Salwa Bakr1,2*, Sherif Edris3,4,5, Nashwa S. Abdel Fattah6, Noha Mohamed Ibrahim7 and Manal F. El-Khadragy8,9,10.
1 Department of Clinical Pathology, College of Medicine, Fayoum University, Fayoum, Egypt.
2 College of Medicine, Princess Nourah bint Abdulrahman University, Riyadh, Saudia Arabia.
3 Department of Biological Sciences, Faculty of Science, King Abdulaziz University (KAU), Jeddah, Saudi Arabia.
4 Department of Genetics, Faculty of Agriculture, Ain Shams University, Cairo, Egypt.
5
Princess Al-Jawhara Al-Brahim Centre of Excellence in Research of
Hereditary Disorders (PACER-HD), Faculty of Medicine, King Abdulaziz
University (KAU), Jeddah, Saudi Arabia.
6 Department of Parasitology, College of Medicine, Ain Shams University, Egypt.
7 College of Medicine, Ain Shams University, Egypt.
8 Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia.
9 Chair Vaccines Research of Infectious Diseases, Faculty of Science, King Saud University, Riyadh, Saudi Arabia.
10 Department of Zoology and Entomology, Faculty of Science, Helwan University, Cairo, Egypt.
Corresponding
author: Salwa Bakr. Department of
Clinical Pathology, College of Medicine, Fayoum University, Fayoum,
Egypt and College of Medicine, Princess Nourah bint Abdulrahman
University, Riyadh, Saudia Arabia. E-mail:
Salwabakr1@hotmail.com
Published: October 25, 2017
Received: August 13, 2017
Accepted: September 25, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017065 DOI
10.4084/MJHID.2017.065
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: Transfusion-transmitted
malaria is undoubtedly a potential health hazard for blood recipients.
Egypt is still on the prevention of reintroduction phase of malaria
control program. Fayoum Governorate is considered one of the high-risk
foci in Egypt due to its geology. However, no studies have been
reported to evaluate the current status of subclinical Plasmodium
infection based on sensitive molecular techniques. Moreover, screening
of malaria is not listed within screening protocols of blood-borne
pathogens in Fayoum blood banks. Objective: To assess the current prevalence of subclinical Plasmodium
infection among blood donors of Fayoum inhabitants for transfusion
biosafety. To predict any possibility of the reemergence of malaria in
the governorate and the effectiveness of malaria control measures.
Methods:
A cross-sectional survey was conducted on 400 apparently healthy
blood-donors in blood transfusion center of Fayoum University hospital
from Jun 2012 to Jan 2013. Conventional PCR was used to detect the 18 S
ssrRNA Plasmodium gene. Results: All Fayoum inhabitants’ blood donors’ samples were negative for Plasmodium infection.
Conclusions: Current
applied control and preventive measures are valid in the context of
blood transfusion biosafety in Fayoum blood banks and, therefore, the
implementation of a routine malaria screening test in Fayoum blood
banks is not merited at this time.
|
Introduction
Malaria caused by Plasmodium parasites is one of health burden blood diseases worldwide, particularly in Africa.[1,2,3]
As according to the latest report from the World Health Organization
(WHO) it is estimated that approximately 212 million people globally
were having malaria. In 2015, most malaria cases (88%) and deaths (90%)
occurred in sub-Saharan Africa.[4] In Egypt, malaria
is a well-known endemic infection and has been reported since ancient
times. Investigators confirmed its presence in Egyptian mummies from
Fayoum Governorate using the highly sensitive PCR tests.[5]
As reported by the WHO, Egypt is one of the countries that has
interrupted malaria transmission and is still in the prevention of
reintroduction stage of malaria control program.[6]
Although malaria has been eliminated successfully from most foci in
Egypt due to an efficient national control program applied by the
Ministry of Health in cooperation with the WHO,[7] there is a high probability of re-emergence of the disease.[8]
Fayoum Governorate was assigned as a high risk of malaria foci in Egypt during the last two decades,[9] due to its geology,[1,10,11] the nature of its irrigation system which depends on a network of small canals,[5] the presence of Quarun Lake, as well as the current changes in climate all over the country.[9] All these conditions may have a direct impact on malaria transmission, facilitating the re-invasion of Anopheles arabiensis into the region which may come from other endemic areas of Africa such Sudan.[12]
Although no autochthonous cases of Plasmodium
infection have been reported officially in Fayoum Governorate or
elsewhere in Egypt since 1998, some sporadic cases from Al-Fayoum, Siwa
