Wonhee So1*, Shuchi Pandya2, Rod Quilitz1, Bijal Shah1 and John N. Greene1.
1 Moffitt Cancer Center, 12902 USF Magnolia Dr, Tampa, FL 33612, USA.
2 Infectious Diseases Associates of Tampa Bay, 4729 N Habana Ave, Tampa, FL 33614, USA.
Corresponding
author: Wonhee So, PharmD, BCPS. Moffitt Cancer Center, 12902 USF
Magnolia Dr, Tampa, FL 33612, USA. Tel: 1-813-745-8529; Fax:
1-813-449-8900. E-mail:
Wonhee.so@moffitt.org
Published: May 1, 2018
Received: February 10, 2018
Accepted: March 23, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018029 DOI
10.4084/MJHID.2018.029
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Blinatumomab is an anti-CD19 immunotherapy approved for
relapsed/refractory B-cell acute lymphoblastic leukemia (ALL) with
significantly increased survival rate. While blinatumomab showed lower
rates of infection, neutropenia and mucosal barrier injury versus
chemotherapy, its infection risks are not well described.
Methods:
All patients who received blinatumomab for ≥ seven days at an academic
cancer center from May 2015 to April 2017 were included. Patient
characteristics pertinent to infectious risks and complications were
examined.
Results: Twenty
patients with refractory (25%), relapsed (70%), or remitted (5%) B-ALL
who received a total of 35 cycles were included. Ten of the 35 cycles
were interrupted, none of which were due to infections. Twenty-six
infections (n) were observed with lower respiratory (9),
gastrointestinal (6) and bacteremia (5) being most common. Compared to
patients without nodular, possible mold pneumonia (n=16), patients with
nodular pneumonia (n=4) had significantly lower baseline absolute
neutrophil count (ANC) (2319 v. 208/µL, p=0.011). There were no
differences in baseline characteristics including ANC between
bacteremic and non-bacteremic patients. One patient was discharged with
no antibacterial prophylaxis since ANC recovered to >500cells/µL,
but developed Pseudomonal bacteremia within a week with ANC
~100cells/µL.
Conclusion:
Despite blinatumomab’s relatively modest myelosuppression and the lack
of mucotoxicity, host factors (e.g., duration and degree of
neutropenia/lymphopenia) play a key role and should be considered when
choosing anti-microbial prophylaxis. In relapsed/refractory disease,
the ANC should be monitored closely post blinatumomab since neutropenia
can unexpectedly develop after treatment which may be compounded by the
underlying disease and recent chemotherapy effects.
|
Introduction
Blinatumomab
is a bispecific monoclonal antibody that enables CD3-positive T-cells
to identify and eradicate CD19 expressed on B-cells in acute
lymphoblastic leukemia (ALL).[1] It activates
endogenous T-cells by connecting CD3 in the T-cell receptor complex
with CD19 on either malignant or benign B-cells, thus forming a
cytolytic synapse between a cytotoxic T-cell and the cancer target
B-cell. Blinatumomab was approved for use in patients with relapsed or
refractory B-cell precursor ALL based on a phase 2 trial which showed a
43% complete remission rate with complete or partial hematologic
recovery and 6.1 months of the median overall survival.[2]
More recently, a multi-institutional phase 3 trial by Kantarjian and
colleagues showed blinatumomab group with a significantly increased
overall survival as compared to chemotherapy group, which led to an
early termination of the study; the median overall survival was 7.7
months in the blinatumomab group v. 4.0 months in the chemotherapy
group (hazard ratio for death blinatumomab vs. chemotherapy, 0.71,
p=0.01).[1]
Interestingly, in the phase 3 trial,
blinatumomab group had numerically lower rates of infection (34.1% v.
52.3%), neutropenia (37.8% v. 57.8%), lymphopenia (1.5% v. 3.7%) and
stomatitis (6.7% v. 12.8%) as compared to chemotherapy group.[1,3]
However, complications from blinatumomab also included cytokine release
syndrome (4.9% v. 0%), which mimics infection by mediating the
production of cytolytic proteins, release of inflammatory cytokines,
and proliferation of T-cells, then ultimately lysis of CD19-positive
cells. Furthermore, numerically higher rates of hypogammaglobulinemia
(6.0% v. 0.9%),[3] upper respiratory tract infection
(7.1% v. 0.9%) and serious pneumonia (3.7% v. 1.8%) were observed with
blinatumomab, which rose concerns for its infectious risks and
complications.[1] Of note, the phase 3 trial required
antifungal prophylaxis primarily using posaconazole for the patients
who previously underwent allogeneic hematopoietic stem-cell
transplantation (HSCT) and presented with a medical history of
graft-versus-host disease (GVHD), but the protocol does not discuss
antibacterial prophylaxis.[4] In the phase 2 trial,
institutional guidelines for infections were followed when patients
became neutropenic, but no details about antimicrobial prophylaxis were
provided.[2]
Currently, antiviral prophylaxis
