Eva Eliassen1*, Gerhard Krueger2, Mario Luppi3 and Dharam Ablashi1.
1 HHV-6 Foundation, Santa Barbara, California, USA.
2 Department of Pathology and Laboratory Medicine, University of Texas, Houston, Texas, USA.
3 Department of Medical and Surgical Sciences, University of Modena and Reggio Emilia, Modena, Italy.
Corresponding
author: Eva Eliassen. HHV-6 Foundation, 1482 East Valley Road,
Suite 619, Santa Barbara, CA 93108, USA. Tel: +1-561-926-8564. E-mail:
eva@hhv-6foundation.org
Published: May 1, 2018
Received: April 24, 2018
Accepted: April 26, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018035 DOI
10.4084/MJHID.2018.035
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Human
herpesvirus 6A and 6B (HHV-6A and HHV-6B) have been noted since their
discovery for their T-lymphotropism. Although it has proven difficult
to determine the extent to which HHV-6A and HHV-6B are involved in the
pathogenesis of many diseases, evidence suggests that primary infection
and reactivation of both viruses may induce or contribute to the
progression of several lymphoproliferative disorders, ranging from
benign to malignant and including infectious mononucleosis-like
illness, drug induced hypersensitivity syndrome/drug reaction with
eosinophilia and systemic symptoms (DIHS/DRESS), and nodular sclerosis
Hodgkin’s lymphoma. Herein, we discuss the conditions associated with
the lymphoproliferative capacity of HHV-6, as well as the potential
mechanisms behind them. Continued exploration on this topic may add to
our understanding of the interactions between HHV-6 and the immune
system and may open the doors to more accurate diagnosis and treatment
of certain lymphoproliferative disorders.
|
Introduction
Human
herpesvirus 6A and 6B (HHV-6A and HHV-6B), collectively known as HHV-6,
are a pair of closely related betaherpesviruses of the genus
Roseolovirus with lymphocytic tropism and immunomodulatory
capabilities.[1,2,3] HHV-6A was discovered in the
peripheral blood mononuclear cells (PBMCs) of patients with
AIDS-related lymphomas and other lymphoproliferative disorders in the
latter part of the 1980s, and its T cell tropism was established in
short order.[1,4-6] Early
investigations found that the available HHV-6 isolates could be split
into two distinct variants, with HHV-6 Type A preferentially infecting
immature T cells and Type B infecting mature T cells.[2,7]
These initial findings were followed by detection of HHV-6 antigen and
DNA in the lymph nodes of patients with lymphoproliferative disorders
and autopsy specimens,[8-12] and a possible role for HHV-6 in lymphomas was examined, with some compelling results but no consensus.[13-15]
Although the virus has been implicated in a range of
lymphoproliferative disorders, efforts are still underway to elucidate
the pathogenic roles of HHV-6 in these conditions. The ubiquitous
nature of the virus was recognized early on as a challenge in
identifying its role in the disorders in which it has been implicated,
as its DNA can be found in the peripheral blood of healthy donors and
low level reactivation may occur without clinical manifestations.
Consequently, serological studies serve primarily to screen patients
for HHV-6 infection, while the most conclusive results are gained
through the use of quantitative PCR, staining techniques applied to
tissue samples, and through comparison with carefully selected
controls. With the technical advancements and growing understanding of
the effects of HHV-6 on the immune response, investigators are
continuing to learn about the potential for HHV-6 to trigger
lymphoproliferative disorders and the possible mechanisms behind it.