Oasis, and Cairo governorate were reported by Zaher et al., 2007[13] and El-Bahnasawy et al., 2010.[14] Moreover, a recent outbreak occurred in southern Upper Egypt; Aswan Governorate during May 2014.[15]
Consequently, this supports the idea that malaria is reemerging in the
country as reported by WHO-EMRO, 2016 and other researchers[2,5,12-17]
Transfusion-transmitted malaria, although uncommon, is considered a potential hazard for blood recipients.[18]
As post-transfusion clinical manifestations of malaria usually simulate
other post-transfusion reactions, as a result of this simulation the
most cases may be misdiagnosed. Moreover, donors who are subclinical
carriers of Plasmodium become a reservoir for the parasite, maintaining its transmission in the community through blood donation.[3] Hence, high sensitivity screening tests should be done to ensure blood transfusion biosafety for the recipient.[19]
As
far as we know, no previous studies have been reported based on
sensitive molecular (PCR) techniques to assess the prevalence of
Plasmodium infection in Fayoum inhabitants. Therefore, the current
status of malaria is unclear in this high risk area of Egypt.[20]
In Egyptian blood transfusion centers, including Fayoum Governorate,
screening of malaria parasite is not currently validated and not listed
within screening protocols of blood-borne pathogens,[21] although the risk of transfusion-transmitted malaria was still poorly documented in this high risk foci of Egypt.[3]
Definitive determination of the prevalence of asymptomatic Plasmodium
infection in a general population and blood-donors, in particular, is
like to help in updating the screening operating policy and procedures
(SOP) for blood recipient safety as well as reviewing health policy
decisions.[21]
Undoubtedly, molecular-based
assays are about 100 fold greater sensitivity compared to microscopy or
RDTs at detecting malaria parasite, especially in subclinical
infections with detection limits as low as 5 parasites/µl.[22] Our study was conducted to detect the 18S rRNA gene of Plasmodium parasite. This study aimed to assess the current prevalence of subclinical Plasmodium
infection in blood donors at a public blood bank of the University
hospital in Fayoum Governorate, Egypt. Furthermore, this study could
provide to give accurate, current epidemiological data on malaria in
Fayoum Governorate considering the changes in climate, so evaluating
the effectiveness of national malaria control measures.
Material and Method
Participants and study area. A cross-sectional study aimed to estimate the prevalence of subclinical Plasmodium
infections among blood donors of Fayoum resident during the expected
malaria transmission season from Jun 2012 to Jan 2013. The study sample
included four hundred asymptomatic apparently healthy volunteers who
enrolled for blood donation in blood transfusion center of Fayoum
University hospital, which is the main transfusion center in Fayoum
Governorate. Donated blood was processed either in the transfusion
center or in mobile blood donation campaigns which targeted different
destination of the governorate. Standard donor history questionnaire
was fulfilled to exclude the potential possibility of the previous
infection with malaria or traveling to endemic regions in the last six
months of donation. Written informed consent for the study was taken
from each participant. The sample size was calculated based on the
prevalence of subclinical malaria infection among blood donors (50%)
and the power of study 0.8 considering alpha 0.05. The study was
committed to ethical committee guidelines approved by the College of
Medicine, Fayoum University.
Sample collection.
Blood samples were obtained from each donor as a whole venous blood on
EDTA, and as a dried blood sample by finger prick, where 2 drops of
capillary blood were spotted onto Whatman®31ETCHR filter paper
(Whatman, Piscataway, NJ). Filtered papers were air dried at room
temperature and kept in separate clean zipper bags until further
processing. All blood samples were screened as routine for ABO grouping
and serological screening tests, including HCV, HBV, HIV, and Syphilis.
Malaria testing. A) Microscopic examination: was done using thick and thin blood films stained with Giemsa according to WHO competency assessment protocol.[23]
Immunological rapid diagnostic test (BinaxNOW Malaria Test; Binax,
Inc., Scarborough, ME, USA), was used for primary detection of malaria
antigen positive cases.
B) DNA extraction:
DNeasy Blood and Tissue kit, Cat no. 69504 (Qiagen, Valencia, CA, USA)
was used as per manufacturer’s instructions with some modifications.