with acyclovir and anti-Pneumocystis jiroveci pneumonia prophylaxis
with sulfamethoxazole-trimethoprim are recommended as per National
Comprehensive Cancer Network (NCCN) guidelines[5] for
patients with active ALL. However, guidelines for anti-bacterial and
anti-fungal prophylaxis are not well established in patients receiving
blinatumomab. In this retrospective review, we intended to describe
infectious risks and complications in these patients to assist in the
supportive care from an Infectious Diseases standpoint including
determination of appropriate antimicrobial prophylaxis regimen.
Patients and Methods
Study Subjects and Design.
A single-center, retrospective, non-interventional study was conducted
among adult patients who received blinatumomab for the treatment of ALL
between May 1, 2015 and April 1, 2017 at Moffitt Cancer Center (Tampa,
FL, USA). All patients who underwent blinatumomab treatment during the
study period were identified from Moffitt Cancer Center Cerner’s
PowerChart. Among these, patients with less than seven days of the
blinatumomab treatment were excluded.
The study was approved by
the Institutional Review Board of University of South Florida. For this
type of study formal consent is not required; an informed consent
waiver was granted as all data were currently in existence and no
patient-specific interventions were conducted for the study. The
collection of data was in compliance with the Health Insurance
Portability and Accountability Act of 1996.
Patient Characteristics and Infectious Risks.
Once patients were identified, the following characteristics were
extracted from the medical records: age; gender; treatment phase
(refractory to primary or salvage therapy, first relapse with remission
<12 months, first relapse with remission >12 months, untreated
second or greater relapse, relapse after HSCT or treatment with
chimeric antigen receptor modified T-cells (CART), remission, or
unspecified); prior chemotherapy regimens; other immunosuppressive
comorbid conditions or treatment; recent infections within 7 days prior
to initiation of blinatumomab; recent use of intravenous antimicrobials
within 90 days prior to initiation of blinatumomab; days between prior
cytotoxic chemotherapy and blinatumomab; total number of blinatumomab
cycles and reasons for interrupted blinatumomab treatment if any;
baseline absolute neutrophils (ANC) and lymphocyte (ALC) count;
incidence, severity and duration of neutropenia and lymphopenia and
whether there was a growth factor support or not.
Infectious Complications.
Microbiological culture-proven infections and clinically diagnosed
infections by imaging and physical exams were recorded. Nodular
pneumonia defined as an opaque macronodule of ≥ 1cm in diameter, which
is by far the most common CT finding in invasive aspergillosis and
present in > 90% of patients,[6] is assessed at
baseline and every two weeks during the duration of neutropenia using
CT thorax without contrast. Mortalities at 30 and 60 days after the end
of the first cycle of blinatumomab were assessed.
Statistical Analysis.
For bacteremia and nodular pneumonia suspicious for mold infection, the
following characteristics were compared between the case and control
groups using Mann-Whitney U test for the ordinal or non-normally
distributed continuous variables and chi-square test for nominal
variables: immunosuppressing conditions or treatments, cytotoxic
chemotherapy prior to blinatumomab within 21 days, baseline neutrophil
and lymphocyte counts, and incidence, severity and duration of
neutropenia and lymphopenia. Multinomial logistic regression tests were
performed after univariate analyses to evaluate risk factors associated
with nodular pneumonia and bacteremia. A two-tailed P value of <0.05
was considered to be statistically significant. All data were analyzed
using SPSS (IBM Corp. Released 2016. IBM SPSS Statistics for Windows,
Version 24.0., Armonk, NY, USA).
Results
Patient Characteristics and Infectious Risks.
Twenty patients who received a total of 35 cycles of blinatumomab
between May 1, 2015 and April 1, 2017 were included in analyses after
excluding two patients who received blinatumomab for less than seven
days. Baseline characteristics are summarized in Table 1.
Ten of the 35 cycles were interrupted for the following reasons: 4
cytokine release syndrome (CRS), 3 liver function test abnormalities (1
transaminitis, 2 hyperbilirubinemia) and 3 patients with disease
progression while on blinatumomab. None of the interruptions were
deemed due to infections although a patient with CRS also had mild
ground glass pneumonia. Most patients had refractory (25%) or relapsed
(70%) disease with a median of two previous chemotherapies. Four
patients had recent infections within 7 days prior to initiation of
blinatumomab: C. difficile
colitis (n=1), viral upper respiratory infection (n=1), Pseudomonal
