Received: October 8, 2017
Accepted: November 29, 2017
Mediterr J Hematol Infect Dis 2018, 10(1): e2018006 DOI 10.4084/MJHID.2018.006
This article is available on PDF format at:

Agustin Avilés, Maria-Jesus Nambo and Natividad Neri.
Oncology Research Unit, Oncology Hospital National Medical Center, IMSS, México D.F. MEXICO

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Background: To
assess maternal and fetal outcome of women and newborns who received
chemotherapy during pregnancy to treat Hodgkin lymphoma (HL)in early
stages (IA, IIA), we performed a retrospective analysis of a cohort of
44 pregnant women with HL and early stages, diagnosed and treated
between 1988 to 2013, at a tertiary reference cancer center. |
Introduction
Material and Methods
Results
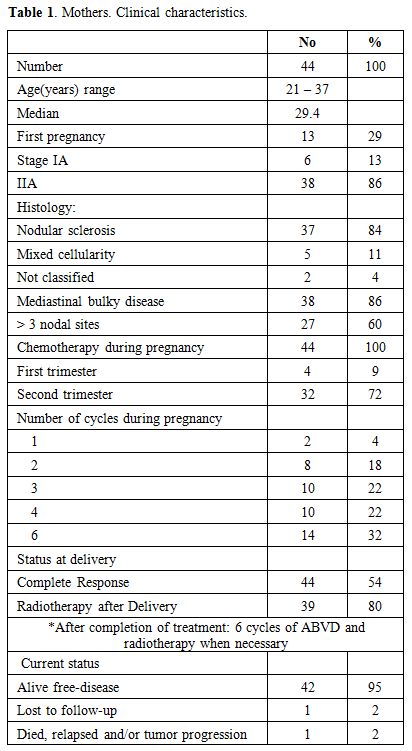 |
Table 1. Mothers. Clinical characteristics. |
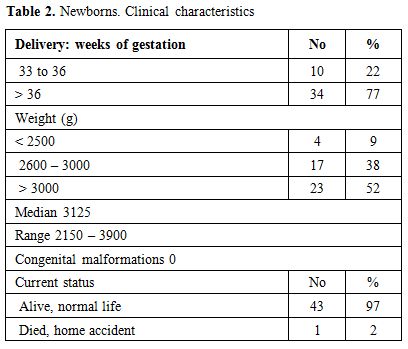 |
Table 2. Newborns. Clinical characteristics |
Discussion
Conclusions
References



















[TOP]