Whatman filter paper was cut into strips (almost 1 cm2)
and put into a 1.5 mL Eppendorf tube. The concentration of extracted
DNA was measured using a Nanodrop 2000 (Thermo Fisher Scientific Inc,
CA, USA).
C) PCR amplification:
Conventional PCR amplification was performed using 10 ng of extracted
DNA with purity 1.7 to 2 in a total volume of 25 μL reaction volume
using master mix (Go Taq green 2x master mix, Promega, USA) with
primers (Bioneer, Korea) rPLU6 (5’- TTA AAA TTG TTG CAG TTA AAA CG 3’)
and rPLU5 (5’-CCT GTT GTT GCC TTA AAC TTC-3); as described by Gal et
al., 2001.[24] Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) with accession number NG 007073, GAPDH-F (5’- AGG
TGG TCT CCT CTG ACT TCA AC-3’), GAPDH-R (5’- CGC CAG ACC CTG CAC TTT
T-3’) was designed in this study with amplicon size 150 bp.
As the
cycle conditions began with an initial denaturing period at 95°C for 5
min, followed by 35 cycles of 94°C for 30 s, 58°C for 1 min, and 72°C
for 1 min, with a final extension for 5 min at 72°C.[23]
PCR amplicon was separated on 1% agarose gel (Agarose, LE, Analytical
Grade, Promega, USA). The presence of the 1200 amplicon was detected in
the gel under UV light after its staining with Invitrogen TM SYBER TM Safe.
D) Statistical methods:
Data were collected, coded and analyzed using IBM SPSS software,
version 23 software (IBM, Armonk, NY, USA). The result of simple
descriptive analysis of numbers and percentages for qualitative data
were tabulated. Graphs were produced using Excel software.
Results
The
descriptive analysis of data revealed that mean age of participants was
29.88 ±7.6 years. The general proportion for male and female was
(91.7%) and (8.3%) respectively. The most common blood group was A+
(33%) and least common was A2 (0.3%). In the Rh blood group, Rh
positive donors (90.3%) were much more than Rh-negative donors (9.7%) (Figure 1). Prevalence of HCV, HBV, and HIV among blood donors was presented in Figure 2. Both microscopically and rapid immunological assays were negative for malaria in all blood samples.
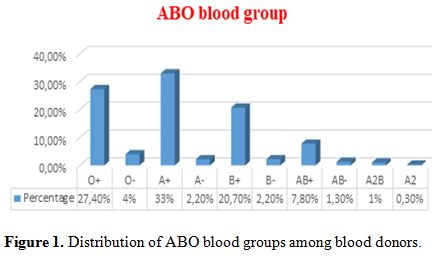 |
Figure 1.
Distribution of ABO blood groups among blood donors. |
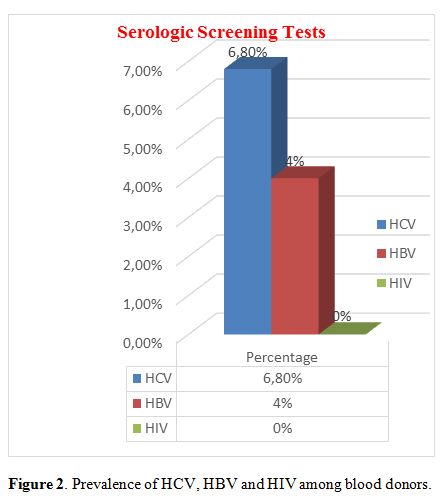 |
Figure 2. Prevalence of HCV, HBV and HIV among blood donors. |
PCR results on Agarose gel showed that all samples were negative for 18 S ssrRNA Plasmodium
gene. For PCR validation, malaria positive control was used for each
run which gives band at 1200 bp. In addition, a housekeeping gene
(GAPDH), which was used as an internal control for PCR negative samples
for testing the integrity of DNA, revealed clear positive PCR band at
150 bp (Figure 3 and 4).
 |
Figure 3.
PCR results on Agarose gel (Gel A and B) showed; (Lane 1): 100 bp
ladder, (EZ Load TM 100 bp Molecular Ruler-BioRad). (Lane 2): Malaria
positive control, (1200 bp). All the samples were malaria negative as
shown from lane 3 up to lane 26 in both gels, but were positive (150
bp) GAPDH internal control. |
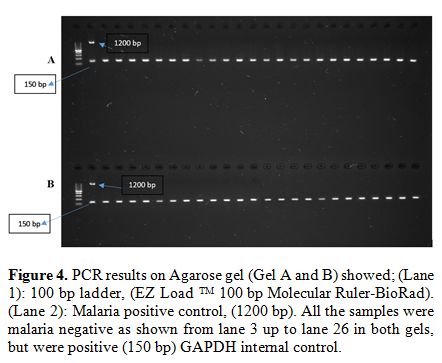 |
Figure 4. PCR results on Agarose gel (Gel
A and B) showed; (Lane 1): 100 bp ladder, (EZ Load TM 100 bp Molecular
Ruler-BioRad). (Lane 2): Malaria positive control, (1200 bp). All the
samples were malaria negative as shown from lane 3 up to lane 26 in
both gels, but were positive (150 bp) GAPDH internal control. |
Discussion
Blood
bank biosafety, especially in endemic malaria regions, is one of the
most important targets for controlling maintenance of the Plasmodium reservoir of malaria transmission.[19]
Many recent reports seemed to suggest that malaria is reemerging in Egypt,[16] due to current climate changes,[9] and the recently discovered malaria outbreak in a specific region of the country.[2]
Recently, the Military Fever Hospital research group recorded that
12.5% out of 36 malaria-infected cases were from Fayoum governorate.[25] Similarly, many studies have reported sporadic malaria-infected cases in Fayoum.[5,13-17,25]
In accordance, Kamel et al. who assessed the prevalence of malaria in Fayoum governorate among patients admitted Fayoum fever hospitals
using rapid diagnostic tests (RDTs- ABON PLUS), pointed out that
malaria control program in South Egypt – including the Fayoum
Governorate – should be strengthened to prevent reintroduction of
malaria. Kamel et al. further recommended the need to evaluate the
validity of the RDT results of their study using more sensitive
technique.[8]
Several studies support the higher
sensitivity of PCR assay compared to microscopy and RDTs to roll out
infection and to monitor the success of control programs in low