bacteremia (n=1) and one patient with both sinusitis and nodular,[6] possible[7]
mold pneumonia per CT scan. Twelve of 20 patients received intravenous
antimicrobials within 90 days prior to blinatumomab treatment. Only two
patients received growth factor support. Mean durations of neutropenia
(ANC <500 cells/µL) and severe neutropenia (ANC <100 cells/µL)
were 11.1 ± 10.0 and 2.7 ± 5.1 days, respectively, and the
corresponding durations for lymphopenia were 12.6 ± 11.3 (ALC <500
cells/µL) and 1.4 ± 1.5 (ALC <100 cells/µL) days.
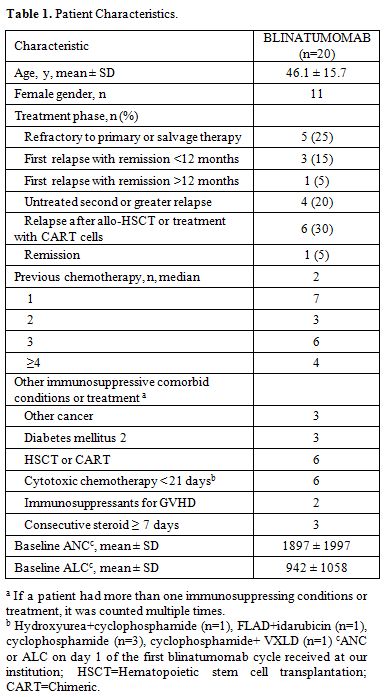 |
Table 1. Patient Characteristics. |
Infectious Complications.
While six patients did not experience infections, 14 patients had a
total of 26 infections, of which lower respiratory (n=9),
gastrointestinal (n=6) and bacteremia (n=5) were most common during or
30 days after the blinatumomab treatment. Four patients had nodular,
possible invasive mold pneumonia, three of which were newly developed
on blinatumomab with normal baseline CT thorax. None of the four
patients had met direct (i.e., cytology, direct microscopy, or
culture-proven) nor indirect [i.e., Aspergillus galactomannan antigen
(GM) or β-D-glucan test (BDG)] microbiological criteria for proven or
probable invasive fungal infection, [7] but had host
factors and radiologic evidence of invasive mold pneumonia (i.e.
nodular consolidation with halo signs). One patient who already had
possible mold pneumonia prior to blinatumomab received isavuconazole
throughout blinatumomab treatment. Two patients were on micafungin and
the last patient was on voriconazole followed by posaconazole as
antifungal prophylaxis before the CT findings of possible mold
pneumonia. Compared to patients without nodular, possible mold
pneumonia, patients with nodular pneumonia had significantly lower ANC
on the first day of blinatumomab (2319 v. 208/µL, p=0.011). In
multinomial logistic regression, ANC on the first day of blinatumomab
remained significantly associated with nodular pneumonia (p= 0.020)
when controlled for other immunosuppressing conditions and cytotoxic
chemotherapy within 21 days prior to blinatumomab.
There were 5 episodes of bacteremia from 4 patients, two of which were polymicrobial (Table 2).
Three of the 4 patients received cytotoxic chemotherapy within 21 days
of blinatumomab, and the timelines between the onset of bacteremia and
cytotoxic chemotherapy are listed in the footnote (Table 2).
One patient was discharged with no antibacterial prophylaxis since ANC
recovered to >500 cells/µL, but developed Pseudomonal bacteremia on
day 35 of blinatumomab with ANC~ 100 cells/µL. One patient expired
within 30 days due to progressive leukemia.
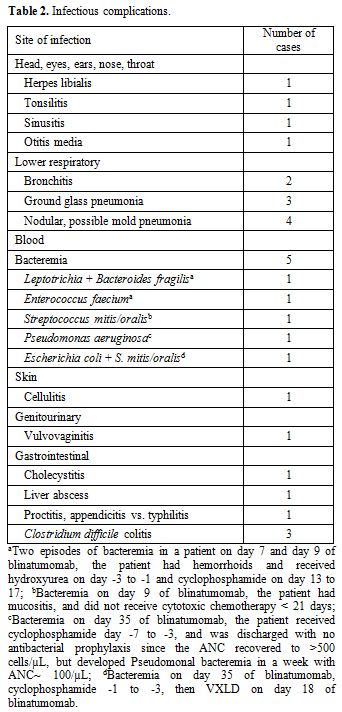 |
Table 2. Infectious complications. |
When
factors associated for nodular, possible mold pneumonia were examined,
baseline ANC and days to become severely neutropenic (ANC < 100
cells/µL) were significantly correlated to nodular pneumonia (Table 3),
but the duration of lymphopenia nor the severity of lymphopenia were
not related (data not shown). The similar analysis for bacteremia found
no significantly associated factors.
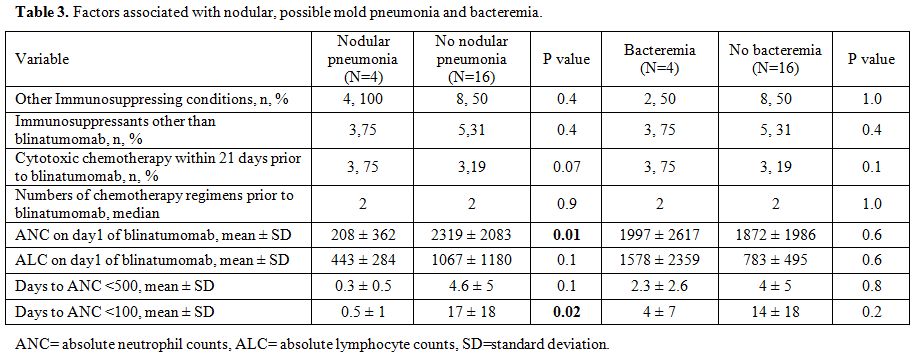 |
Table 3. Factors associated with nodular, possible mold pneumonia and bacteremia. |
Two
patients were sent to hospice care by 30 days post blinatumomab with
progressive disease, one of whom also had nodular, possible mold
pneumonia and bacteremia. There were two other patients who expired in
the 30 to 60-day range, one of whom experienced multiple infectious
complications (i.e., pansinusitis, possible mold pneumonia, P. aeruginosa bacteremia and C. difficile colitis). Additional two patients were sent to hospice care by 60 days post blinatumomab with progressive disease.
Discussion
Blinatumomab
is an anti-CD19 immunotherapy, newly approved for relapsed or
refractory B-cell ALL with significantly increased survival rate. While
it showed lower rates of infection, myelosuppression and mucosal
barrier injury as compared to conventional chemotherapy in phase 3
clinical trial, there are yet concerns about its infectious risks
due to hypogammaglobulinemia, severe pneumonia as well as
infection-mimicking complications such as cytokine release syndrome. In
our small retrospective chart review of 20 patients who received
blinatumomab, lower respiratory infections were most commonly observed
followed by intra-abdominal infections and bacteremia. We report a
relatively high incidence rate of 15% for nodular, possible mold
pneumonia newly developed on blinatumomab treatment, which was
associated with duration and degree of neutropenia. Host factors in
hematologic malignancies, i.e., impaired antifungal defenses, have been
recognized as important risks for invasive fungal disease,[7] which have accounted for a disproportionate number of fungal pneumonia in North America and Europe.[8,9]
