B.
Entasoltan1, M.A. Bekadja1,
H. Touhami2, N. Mehalhal3,
Z. Zouaoui4, N. Mesli5,
M. Talbi6, A. Bachiri7
and M. Michallet8
1
Service d’Hématologie et Thérapie Cellulaire, EHU1er Novembre, Oran,
Algérie
2 Service d’Hématologie, CHU Oran, Algérie
3 Service d’Hématologie, EPH Mascara, Algérie
4 Service d’Hématologie, CHU Sidi-Bel-Abbès,
Algérie
5 Service d’Hématologie, CHU Tlemcen, Algérie
6 Service d’Hématologie, CHU Béchar, Algérie
7 Service d’Hématologie, HMRU Oran, Algérie
8 Service d’Hématologie, CHU Lyon, France
Corresponding
author: Mohamed Amine Bekadja. Service d’Hématologie et Thérapie
Cellulaire, EHU1er Novembre, Oran, Algérie. E-mail:
mabekadja@yahoo.fr
Published: October 25, 2017
Received: July 23, 2017
Accepted: September 27, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017062 DOI
10.4084/MJHID.2017.062
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Introduction: In
a developing country like Algeria, such expensive therapy is not
available. Alternative approaches are needed to help these adult. In
Algeria ‘imatib’ (CIPLA-India) was introduced in 2006; but no study has
been published yet in the North Africa region regarding response and
outcome of this copy in CML patients. The goal of this multicenter
study is to characterize newly adult CML in the western region of
Algeria and to assess the effectiveness and safety of imatib (IM, copy)
as frontline therapy for patients with CML.
Patients and Methods:
The study was carried out in 7 hematology centers in the western
Algeria. Patients, who were diagnosed to be suffering from CML between
January 1st, 2007 and December 31st,
2014 were selected for data analysis. All patients received a copy
preparation, consisting of the alpha crystal form of imatinib, (IM,
copy) at an oral dose of 400 mg daily and monitored for tolerance and
side effects while on therapy.
Results:
Between January 2007 and December 2014, 355 patients with CML were
treated with imatib (Copy). The median follow- up of the study was 46
months (range: 13–107 months). Complete hematological response (CHR)
was seen in 83% of patients within 3 months. According to the Sokal
score, 72% patients with low, 78% with intermediate and 69% with high
risk disease achieved a CHR in 3 months (p=0.26) and according to the
EUTOS score, 81% of patients with low and 70% with high risk disease
achieved a CHR in 3 months (p=0.08). The major molecular response (MMR)
at six months (M6), M9, M12, M18 and M24 was 21%, 38%, 35%, 51% and 67%
respectively and 34% of patients achieved a complete molecular response
(CMR). The projected 5-year overall survival (OS) rate was 83%. Side
effects of imatib (copy) in this study were similar to those reported
previously for the entire imatinib mesylate treatment study and only 8%
of patients were intolerant to imatib (copy) and treated with a second
generation of BCR-ABL inhibitor.
Conclusion: This
study reflects real world experience treating patients with CML in a
developing country and thus sheds light on differences in this
population compared to Western countries. In conclusion, imatib (copy)
is effective and safe in treating patients with CML in chronic phase
and proves to have a durable outcome. To our knowledge this is the
first study reporting the response to imatib (copy) in an Algerian
population.
|
Introduction
Chronic
myeloid leukemia (CML) is a malignant disorder of the stem cell due to
reciprocal balanced translocation of genetic material between the long
arms of chromosomes 9 and 22 t (9; 22) (q34; q11).[1]
The translocation causes the production of a new hybrid gene (BCR/ABL)
that codes for a 210 Kb cytoplasmic protein (P210), which by
autophosphorylation activates some signaling pathways involved in cell
proliferation, maturation, apoptosis, and adhesion, leading to the
malignant cell transformation.[2]
Imatinib mesylate
(Glivec®/Gleevec®) is the standard first-line therapy for the treatment
of CML due to its high hematologic, cytogenetic, and molecular response
rates and favorable long-term safety profile.[3]
It
has been some years since TKI were introduced in CML therapeutic
strategy either in case of intolerance or resistance to Imatinib or as
a first line drug when it was observed that it has a more rapid and