transmission areas.[26] Hence, a study highlighted
the urgency to use a sensitive PCR based molecular assay to determine
the current malaria status in the high risk areas of Egypt.[20]
Many molecular assays are available for detection of Plasmodium
pathogens. However, it is challenging to select the most ideal and
appropriate method for screening of blood donors, that can screen a
large number of samples with a higher sensitivity and easily performed
technique.[27] Since using PCR based pooling strategy reduces the number of assays needed when screening for infectious diseases,[10] individual PCR testing of dried blood spot samples allows pathogen detection despite its higher efforts and cost.[27]
Therefore, in the present study, PCR of dried blood spot samples was
used, due to its sensitivity, to validate the results obtained from
microscopic evaluation and rapid immunologic assay for malaria antigen.
Regarding the sensitivity of detection, Bharti et al. (2009) who
assessed PCR based pooling strategy with a pool size of 10, reported
six positives cases out of 200 ‘negative’ malaria samples using
microscopic detection.[22] Furthermore, Taylor et al. (2010) reported 35 positive samples out of 1,092 negative cases with microscopy.[28] Lima GF et al. (2016) reported in a study conducted to assess four different molecular assays in detecting Plasmodium
pathogens that the sensitivity of pooled samples is not ideal (86.7%)
mainly when used for blood transfusion screening, and more efforts
still need to improve the assays used for detection low-density
parasitemia.[27] Another study pointed out that PCR
using dried blood spot from filter paper was able to detect almost
one-fifth and one-third of Plasmodium presences that were missed by RDT and microscopy, respectively.[29]
In
contrast to the previously mentioned studies which were claimed the
high probability of reemergence of malaria in Upper Egypt (including
Fayoum), the present study’s results made it clear by using PCR based
assay for detecting 18S ssrRNA Plasmodium gene. The results demonstrated no subclinical malarial infection in the sample group as no Plasmodium DNA
genome was detected in any of the donors’ blood samples. The results
obtained in our study were confirmed by using an internal control gene
(GAPDH) to check the quality of DNA extraction and to assure that there
were not misleading results due to the poor DNA quality or no DNA at
all.
In response to recent studies suggesting the possibility of re-emergence of malaria in the area,[5,9,13-17] the results of our study support the idea that all cases of malaria previously reported in the Fayoum governorate[8,25] are imported from outside.
In
the present study, the prevalence of blood groups O+, O-, A+, A-, B+,
B-, AB+, AB-, A2B, and A2 among donors was 27.4 %, 4%, 33%, 2.2%,
20.7%, 2.2%, 7.8%, 1.3%, 1%, and 0.3% respectively. Similar prevalence
have been reported in another study carried out all over 26
governorates of Egypt,[30] as the frequency of groups
O+, O-, A+, A-, B+, B-, AB+, and AB- was 27.5%, 2.3%, 33.6%, 2.7%, 22%,
1.8%, 9.3%, 0.7% respectively.
Conclusions
Currently
applied control and preventive measure are effective in the context of
blood transfusion biosafety in Fayoum blood banks and, therefore, the
implementation of a routine malaria screening test in Fayoum blood
banks is not merited at this time.
In light of the results of
our study, we may assume that the current control and preventive
measures applied under the umbrella of the WHO and Egyptian MOH were
able to control malaria, at least during the time of the study, in the
Fayoum Governorate. However further comprehensive study is recommended
to screen and stratify the results according to age to substantiate the
assume eradication of the infection. Regular monitoring is still
needed.
Acknowledgements
The
authors thank all staffs of Fayoum University blood bank for data and
sample collection. Sincere appreciation for Prof. Hala Elmorshedy,
Professor of public health, hiph, Alexandria University for her
valuable time and comments.
References
- WHO. Africa Malaria Report 2003. Geneva (Switzerland): World Health Organization, 2004. http://apps.who.int/iris/bitstream/10665/67869/1/WHO_CDS_MAL_2003.1093.pdf
- WHO- EMRO. Vector- borne diseases. Eastern Mediterranean Regional Office: World Health Organization, 2016. http://www.emro.who.int/egy/programmes/neglected-tropical-diseases.html
- Noubouossie
D, Tagny CT, Same-Ekobo A, Mbanya D. Asymptomatic carriage of malaria
parasites in blood donors in Yaoundé. Transfusion Medicine. 2012 Feb
1;22(1):63-7. https://doi.org/10.1111/j.1365-3148.2011.01121.x PMid:22141368