Interactions between HHV-6 and the Immune System
HHV-6A and B infect hematopoietic stem cells (CD34+, CD32+), cord blood cells, and several immune cell populations in vitro and in vivo, including T lymphocytes, NK cells,[16] monocytes and macrophages,[17,18] and dendritic cells,[19] and HHV-6A has also been found to infect EBV-immortalized B cells.[20]
However, as the two species utilize different cellular receptors and
impact chemokine/cytokine signaling in different ways, their tropism
differs. This matter is further complicated as the presence of cellular
receptors for HHV-6 does not guarantee infection of such cells in
tissues.[21] Viral DNA is found in 9-25% of peripheral blood mononuclear cells (PBMCs) from healthy donors,[13,22] and HHV-6B is found more frequently than HHV-6A, which has been detected in the PBMCs of 3-10% of healthy individuals.[13] In most healthy adults, a T cell response to HHV-6 is present,[23] but fewer than 0.12% of CD4+ T cells in PBMC samples are reactive to HHV-6,[24] and fewer than 1 in 105 CD8+ T cells in PBMCs are HHV-6-specific.[25]
Reactivation of HHV-6 occurs during T cell activation by various
stimuli, including endotoxins, endocrine stimulation, certain
cytokines, and food components (e.g. agglutinins, phorbol esters
etc.). Immunosuppressive conditions, including those involving stress,
transient immunosuppression, and/or stimulation resulting from
infection with other viruses such as measles virus, support the
persistent activity of HHV-6 once reactivated. Similar to EBV
infections, persistent HHV-6 activity can also be found after organ and
hematopoietic stem cell transplantation.
As our understanding of
HHV-6A and B has grown, their immunomodulatory activities have emerged
as key contributors to many of the clinical manifestations linked to
HHV-6 infection. HHV-6 infection is most commonly associated with
exanthema subitum (roseola infantum),[26] a
manifestation of primary infection that occurs during early childhood.
Intense reactivation of the virus is frequently observed in the
transplantation setting, resulting in a host of complications post-
solid organ and hematopoietic stem cell transplantation (HSCT). These
conditions, as well as others associated with HHV-6 infection and
reactivation, are often mediated to a great extent by the immune
response to the virus and by the effects of the virus on various immune
cell populations and cytokine/chemokine expression. The influences
exerted by HHV-6A/HHV-6B differ by species, but both trigger
inflammatory reactions while also employing mechanisms by which they
can suppress an immune response and avoid detection.
In vitro experimentation has demonstrated that HHV-6B and HHV-6A induce myelosuppression,[27,28] infection of T cells and PBMCs inhibit immune responses against a tuberculin protein derivative or mumps antigen,[29]
and infection of PBMCs results in reduced IL-2 mRNA and protein
synthesis- 50% less than mock-infected cells- sharply reducing cellular
proliferation and indicating that the functions of T cells are
suppressed.[30] In the clinical setting, thymic atrophy and severe T lymphocytopenia have been reported,[31-33] and in transplant recipients, delayed engraftment, myelosuppression,[34] and graft failure[35]
have been known to occur in response to active HHV-6. An inverse
correlation has been identified between reconstitution of CD4+ cells
after HSCT and reactivation of HHV-6,[36] as well as CD3+ cells[37] and CD8+ cells,[38] and indeed, proliferation of lymphocytes has been inhibited by persistent HHV-6 infections after allo-HSCT.[39] However, transplantation of cord blood, which is associated with a higher risk of HHV-6 reactivation and higher HHV-6 DNAemia[40]
compared to transplantation of other sources of hematopoietic stem
cells, have also been found to have faster reconstitution of B
lymphocytes with higher B cell counts, a phenomenon that has been
hypothesized to arise as a result of an immune response against viral
reactivation.[40] As immune suppression enables HHV-6
to better avoid detection and clearance, this phenomenon is
advantageous for the persistence of the virus, but proliferation of
infected lymphocytes may also be beneficial for its dissemination.
Accordingly, immune suppression and immune activation are but two sides
of the same coin during HHV-6 infection. When either suppression or
proliferation is unchecked, severe clinical manifestations may result.