While we did not find statistical differences in other host factors
such as receipt of immunosuppressive agents or other immunosuppressing
conditions including history of HSCT between the patients with and
without nodular pneumonia, we found the differences in baseline ANC,
which is a well-known risk factor for fungal pneumonia.[10] Historically, the incidence of invasive fungal infection in hematologic malignancy or HSCT ranges from 0.8-11.3%.[11-14]
The high incidence rates of 15% (i.e., 3 of 20 patients excluding one
patient who already had possible mold pneumonia prior to blinatumomab)
for possible mold pneumonia in our patient echoed the role of the
compromised host factors in the patients with relapsed or refractory
ALL for whom blinatumomab is approved. This is well reflected in our
patients’ treatment phase and numbers of previous chemotherapies, 50%
of which were comprised of untreated second or greater relapse or post
HSCT and who received ≥ three previous chemotherapies,
respectively. Although blinatumomab itself causes less myelosuppression
than conventional chemotherapy, when designing antimicrobial
prophylaxis in these patients with relapsed or refractory ALL,
compromised host factors should be considered, and we advocate for
anti-mold coverage when the baseline ANC is < 500 cells/µL.
On
the other hand, the incidence of bacteremia was not significantly
associated with the baseline ANC. Since chemotherapy-induced mucositis
is associated with early onset of bacteremia[15] and blinatumomab causes less stomatitis than conventional chemotherapy (6.7% v. 12.8%),[3] we investigated the timing between the onset of bacteremia and other cytotoxic chemotherapy given pre and post blinatumomab (Table 2).
While hydroxyurea is not considered conventional chemotherapy, it could
cause severe mucositis, thus was counted as cytotoxic chemotherapy.
Although 3 of the 4 bacteremic patients received cytotoxic chemotherapy
prior to initiation of blinatumomab within 21 days, 2 of those 3
patients had bacteremia on day 35 of blinatumomab, which makes it less
likely that the episodes of bacteremia were related to the cytotoxic
chemotherapy that was received prior to blinatumomab. Furthermore, one
of these two patients who had bacteremia on day 35 of blinatumomab,
also received VXLD (dexamethasone, doxorubicin, vincristine, bortezomib
and peg-asparaginase) on day 18 of blinatumomab, which is known to
induce mucositis and bacterial translocation from the gut. Nonetheless,
compared to non-bacteremic patients, numerically higher number of
patients with bacteremia received cytotoxic chemotherapy ≤21 days of
blinatumomab (Table 3). Other than mucositis, chemotherapy dose gram/m2,[16] severe neutropenia of ANC <100 cell/µL,[17] or previous use of antibacterial prophylaxis for neutropenia[18]
have been identified as risks for bacteremia. Understandably, one of
our patients had hemorrhoids and developed two episodes of
polymicrobial bacteremia. Another patient developed pseudomonal
bacteremia when ANC dropped to around 100 cells/µL after being
discharged post count recovery, i.e. ANC>500 cell/µL, without
antibacterial prophylaxis. Fluctuation in ANC is not uncommon in
relapsed or refractory disease, and ANC should be monitored closely
during both inpatient and outpatient stays as neutropenia can
unexpectedly develop after blinatumomab which may be compounded by the
underlying disease and recent chemotherapy effects. While we did not
identify specific risk factors associated with breakthrough bacteremia,
we propose antibacterial prophylaxis to be individualized based on the
degree of neutropenia, other recent cytotoxic chemotherapy, mucositis,
previous bacterial infections and other risk factors such as
hemorrhoids. For example, antibacterial prophylaxis may be initiated
when ANC <100 cell/µL for most patients receiving blinatumomab
treatment, but the ANC cut-off may be changed to <500 cell/µL if
other risk factors coexist.
Conclusions
Based
on the findings herein, we advocate for anti-mold coverage when ANC
< 500/µL in this patient population considering compromised host
factors. While we need more data to support our recommendation for
anti-bacterial prophylaxis in this population, it may be prudent to
individualize based on well-known risk factors. In relapsed or
refractory disease, the ANC should be monitored closely post
blinatumomab since neutropenia can unexpectedly develop after treatment.
References
- Kantarjian H, Stein A, Gokbuget N, Fielding AK,
Schuh AC, Ribera JM, Wei A, Dombret H, Foa R, Bassan R, Arslan O, Sanz
MA, BergeronJ, Demirkan F, Lech-Maranda E, Rambaldi A, Thomas X, Horst
HA, Bruggemann M, Klapper W, Wood BL, Fleishman A, Nagorsen D, Holland
C, Zimmerman Z, Topp MS. Blinatumomab versus chemotherapy for advanced
acute lymphoblastic leukemia. N Engl J Med 2017;376:836-47. https://doi.org/10.1056/NEJMoa1609783 PMid:28249141 PMCid:PMC5881572