- WHO. World Malaria Report 2016. Geneva (Switzerland): World Health Organization, 2016. http://www.who.int/mediacentre/factsheets/fs094/en/
- Lalremruata
A, Ball M, Bianucci R, Welte B, Nerlich AG, Kun JF, Pusch CM. Molecular
identification of falciparum malaria and human tuberculosis
co-infections in mummies from the Fayum depression (Lower Egypt). PloS
one. 2013 Apr 2;8(4):e60307. https://doi.org/10.1371/journal.pone.0060307 PMid:23565222 PMCid:PMC3614933

- WHO-Global Malaria Programme. World Malaria Report 2012, Geneva (Switzerland): World Health Organization, 2012. http://www.who.int/malaria/publications/.
- Hassan
A.N., Kenawy M.A., Kamal H., Abdel Sattar A. A., and Sowilem M.M.
GIS-based prediction of malaria risk in Egypt, Eastern Mediterranean
Health Journal 2003; 9: 548-558. PMid:15748052

- Kamel
MM, Attia SS, Emam GD, Al Sherbiny NA. The Validity of Rapid Malaria
Test and Microscopy in Detecting Malaria in a Pre-elimination Region of
Egypt. Scientifica. 2016 Mar 21; 2016.

- Lotfy
WM. Climate change and epidemiology of human parasitosis in Egypt: A
review. Journal of advanced research. 2014 Nov 30;5(6):607-13. https://doi.org/10.1016/j.jare.2013.06.009 PMid:25685530 PMCid:PMC4293906