Both
HHV-6A and HHV-6B are able to affect chemokine/cytokine pathways, which
are dysregulated in lymphoproliferative disorders. Infection by either
virus impairs production of IL-12 in macrophages[41] and in dendritic cells,[42]
which may allow the viruses to suppress activation of cytotoxic
effectors. In combination with lower IL-2 expression,30 TNF-alpha,
IL-1beta, IL-8, and IL-15 have been found to be upregulated,[43,44,45]
coinciding with a shift from a Th1 to Th2 cytokine profile. HHV-6A
infection of astrocytes in patients with glioma has been associated
with significantly upregulated TGF-beta, IL-6, and IL-8,[46] and in vitro, HHV-6B infection of astrocytes has increased production of the proinflammatory cytokines IL-6 and IL-1beta.[47]
Notably, cytokine expression patterns vary temporally and in relation
to the infected cell’s environment. IL-10, IL-11, and other
anti-inflammatory mediators, for example, are expressed at high levels
after exposing HHV-6 infected astrocytes to proinflammatory cytokines.[48]
Viral
proteins are also integral to HHV-6-mediated immunomodulation. The
HHV-6B encoded chemokine U83B induces chemotaxis and activation of
leukocytes expressing CCR2, which is expressed under proinflammatory
conditions.[49] Similarly, endometrial epithelial
cells infected with HHV-6A have shown increased cell surface expression
of CCL2, IP-10, and CCL26.[50] On the other hand, the U83A chemokine (HHV-6A-specific), as well as the U51A chemokine receptor, target CCR5/CCL5 (RANTES),[51]
another receptor/ligand pair involved in inflammation, resulting in
down-modulation of their activity. U51A also binds four other
inflammatory modulating chemokines that bind to and stimulate several
immune cell populations, including B and T lymphocytes- CCL2, CCL7,
CCL11, and CCL13- and the inflammatory cells expressing the U51A
receptor exhibit chemotaxis toward, and internalization of, target
chemokines.[52] In contrast, primary HHV-6B infection results in upregulation of CCL2, CXCL11, CXCL10, and CXCL16.[53]
Another
mechanism by which the pair of viruses affect their host cells is the
alteration of cell membrane fluidity and the dysregulation of cellular
receptors.[54,55] Both species downregulate MHC class I[56,57] and CD46, which may result in activation of autologous complement and cellular damage in lymphoid tissue and beyond,[58]
and HHV-6A impairs expression of the T cell receptor/CD3 complex at the
cell membrane, rendering the affected cells ineffective in responding
to antigen-presenting cells.[4,59,60] In addition, HHV-6A triggers expression of CD4 mRNA and protein production in CD4 negative NK[16] and T cells[61] and can reduce the cytotoxicity of CD4+ T cells.[62]
Both species interact with toll-like receptors and may exert their
effects on cytokine/chemokine expression and the Th1/Th2 balance
through them.[63-67]
Benign/Reactive Lymphoproliferative Disorders
Primary
HHV-6B infection was identified as a causative agent of exanthema
subitum (roseola infantum) in 1988, when the onset of the illness was
linked to HHV-6 seroconversion, and the presence of viral antigen was
observed in patients’ lymphocytes.[26] In some cases observed by Krueger et al., exanthema subitum was also seen during primary HHV-6A infection.[68] In addition to the characteristic rash, fever, and occasionally febrile seizures and encephalitis,[69,70]
children with exanthema subitum can experience lymphadenopathy,
relative lymphocytosis with increased CD38+ (immature) T cells, and
hepatomegaly.[69,71,72]
Thrombocytopenia, neutropenia, and leukopenia are commonly found as well.[73] Notably, reactive “atypical” lymphocytes and hemophagocytosis may be observed in bone marrow,[74,75] and a mononucleosis-like illness, with reactive lymphocytosis and liver dysfunction, has been described.[76]
Two unique cases documenting HHV-6 infection with unusual
lymphoproliferation in very young children have been reported: One
infant, 7 months old, died suddenly after developing otitis media, and
HHV-6 was found by PCR in tissue samples and in atypical lymphoid
infiltrate via ISH.[77] Abnormal, inflammatory
lymphocytic infiltration was present in the liver, kidney, heart,
spleen, bone marrow, and lymph nodes of the child, and interstitial
pneumonitis was reported. In the other case, a 2-week-old with HHV-6
DNA present in PBMCs, and who perhaps had congenital HHV-6, developed
bone marrow cell proliferation, hypersensitivity to
granulocyte-macrophage colony-stimulating factor (GM-CSF),
hepatosplenomegaly, and was thought to have juvenile myelomonocytic
leukemia. However, the symptoms resolved.[78] Generally, lympho-proliferative responses during primary infection are limited in scope and resolve without issue.