profound molecular response when compared to Imatinib.[4]
More recently a third generation of TKI has been available in case of
intolerance or resistance to TKI of the second generation and
especially for patients who developed the resistance with a mutation
T315I.[5] Due to the fundamental
role of Imatinib in
first-line therapy of CML, as well as the high cost of Imatinib, which
is not affordable in some countries, a copy preparation, consisting of
the alpha crystal form of imatinib, has become commercially available
under the name ‘imatib’ (CIPLA-India). It is currently available at a
markedly reduced price in several countries. The manufacturer of imatib
(IM, copy) lists the product as being ‘comparable’ to imatinib
mesylate.[6] However, the safety
and efficacy of these
molecules have not been widely assessed and for which patient data are
limited, and the safety and efficiency of this drug have not been
demonstrated yet in a randomized clinical trial.
In Algeria
‘imatib’ (CIPLA-India) was introduced in 2006, but no study has been
published yet in the North Africa region regarding the response and the
outcome of this copy in CML patients. The goal of this multicenter
study is to characterize newly adult CML in the western region of
Algeria and to assess the effectiveness and safety of imatib as
frontline therapy for patients with CML.
Patients and Methods
In
Algeria, the incidence of CML was 0.53/100,000 inhabitants with a
prevalence of 1030 cases in 2014. The median age is 48 years (03-90)
with a peak incidence in the age group (45-49 years) and slight male
predominance (sex ratio: 1,2).
Patients’
characteristics.
The study was carried out in all the 7 hematological services of
university hospitals, in the western Algeria and data were collected
using electronic medical records from patients' clinic visits. All
patients aged over 15 years with de
novo CML were included.
It is a longitudinal study, multicentric and retrospective. Patients,
who were diagnosed to be suffering from CML between 1st
January 2007 and 31st
December 2014, were selected for data analysis. In all patients, the
diagnosis of CML was confirmed by morphologic review of peripheral
blood (PB) and by RT-PCR based BCR-ABL analysis (Applied Biosystems
7500 Real-time PCR system).[7]
Staging and evaluations of response were determined according to the
current European LeukemiaNet (ELN) recommendations.[8]
The prognosis of CML patients in chronic phase was determined by either
Sokal and/or EUTOS prognostic scoring systems at initial presentation,
using European LeukemiaNet calculator.[9]
Patients
with acceleration and blastic phase were not included in our study. All
patients received a copy preparation, consisting of the alpha crystal
form of imatinib, (Imatib) at an oral dose of 400 mg daily and
monitored for tolerance and side effects according to the ELN
recommendations adapted to our conditions and capabilities in Algeria.
Doses were reduced in the presence of grade 3-4 thrombocytopenia or
grade 3-4 neutropenia. Wherever it was possible the dose was maintained
above 300 mg/day. Patients were treated as long as they continued to
respond.
Response
assessment.
All patients were assessed for response to treatment by weekly physical
examination, full blood count, and biochemistry for the first 6-weeks
of treatment and were assessed every 3 months during their follow-up.
During the treatment, quantitative, real-time PCR (RQ-PCR) for the
determination of BCR/ABL1 transcripts level using the international
scale, has been performed every 3 months until achievement of an MMR,
then every 3 to 6 months. The complete hematologic response (CHR) at 03
months and molecular response at 03, 06, 12, 18, 24 months and more
according to capabilities. At 03 months and/or 6 months we looked for a
BCR/ABL1 rate <10%. At 12 months we looked for a major molecular
response (MMR), defined by BCR/ABL1 ratio lower than 0,1% according to
the ELN. A ratio between 0,1 to 1% is considered a good response
according to GAT-LMC (the CML study Algerian group), so the Imatinib
treatment is continued.
Statistical
Analysis.
The statistical analysis used the calculation of averages and the Khi2