- Topp
MS, Gokbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, Dombret H,
Fielding AK, Heffner L, Larson RA, Neumann S, Foa R, Litzow M, Ribera
JM, Rambaldi A, Schiller g, Bruggermann M, Horst HA, Holland C, Jia C,
Maniar T, Huber B, NagorsenD, Forman SJ, Kantarjian HM. Safety and
activity of blinatumomab for adult patients with relapsed or refractory
B-precursor acute lymphoblastic leukaemia: a multicenter, single-arm,
phase 2 study. Lancet Oncol 2015;16:57-66. https://doi.org/10.1016/S1470-2045(14)71170-2

- Supplement
to: Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera
JM, Wei A, Dombret H, Foa R, Bassan R, Arslan O, Sanz MA, BergeronJ,
Demirkan F, Lech-Maranda E, Rambaldi A, Thomas X, Horst HA, Bruggemann
M, Klapper W, Wood BL, Fleishman A, Nagorsen D, Holland C, Zimmerman Z,
Topp MS. Blinatumomab versus chemotherapy for advanced acute
lymphoblastic leukemia. N Engl J Med 2017;376:836-47 https://doi.org/10.1056/NEJMoa1609783 PMid:28249141 PMCid:PMC5881572

- Protocol
Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM,
Wei A, Dombret H, Foa R, Bassan R, Arslan O, Sanz MA, BergeronJ,
Demirkan F, Lech-Maranda E, Rambaldi A, Thomas X, Horst HA, Bruggemann
M, Klapper W, Wood BL, Fleishman A, Nagorsen D, Holland C, Zimmerman Z,
Topp MS. Blinatumomab versus chemotherapy for advanced acute
lymphoblastic leukemia. N Engl J Med 2017;376:836-47. https://doi.org/10.1056/NEJMoa1609783 PMid:28249141 PMCid:PMC5881572