After
primary infection, HHV-6A and HHV-6B remain latent in many organs,
including the heart, lungs, gastrointestinal tract, and the brain, in
addition to circulating lymphocytes. In immunocompetent individuals,
low level reactivation may occur without major clinical symptoms. In
those with immune deficiencies,[79] incomplete
clearance and persistent activation cause various clinical diseases
(e.g. post-transplant syndromes, autoimmune disorders etc.) During
persistent or frequently recurrent reactivation in immune deficient
persons (which is usually the case in studying adult patients), the
virus has been associated with generalized reactive lymphadenopathy
lasting for several days to several weeks.[80-84]
Among patients with reactive lymphadenopathy, scattered positivity
(less than 1% of total cells) when staining for the HHV-6 antigens
p101K, gp106, and gp116, has been observed among cells, namely plasma
cells and histiocytes, from lymph node biopsies.[85]
The virus has been detected by PCR in PBMCs at a higher prevalence
among patients with lymphoproliferative disorders than in healthy
volunteers,[86] and in Brazilian patients with
lymphadenopathy and fever (but without skin rash), 8.7% had active
HHV-6 infection (plasma viremia).[87] While these
results indicate that HHV-6 may contribute to reactive lymphadenopathy,
they may not implicate HHV-6 as the sole virus involved in its
development, as it is difficult to rule out its reactivation in
response to a different underlying cause of lymphadenopathy, including
infections by other viruses. However, strong evidence of a role for
HHV-6 in chronic/recurrent lymphadenopathy, backed by
immune-histochemical staining- a more specific technique- has been
reported. Of 111 cases of benign/reactive lymphadenopathies with
unknown etiology in a recent study, 7 (6.3%) demonstrated
recurrent/chronic behavior, and intense staining for HHV-6B was
detected in follicular dendritic cells in all cases (Figure 1).[88]
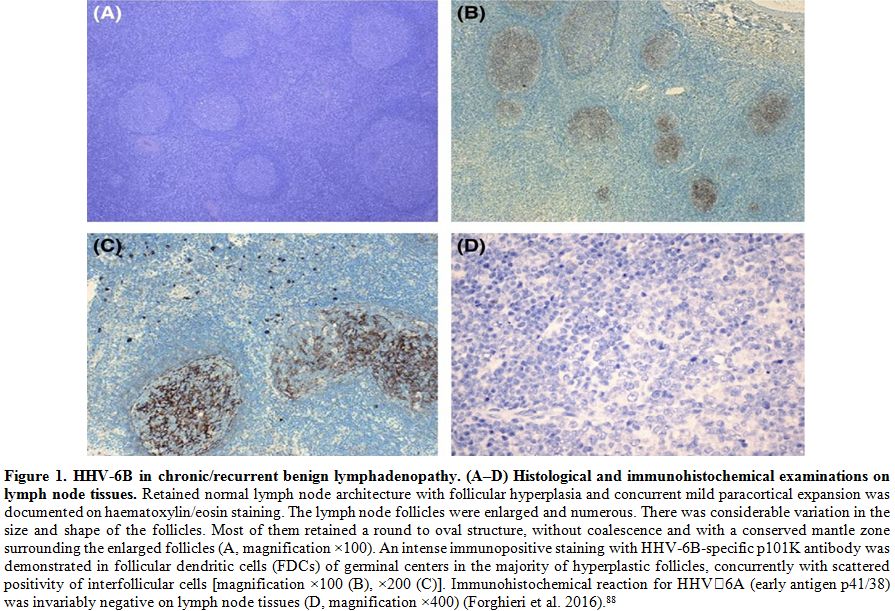 |
Figure 1. HHV-6B in
chronic/recurrent benign lymphadenopathy. (A–D) Histological and
immunohistochemical examinations on lymph node tissues. Retained normal
lymph node architecture with follicular hyperplasia and concurrent mild
paracortical expansion was documented on haematoxylin/eosin staining.