test. Overall survival (OS) was calculated from the time of diagnosis
to the date of death or last follow-up. The Kaplan–Meier method was
used to estimate survival rates and the log-rank test to determine
differences between subgroups. For all tests, a p-value of less than
0.05 was considered statistically significant. Statistical calculations
were performed using the software package SPSS 20 (SPSS, Inc., Chicago,
IL). The median follow-up of patients in December 2014 is 46 months
(13-107 months).
Results
From
January 2007 to December 2014, 387 patients with CML were treated with
imatib. Among them 355 patients are evaluable. The clinical
characteristics of patients are shown in Table 1.
The median age of patients was 48 years (range: 16– 85 years), 182
patients were male, and 173 were female (sex ratio= 1,05). The median
time from diagnosis to treatment was 3 months (0.1–4). Splenomegaly was
the most common clinical feature present in 86% of patients. At
diagnosis, the median number of white blood cells (WBC) was 346 G/L
(range: 78-588 G/L). The prognostic classification, according to the
Sokal score, found a low risk in 18%, intermediate risk in 40% and a
high risk in 42%. The Eutos score is less than 87 in 75,5% and more
than 87 in 24,5%. A CHR at 03 months was found in 295 patients (83%)
without any significant differences considering either the Sokal groups
[72% in low-risk group, 78% in intermediate risk group, and 69% in
high-risk group (p=0.26)] or the EUTOS score [81% in low-risk
group, and 70% in high-risk group (p=0.08)]. A major molecular response
(MMR) was achieved at six months (M6), M9, M12, M18, and M24 in 21%,
38%, 35%, 51% and 67% respectively and 34% of patients achieved a
complete molecular response (CMR). The 5-year overall survival (OS)
rate was 83% (Figure 1).
According to Sokal groups, we observed a significant difference in OS
with at 5-years, 93% for low-risk group, 84% for intermediate risk
group and 78% in high-risk group (p = 0.009) (Figure 2).
When patients were stratified according to EUTOS risk classification, a
significant difference in OS rates was observed (p=0.02), and the
5-year OS rates for low, and high-risk groups were 90% and 74%
respectively (Figure 3).
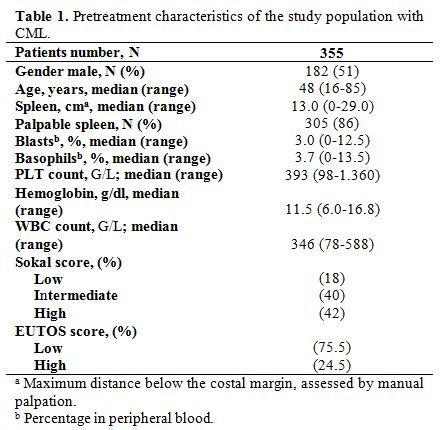 |
Table 1.
Pretreatment characteristics of the study population with CML. |
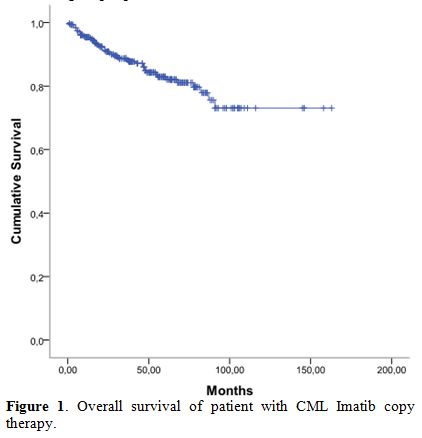 |
Figure 1.
Overall survival of patient with CML Imatib copy therapy. |
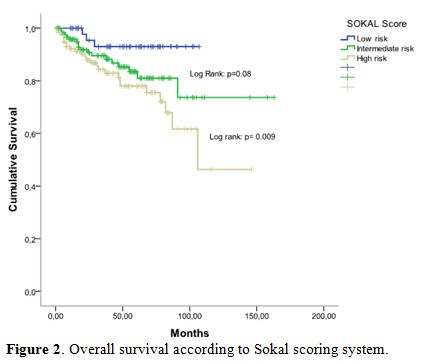 |
Figure 2.
Overall survival according to Sokal scoring system. |
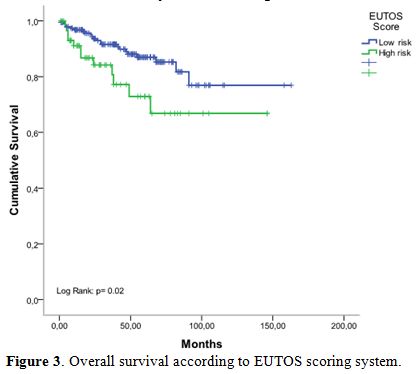 |
Figure 3.
Overall survival according to EUTOS scoring system. |
Side effects of
imatib (copy) in this study are shown in Table 2,
and no adverse effects related deaths have occurred. At a median
follow-up duration of at least 48 months (12-84 months), 81% of
patients are alive and are still taking imatib, 10% of patients
relapsed among them 50% because of low adherence, 42% because of
resistance and only 8% developed intolerance to imatib. 9% of patients
died after developing blastic phase disease. 6% of patients
discontinued their follow-up, and 4% of patients died due to other
reasons.
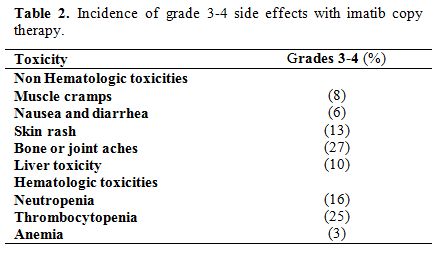 |
Table 2.
Incidence of grade 3-4 side effects with imatib copy therapy. |
Discussion
CML
constitutes the most common myeloproliferative disorder in Algeria. The
incidence of CML was 0.53/100,000 inhabitants with a prevalence of 1030
cases in 2014 in the country, with 472 newly cases/year.[10]
The median age is 48 years with a peak incidence in the age group
(45-49 years) and slight male predominance (sex ratio: 1,2).[10]
The median age of patients with CML in Algeria and other African
countries with a similar demographic pattern is over 40 years and lower
than that reported in Europe and United States.[11-12]
Our
multicenter study of patients with newly diagnosed CML is unique in
Northern Africa. Regarding the clinical and biological feature, CML of
Algerian patients seems to be frequently aggressive with anemia and a
massive splenomegaly associated in a majority of cases with a high-risk
Sokal.
After ITK therapy, results were very well known and
