
of Bing-Neel syndrome. Am J Hematol, 2016; 91(3): E17-19 https://doi.org/10.1002/ajh.24279 PMid:26689870

- Mason
C, Savona S, Rini JN, et al. Ibrutinib penetrates the blood brain
barrier and shows efficacy in the therapy of Bing Neel syndrome. Br J
Haematol, 2016; https://doi.org/10.1111/bjh.14218

- Stone MJ, Bogen SA. Evidence-based focused review of management of hyperviscosity syndrome. Blood 2012; 119(10): 2205-2208 https://doi.org/10.1182/blood-2011-04-347690 PMid:22147890

- Stork
AC, van der Pol WL, Franssen H, et al. Clinical phenotype of patients
with neuropa-thy associated with monoclonal gammopathy: a comparative
study and a review of the literature. J Neurol 2014; 261(7): 1398-1404 https://doi.org/10.1007/s00415-014-7354-3 PMid:24781837

- Grewal
JS, Brar JK, Sahijdak WM et al. Bing Neel syndrome: a case report and
systematic review of clinical manifestations, diagnosis and treatment
options. Clin Lymphoma Myeloma 2009; 9(6): 462-466 https://doi.org/10.3816/CLM.2009.n.091 PMid:19951888

- Gupta
N, Gupta S, Al Ustwani O, et al. Bing-Neel syndrome in a patient with
Walden-strom's macroglobulinemia: a challenging diagnosis in the face
of normal brain imaging. CNS Neurosci Ther 2014;20(10): 945-946 https://doi.org/10.1111/cns.12311 PMid:25102920

- Zetterberg. Patognomonic cerebrospinal fluid findings in Bing-Neel syndrome. J Neurooncol 2011; 104 (2): 615 https://doi.org/10.1007/s11060-010-0510-4 PMid:21221713

- Hughes
MS, Atkins EJ, Cestari DM et al. Isolated optic nerve, chiasm, and
tract involve-ment in Bing Neel syndrome. J Neuroophtalmol 2014;
34(4):340-345 https://doi.org/10.1097/WNO.0000000000000138 PMid:25409481

- Stacy RC, Jakobiec FA, Hochberg FH, et al. Orbital involvement in Bing Neel syndrome. J Neuroophtalmol, 2010; 30(3): 255-259 https://doi.org/10.1097/WNO.0b013e3181dee96c PMid:20548243

- Treon SP, Xu L, Yang G et al. MYD88 L265P somatic mutation in Waldenstrom's macro-globulinemia. N Engl J Med 2012; 367: 826-833 https://doi.org/10.1056/NEJMoa1200710 PMid:22931316

- Poulain
S, Boyle EM, Roumier C, et al. MYD88 L265P mutation contributes to the
diag-nosis of Bing Neel syndrome. Br J Haematol 2014; 167: 506-513 https://doi.org/10.1111/bjh.13078 PMid:25160558

- Montesinos-Rongen
M, Godlewska E, Brunn A, et al. Activating L265P mutations of the MYD88
gene are common in primary central nervous system lymphoma. Acta
Neuropathol 2011 Dec; 122(6):791-792 https://doi.org/10.1007/s00401-011-0891-2 PMid:22020631

- Elliott
MA, Letendre L, Li CY, et al. Chronic lymphocytic leukemia with
symptomatic dif-fuse central nervous system infiltration responding to
therapy with systemic fludarabine. Br J Haematol 1999; 104: 689-694 https://doi.org/10.1046/j.1365-2141.1999.01245.x PMid:10192427

- Knop
S, Herrlinger U, Ernemann U, et al. Fludarabine may induce durable
remission in patients with leptomeningeal involvement of chronic
lymphocytic leukemia. Leuk Lymphoma 2005; 46: 1593-1598 https://doi.org/10.1080/10428190500178472 PMid:16236614

- Vos
JM, Kersten MJ, Kraan W et al. Effective treatment of Bing Neel
syndrome with oral fludarabine: a case series of four consecutive
patients. Br J Haematol 2016; 172 (3): 461-464 https://doi.org/10.1111/bjh.13483 PMid:25944724

- Bernard
S, Goldwirt L, Amorim S et al. Activity of ibrutinib in mantle cell
lymphoma pa-tients with central nervous system relapse. Blood 2015;
126(14):1695-1698 https://doi.org/10.1182/blood-2015-05-647834 PMid:26239089 PMCid:PMC4591793

- Saad S, Wang TJ. Neurocognitive deficits after radiation therapy for brain malignancies. Am J Clin Oncol 2015; 38(6):634-640 https://doi.org/10.1097/COC.0000000000000158 PMid:25503433

- Tallet
AV, Azria D, Barlesi F et al. Neurocognitive function impairment after
whole brain radiotherapy for brain metastases: actual assessment.
Radiat Oncol 2012; 7:77 https://doi.org/10.1186/1748-717X-7-77 PMid:22640600 PMCid:PMC3403847

[TOP]




