- National Comprehensive Cancer Network. Prevention and treatment of cancer-related infections. https://www.nccn.org/professionals/physician_gls/pdf/infections.pdf. Accessed Nov 2, 2017.
- Greene R. The radiological spectrum of pulmonary aspergillosis. Med Mycol 2005;43 Suppl 1:S147-54.

- De
Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T,
Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson
TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler
CC, Kullberg BJ, Marr KA, Mu-oz P, Odds FC, Perfect JR, Restrepo A,
Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR,
Zaoutis T, Bennett JE; European Organization for Research and Treatment
of Cancer/Invasive Fungal Infections Cooperative Group; National
Institute of Allergy and Infectious Diseases Mycoses Study Group
(EORTC/MSG) Consensus Group. Revised definitions of invasive fungal
disease from the European Organization for Research and Treatment of
Cancer/Invasive Fungal Infections Cooperative Group and the National
Institute of Allergy and Infectious Diseases Mycoses Study Group
(EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813-21. https://doi.org/10.1086/588660 PMid:18462102 PMCid:PMC2671227

- Bitar
D, Lortholary O, Le Strat Y, Nicolau J, Coignard B, Tattevin P, Che D,
Dromer F. Population-based analysis of invasive fungal infections.
France, 2001-2010. Emerg Infect Dis 2014;20:1149-55. https://doi.org/10.3201/eid2007.140087 PMid:24960557 PMCid:PMC4073874

- Azie
N, Neofytos D, Pfaller M, Meier-Kriesche HU, Quan SP, Horn D. The PATH
(Prospective antifungal therapy) Alliance® registry and invasive fungal
infections: update 2012. Diagn Microbiol Infect Dis 2012;73:293-300. https://doi.org/10.1016/j.diagmicrobio.2012.06.012 PMid:22789847

- Gerson
SL, Talbot GH, Hurwitz S, Strom BL, Lusk EJ, Cassileth PA. Prolonged
granulycytopenia: the major risk factor for invasive pulmonary
aspergillosis in patients with acute leukemia. Ann Intern med
1984:100:345-51. https://doi.org/10.7326/0003-4819-100-3-345 PMid:6696356