described regarding Glivec®/Gleevec® used in multiple clinical trials
showing cumulative OS rates of 86%, after 7-years of follow-up.[3]
In
low-income countries like Algeria, such expensive treatment is not
available, and physicians in charge of adult CML patients are obliged
to use a copy form, which could appear as less effective therapy in
this setting. To our knowledge, there are only a few data regarding
generic forms or imatib for Algerian patients with CML. A.H. Goubran,[13] Z. Chouffai,[14]
I.A. Asfour[15] and M. Mattar[16]
published only case reports and showed outcomes failure with Imatib
copy. Ostojic A, showed that when taken at equivalent doses, imatinib
generics are bioequivalent, comparable in clinical efficacy and have
the potential for substantial savings in the treatment cost for CML.[17]
In a prospective, multicenter clinical trial to evaluate the early
clinical efficacy and safety of a generic imatinib in treating patients
with CP of CML in China, the authors showed that among 107 patients at
3-month, the CHR rate was 98.1%(105/107). The BCR-ABL transcript was
≤10% in 77/106 patients (72, 6%), 11 of them (10, 4%)achieved MMR
(BCR-ABL≤0,1%). At 6-month, the CHR rate was 100%; BCR-ABL was ≤1% in
68,5%, and 33% of them achieved MMR. Grade 3 leukopenia,
thrombocytopenia and anemia rates were 19,5%, 23% and 13,8%,
respectively. No patient experienced grade 4 non-hematologic toxicity.
No adverse effects related deaths have occurred.[18]
Our
study is the first study concerning a large cohort of patients
receiving Imatib for CML and which has analyzed the safety, efficacy,
OS at short, medium and long-term. With a median follow-up of 46 months
(range: 13–107), we found 83% of CHR with 67% of MMR at 2 years, and
34% of CMR. The IRIS trial at 7-years has demonstrated better overall
survival rate of 86%.[19] At
5-years and 9-years, OS
in our study was 83% and 67% respectively. Respect to literature data
we observed a larger group of patients (17%) who have never achieved a
CHR probably related to the existence of higher proportion (42%) with
high-risk Sokal score and it was demonstrated that there was a
relationship between prognosis and disease response. In parallel, the
delayed diagnosis is one of the arguments in favor of the disease
extension.
Side effects of imatib in our study were similar to
those reported previously for the imatinib mesylate treatment study and
only 8% of patients showed intolerance to imatib and switch to the
second generation of ITK. There was a previous report from Canada with
similar conclusions about the efficacy and tolerability of generic
imatinib, although they used a different source of generic imatinib
from our study.[20]
Scarce compliance in CML
patients treated with BCR-ABL inhibitors is common and associated with
critical outcomes. Poor adherence to therapy was associated with a
negative impact on both clinical and economic outcomes.[21]
In our study, more than 15% of patients presented poor adherence to CML
treatment, and they had a lower CHR response at 3 months and MMR at 6
months. The estimated 5-year OS in our patients was comparable to the
72% reported in the study from Côte d’Ivoire[22]
and the 80% in the study from Nepal,[23]
whom patients were treated with imatinib mesylate (Gliveec®).
Conclusion
This
study reflects a real-world experience by treating patients with CML in
a developing country and thus sheds light on differences in this
population compared to Western countries. A higher proportion of
patients were diagnosed at later stages of CP compared to reports from
Western countries, and that may impact response rates seen with
frontline imatib. Imatib (copy) is effective and safe in treating
patients with CML in chronic phase and proves to have a durable
outcome. To our knowledge, this is the first study reporting the
response to imatib (copy) in an Algerian population.
Acknowledgements
We
thank Pr Bouhass Rachid, Dr. Taibi Karima, Dr. Siali Nadia, Dr.
Benlazar Mohamed, Dr. Benzineb Brahim, and Dr Yachkour Toufik for
collection and assembly database.
References
- Hehlmann R, Hochhaus A,
Baccarani M. Chronic myeloid leukaemia. Lancet 2007; 370: 342–350. https://doi.org/10.1016/S0140-6736(07)61165-9