- Kenawy
MA. Anopheles sergentii (Diptera: Culicidae): seasonal variation in the
development rates of immatures from El Faiyum and Siwa Oasis, Egypt. J
Egypt Soc Parasitol 1995;25:257–68. PMid:7602168

- Morsy
TA, El Kadry AA, Salama MMI, Sabry AA, El Sharkawy IMA. Studies on
bionomics and vector competence of adult anopheline mosquitoes in El
Faiyum Governorate, Egypt. J Egypt Soc Parasitol 1995;25 213–44.
PMid:7602165

- Fuller
DO, Parenti MS, Hassan AN, Beier JC. Linking land cover and species
distribution models to project potential ranges of malaria vectors: an
example using Anopheles arabiensis in Sudan and Upper Egypt. Malaria
journal. 2012 Aug 6;11(1):1. https://doi.org/10.1186/1475-2875-11-264 PMid:22866895 PMCid:PMC3483279

- Zaher
T., Ahmadi M., Ibrahim A., El-Bahnasawy M., Gouda H., and Shahat S.A.,
2007, Malaria in Egypt, Saudi Arabia and Yemen: a clinical pilot study,
Journal of the EgyptianSociety of Parasitology, 37: 969-976.
PMid:18383796

- El-Bahnasawy
MM, Saleh NM, Khalil MF, Morsy TA. The impact of three anopheline
mosquito species in Toshka, on the introduction of chloroquine
resistant P. falciparum to Egypt. J Egypt Soc Parasitol. 2011
Dec;41(3):573-92. PMid:22435151

- WHO. Egypt: vector-borne diseases, Geneva (Switzerland): World Health Organization, 2014. http://www.emro.who.int/egy/programmes/neglected-tropical-diseases.html
- Kenawy
MA. Review of Anopheles Mosquitoes and Malaria in Ancient and Modern
Egypt. Journal of Mosquito Research. 2015 Feb 15;5(4). https://doi.org/10.5376/jmr.2015.05.0004

- Dahesh
SM, Bassiouny HK, El-Masry SA. Malariometric parasitological survey in
El-Fayoum Governorate, Egypt. Journal of the Egyptian Society of
Parasitology. 2009 Apr;39(1):213-25. PMid:19530623

- Kazemi
B, Najari M, Saneimoghaddam E, Bandehpour M, Seyed N. Detection of
Plasmodium parasites in healthy blood donors using polymerase chain
reaction. Archives of Iranian Medicine. 2005 Apr 1;8(2):135-8.

- Maselli
LM, Levy D, Laporta GZ, Monteiro AM, Fukuya LA, Ferreira-da-Cruz MF,
Daniel-Ribeiro CT, Dorlhiac-Llacer PE, Sallum MA, Bydlowski SP.
Detection of Plasmodium falciparum and Plasmodium vivax subclinical
infection in non-endemic region: implications for blood transfusion and
malaria epidemiology. Malaria journal. 2014 Jun 6;13(1):1. https://doi.org/10.1186/1475-2875-13-224 PMid:24906577 PMCid:PMC4059091

- Abdullah. Malaria in Egypt: a perplexing behavior with no successful surveillance. Infectious diseases. 2015.