The lymph node follicles were enlarged and numerous. There was
considerable variation in the size and shape of the follicles. Most of
them retained a round to oval structure, without coalescence and with a
conserved mantle zone surrounding the enlarged follicles (A,
magnification ×100). An intense immunopositive staining with
HHV‐6B‐specific p101K antibody was demonstrated in follicular dendritic
cells (FDCs) of germinal centers in the majority of hyperplastic
follicles, concurrently with scattered positivity of interfollicular
cells [magnification ×100 (B), ×200 (C)]. Immunohistochemical reaction
for HHV‐6A (early antigen p41/38) was invariably negative on lymph node
tissues (D, magnification ×400) (Forghieri et al. 2016).[88] |
In contrast, only
three non-recurrent/chronic cases showed staining for HHV-6B in
germinal centers of follicular dendritic cells, and 7 others showed
scattered positivity in cells of interfollicular areas. Control lymph
node tissues (n=134), which were obtained from patients with benign
lymphadenopathy with known etiology- including other infections, solid
cancer without metastasis, sarcoidosis, Kikuchi-Fujimoto disease,
Wegener granulomatosis, dermatopathic lymphadenopathy, and unspecified
autoimmune disorders- or malignant tumors, were negative for HHV-6.
While recurrent or chronic activity is not usually observed in
reactive/benign conditions, it is more commonly found in atypical or
malignant disorders.[89] Although atypical
lymphocytosis was not observed and there were no systemic symptoms,
characteristics of abnormal/malignant lymph nodes, including irregular
margin and hypoechoic center, were noted among the HHV-6+ patients with
chronic/recurrent lymphadenopathy. Taken together, the data suggest
that HHV-6 may primarily be involved in prompting benign
lymphoproliferative conditions of a certain type, and that the features
discussed by Forghieri et al. may prove to be useful in differentiating
between HHV-6-associated lymphoproliferation and that caused by other
agents.[88]
Rosai-Dorfman Disease.
HHV-6 has been implicated as a trigger for several specific
benign/reactive lymphoproliferative disorders, including Rosai-Dorfman
(RD) disease. The first report of a possible association between the
virus and development of RD described the detection of HHV-6 in
involved tissues by ISH in the majority of a small cohort of patients
(n=9).[90] HHV-6B DNA was later identified using PCR
and Southern blotting in the skin of a patient with RD presenting as
giant lesions of granuloma annulare with multiple soft tissue tumors.[91]
The patient did not experience spontaneous clearing, which is usually
observed for this disease. Notably, the HHV-6 late antigen p101k was
detected in the lymph nodes of two RD patients (of two tested) in a
pattern similar to that noted in the cases of chronic/recurrent
lymphadenopathy described previously.[85,88]
In particular, intense staining was noted in follicular dendritic cells
of reactive germinal centers in areas of normal lymph node
architecture. Moreover, gp106 staining of many abnormal histiocytes
within distended sinuses was observed, with an intense granular
positive reaction in the cytoplasm, while weak positivity was observed
for both antibodies in few plasma cells. In comparison, the lymph node
biopsies from five cases of florid follicular hyperplasia, four cases
with a predominantly paracortical lesion, four cases with sinus
histiocytosis, and one histiocytic necrotizing lymphadenitis were all
HHV-6 positive by PCR, but only isolated plasma cells and histiocytes,
most often scattered in interfollicular areas, were positive for late
antigens by IHC (<1% of cells in the lymph node) [85].