- Kurosawa
M, Yonezumi M, Hashino S, Tanaka J, Nishio M, Kaneda M, Ota S, Koda K,
Suzuki N, Yoshida M, Hirayama Y, Takimoto R, Torimoto Y, Mori A,
Takahashi T, Iizuka S, Ishida T, Kobayashi R, Oda T, Sakai H, Yamamoto
S, Takahashi F, Fukuhara T. Epidemiology and treatment outcome of
invasive fungal infections in patients with hematological malignancies.
Int J Hematol 2012;96:748-57. https://doi.org/10.1007/s12185-012-1210-y PMid:23111539

- Sun
Y, Huang H, Chen J, Li J, Ma J, Li J, Liang Y, Wang J, Li Y, Yu K, Hu
J, Jin J, Wang C, Wu D, Xiao Y, Huang X. Invasive fungal infection in
patients receiving chemotherapy for hematological malignancy: a
multicenter, prospective, observational study in China. Tumour Biol
2015;36:757-67. https://doi.org/10.1007/s13277-014-2649-7 PMid:25293517

- Pagano
L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, Pastore D,
Picardi M, Bonini A, Chierichini A, Fanci R, Caramatti C, Invernizzi R,
Mattei D, Mitra ME, Melillo L, Aversa F, Van Lint MT, Falcucci P,
Valentini CG, Girmenia C, Nosari A. The epidemiology of fungal
infections in patients with hematologic malignancies: the SEIFEM-2004
study. Haematologica 2006;91:1068-75. PMid:16885047

- Auberger
J, Lass-Florl C, Ulmer H, Nogler-Semenitz E, Clausen J, Gunsilius E,
Einsele H, Gastl G, Nachbaur D. Significant alterations in the
epidemiology and treatment outcome of invasive fungal infections in
patients with hematological malignancies. Int J Hematol 2008;88:508-15.
https://doi.org/10.1007/s12185-008-0184-2 PMid:18982251

- Van
der Velden WJ, Herbers AH, Netea MG, Blijlevens NM. Mucosal barrier
injury, fever and infection in neutropenic patients with cancer:
introducing the paradigm febrile mucositis. Br J Haematol
2014;167:441-52. https://doi.org/10.1111/bjh.13113 PMid:25196917

- Lewis
V, Yanofsky R, Mitchell D, Dix D, Ethier MC, Gillmeister B, Johnston D,
Michon B, Stobart K, Portwine C, Silva M, Cellot S, Price V, Bowes L,
Zelcer S, Brossard J, Beyene J, Sung L. Predictors and outcomes of
viridans group streptococcal infections in pediatric acute myeloid
leukemia: from the Canadian infections in AML research group. Pediatr
Infect Dis J. 2014;33:126-9. https://doi.org/10.1097/INF.0000000000000058 PMid:24064558

- Girmenia
C, Bertaina A, Piciocchi A, Perruccio K, Algarotti A, Busca A, Cattaneo
C, Raiola AM, Guidi S, Iori AP, Candoni A, Irrera G, Milone G, Marcacci
G, Scimè R, Musso M, Cudillo L, Sica S, Castagna L, Corradini P,
Marchesi F, Pastore D21, Alessandrino EP, Annaloro C, Ciceri F,
Santarone S, Nassi L, Farina C, Viscoli C, Rossolini GM, Bonifazi F,
Rambaldi A; Gruppo Italiano Trapianto di Midollo Osseo (GITMO) and
Associazione Microbiologi Clinici Italiani (AMCLI). Incidence, risk
factors and outcome of pre-engraftment Gram-negative bacteremia after
allogeneic and autologous hematopoietic stem cell transplantation: an
Italian prospective multicenter survey. Clin Infect Dis 2017;
65:1884-96. https://doi.org/10.1093/cid/cix690

- De
Rosa FG, Motta I, Audisio E, Frairia C, Busca A, Di Perri G, Marmont F.
Epidemiology of bloodstream infections in patients with acute myeloid
leukemia undergoing levofloxacin prophylaxis. BMC Infect Dis
2013;13:563-7. https://doi.org/10.1186/1471-2334-13-563 PMid:24289496 PMCid:PMC4219399.

[TOP]





