- Faderl
S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM: The
biology of chronic myeloid leukemia. N Engl J Med 1999, 341:164-172. https://doi.org/10.1056/NEJM199907153410306
PMid:10403855

- O'Brien
SG, Guilhot F, Goldman JM, et al: International randomized study of
interferon versus STI571 (IRIS) 7-year follow-up: sustained survival,
low rate of transformation and increased rate of major molecular
response (MMR) in patients (pts) with newly diagnosed chronic myeloid
leukemia in chronic phase (CML-CP) treated with imatinib (IM). Blood
2008; 112:76.

- Saglio
G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly
diagnosed chronic myeloid leukemia. N Engl J Med. 2010; 362:2251-9. https://doi.org/10.1056/NEJMoa0912614
PMid:20525993

- O'Hare
T, Shakespeare WX, Zhu X, et al. AP24534, a pan-BCR- ABL inhibitor for
chronic myeloid leukemia, potently inhibits the T315I mutant and
overcomes mutation-based resistance. Cancer Cell 2009; 16: 401-412. https://doi.org/10.1016/j.ccr.2009.09.028
PMid:19878872 PMCid:PMC2804470

- Cipla Imatib Product
Website. Accessed November, 2008 at www.cipla.com
- Gabert
J, Beillard E, van der Velden VH, et al. Standardization and quality
control studies of 'real-time' quantitative reverse transcriptase
polymerase chain reaction of fusion gene transcripts for residual
disease detection in leukemia - a Europe Against Cancer program.
Leukemia. 2003; 17: 2318–57. https://doi.org/10.1038/sj.leu.2403135
PMid:14562125

- Baccarani
M, Cortes J, Pane F, et al: Chronic myeloid leukemia: an update of
concepts and management recommendations of European LeukemiaNet. J Clin
Oncol 2009 [published online ahead of print November 2, 2009]. https://doi.org/10.1200/JCO.2009.25.0779

- Baccarani M. Calculation
of relative risk of CML patients [online] 2010 [last changed
2010/11/08]. Available from: http://www.leukemia-net.org/content/leukemias/cml/cml_score/
- K
Djouadi, N Abdennebi, F Harieche, R Ahmed Nacer, RM Hamladji, A
Bouchakour, et al. Epidemiological approach of chronic myeloid
leukemia. Algerian-tunisian study. Blood 2016 128:5440 http://www.bloodjournal.org/content/128/22/5440