- Gelaw
B, Mengitsu Y. The prevalence of HBV, HCV and malaria parasites among
blood donor in Amhara and Tigray regional states. Ethiopian Journal of
Health Development. 2008;22(1):3-7. https://doi.org/10.4314/ejhd.v22i1.10056

- Bharti
AR, Letendre SL, Patra KP, Vinetz JM, Smith DM. Malaria Diagnosis by a
Polymerase Chain Reaction–Based Assay Using a Pooling Strategy. The
American journal of tropical medicine and hygiene. 2009 Nov
1;81(5):754-7. https://doi.org/10.4269/ajtmh.2009.09-0274 PMid:19861605 PMCid:PMC2770880

- Doni
NY, Zeyrek FY, Seyrek A. Detection of Plasmodium using filter paper and
nested PCR for patients with malaria in Sanliurfa, in Turkey. Malaria
Journal. 2016 May 28;15(1):1.

- Gal
S, Fidler C, Turner SU, LO YD, Roberts DJ, Wainscoat JS. Detection of
Plasmodium falciparum DNA in plasma. Annals of the New York Academy of
Sciences. 2001 Sep 1;945(1):234-8. https://doi.org/10.1111/j.1749-6632.2001.tb03891.x PMid:11708485

- El-Bahnasawy
MM, Dabbous H, Morsy TA. Imported malaria as a threat to Egypt. Journal
of the Egyptian Society of Parasitology. 2010 Dec;40(3):773-88.
PMid:21268544

- Hsiang
MS, Lin M, Dokomajilar C, Kemere J, Pilcher CD, Dorsey G, Greenhouse B.
PCR-based pooling of dried blood spots for detection of malaria
parasites: optimization and application to a cohort of Ugandan
children. Journal of clinical microbiology. 2010 Oct 1;48(10):3539-43. https://doi.org/10.1128/JCM.00522-10 PMid:20686079 PMCid:PMC2953134

- de
Castro Lima GF, Lucchi NW, Silva-Flannery L, Macedo-de-Oliveira A,
Hristov AD, Inoue J, de Jesus Costa-Nascimento M, Udhayakumar V, Di
Santi SM. Still Searching for a Suitable Molecular Test to Detect
Hidden Plasmodium Infection: A Proposal for Blood Donor Screening in
Brazil. PloS one. 2016 Mar 9;11(3):e0150391. https://doi.org/10.1371/journal.pone.0150391 PMid:26959994 PMCid:PMC4784969

- Taylor
SM., Juliano JJ, Trottman PA, Griffin JB, Landis SH, Kitsa P, Tshefu AK
and Meshnick SR. High-throughput pooling and real-time PCR-based
strategy for malaria detection. J. Clin. Microbiol. 2010; 48: 512–519. https://doi.org/10.1128/JCM.01800-09 PMid:19940051 PMCid:PMC2815636

- Matangila
JR, Lufuluabo J, Ibalanky AL, da Luz RA, Lutumba P, Van Geertruyden JP.
Asymptomatic Plasmodium falciparum infection is associated with anaemia
in pregnancy and can be more cost-effectively detected by rapid
diagnostic test than by microscopy in Kinshasa, Democratic Republic of
the Congo. Malaria journal. 2014 Apr 2;13(1):1. https://doi.org/10.1186/1475-2875-13-132 PMid:24690179 PMCid:PMC3976674

- Eissa
SA, Abdel Meguid LM, Ebeid SM, Abou Elfetouh RM, Abdel Moneim GM.
National Cancer Institute experience in healthy Egyptian blood donors
as regards blood group frequencies and seroprevalence of hepatitis b
virus, hepatitis C Virus & HIV: 10 year evaluation. J Egypt Natl
Canc Inst. 2007 Mar;19(1):71-6. PMid:18839037

[TOP]




