- Fleming AF
and Menendez C, "Blood," in Principles of Medicine in Africa,
E.
Parry, R. Godfrey, D. Mabey, and G. Gill, Eds., vol. 78, Cambridge
University Press, Cambridge, UK, 2004.
- Groves
FD, Linet MS and Devesa SS. Patterns of occurrence of the
leukaemias. European Journal of Cancer Part A, vol. 31, no. 6, pp.
941–949, 1995. https://doi.org/10.1016/0959-8049(95)00024-0

- Hadi
Alphonse Goubran. Failure of a non-authorized copy product to maintain
response achieved with imatinib in a patient with chronic phase chronic
myeloid leukemia: a case report. Journal of Medical Case Reports 2009,
3:7112 https://doi.org/10.1186/1752-1947-3-7112
PMid:19830137 PMCid:PMC2726486

- Chouffai
Z. Hematologic Relapse after? 2 Years on a Non-Authorized Copy Version
of Imatinib in a Patient with Chronic Myeloid Leukemia in Chronic
Phase: A Case Report. Case Rep Oncol 2010; 3: 27 https://doi.org/10.1159/000319150
PMid:21045935 PMCid:PMC2968768

- Asfour
IA and Elshazly SA. Changing therapy from Glivec® to a "copy" imatinib
results in a worsening of chronic myeloid leukemia disease status: two
case reports. Cases Journal 2009, 2:9342 https://doi.org/10.1186/1757-1626-2-9342
PMid:20062598 PMCid:PMC2803998

- Mattar
M. Failure of copy Imatib (CIPLA, India) to maintain hematologic and
cytogenetic responses in chronic myeloid leukemia in chronic phase. Int
J Hematol. 2010 Jan; 91(1):104-6. https://doi.org/10.1007/s12185-009-0431-1
PMid:20054670

- Ostojic
A, Sertic D, Roncevic P, Peric Z, Granic P, et al. Comparison
of
Branded and Generic Imatinib Plasma Concentrations in Patients With
Chronic Myelogenous Leukemia: Unicentric Study. Clin Lymphoma Myeloma
Leuk. 2016 Aug;16(8):472-6. https://doi.org/10.1016/j.clml.2016.04.003
PMid:27245313

- Jiang
Q, Zhao D, Jin J, Wu D, Meng F, Hu J, et al. A prospective,
multi-centre clinical trial to evaluate the early clinical efficacy and
safety of a generic imatinib in treating patients with chronic phase of
chronic myelogenous leukemia. Zhonghua Xue Ye Xue Za Zhi. 2015
Aug;36(8):651-5. PMid:26462633

- Druker
BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gatter- mann N,
Deininger MW, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A,
Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ,
Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl
A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C,
Letvak L, Larson RA, IRIS Investigators: Five-year follow-up of
patients receiving imatinib for chronic myeloid leukemia. N Engl J Med
2006, 355(23): 2408-2417. https://doi.org/10.1056/NEJMoa062867
PMid:17151364

- Kang
M, Xenocostas A, LazoLangner A, ChinYee IH, Howson-Jan K, and
Abouzeenni M. Impact of Transition to Generic Imatinib in the Molecular
Response Among Patients with Chronic Myeloid Leukemia, Blood 2014,
vol.124: 5527.

- Kapoor
J, Agrawal N, Ahmed R, Sharma SK, Gupta A and
Bhurani D.
Factors Influencing Adherence to Imatinib in Indian Chronic Myeloid
Leukemia Patients: A Cross-Sectional Study. Mediterr J Hematol Infect
Dis 2015, 7(1): e2015013. https://doi.org/10.4084/mjhid.2015.013

- Koffi KG, Nanho DC ,
N'dathz E, Kouehion P, Dissieka R, Attia A
et al. The Effect of Imatinib Mesylate for Newly Diagnosed Philadelphia
Chromosome-Positive, Chronic-Phase Myeloid Leukemia in Sub-Saharan
African Patients: The Experience of Côte d'Ivoire. Advances in
Hematology. Volume 2010, Article ID 268921, 6

- Gyan
K Kayastha, Padma Gurung, Paras K Acharya, & al. Patan hospital
experience in treating philadelphia chromosome/BCR-ABL1 positive
chronic myeloid leukemia patients with gleevec (imatinib mesylate); the
first generation specific tyrosine kinase inhibitor. BMC Blood
Disorders 2010, 10:8.

[TOP]


