Few granulocytes were positive in the dilated sinuses of 2/4 cases with
sinus histiocytosis. HHV-6B late antigen was also found by IHC in the
lesion of a young boy with extranodal renal RD, a rare manifestation of
the disease.[92] It may be the case that only a subset of RD cases are associated with HHV-6,[93]
but the distinctive pattern of late antigen expression, indicating
active infection in the follicular dendritic cells of germinal centers
argues for the involvement of reactivated HHV-6 in the development of
some cases of RD, especially as the pattern was later identified among
patients with chronic/recurrent lymphadenopathy.[88]
Kikuchi-Fujimoto Disease.
Viral etiology has been investigated in Kikuchi-Fujimoto disease (KFD),
another self-limited benign disorder. Reactivation of HHV-6 has been
determined serologically[94,95] during the course of KFD, and affected lymph nodes have been found to harbor HHV-6 DNA.[94]
However, few studies have performed testing for HHV-6, and not all data
has supported involvement of the virus. Twenty lymph nodes tested for
both EBV and HHV-6, for instance, did not reveal any positivity via
PCR.[96] Similarly, only 1/18 lymph node biopsies
from another study were HHV-6 positive by PCR- as were 2/18 control
lymph nodes from asymptomatic patients with papillary thyroid carcinoma
who had not received chemo/radiotherapy, 1 patient with Warthin tumor,
and 2 cases of paraganglioma.[97] An earlier study
found that the lymph nodes of all but one patient were PCR positive,
and all samples tested by ISH were positive as well. On the other hand,
the positivity by ISH was not limited to these specimens but was also
present in patients with reactive paracortical hyperplasia (60%),
nonspecific lymphadenitis (60%), and tuberculosis lymphadenitis
(22.2%).[98] Because it is often difficult to procure
tissue samples from healthy persons, it is important to note that HHV-6
could contribute to illnesses affecting controls or may reactivate in
response to an illness, which may complicate analysis of the role of
HHV-6. This obstacle, as well as variation in assays, carries the
potential to skew the interpretation of results.
Ocular Lymphoproliferation.
Several herpesviruses, including HHV-6, have been isolated from ocular
tissue removed from patients with lymphoproliferative disorders.
Whereas malignant orbital and conjunctival tissues were infrequently
positive for the virus,[99] 23% (mean viral load 1.7x103copies/microgram
DNA) of samples from IgG4-related ophthalmic disease and 43.9% of
samples from reactive lymphoid hyperplasia of the ocular adnexa were
HHV-6+. HHV-6A[100] and HHV-6B[101] have been linked to ocular inflammation, perhaps through persistent reactivation and stimulation of immune cells. Atypical/Unusual Lymphoproliferative Disorders and Lymphocytic Infiltration
Atypical
lymphoproliferative disorders are characterized by extensive
lymphoma-like persistent and/or progressive lymphoproliferation, which,
while still polyclonal, may progress to open malignant lymphoma. It may
thus be considered a pre-lymphomatous condition.[11] HHV-6 has been cited as a trigger for atypical polyclonal lymphoproliferation,[11,21]
although it is more commonly involved in non-neoplastic lymphocytosis
with lymphocytes that are often labeled “atypical” but do not show
potential for malignant transformation, such as those frequently
observed during infectious mononucleosis (IM)-like illnesses. The virus
has been detected in reactive CD4+ lymphocytes characteristic of these
illnesses,[102] which, though ultimately not malignant, can strongly resemble lymphoma.[103]
Specifically, HHV-6 has been localized to CD3+ and CD4+ T cells in the
lymph node, characterized by intranuclear eosinophilic viral inclusions
and bearing resemblance to lymphocytes present in Hodgkin’s lymphoma
and anaplastic large cell lymphoma. Large reactive lymphocytes with a
sinusal and paracortical distribution have been found among
eosinophils, plasma cells, and scattered immunoblasts.
Infectious Mononucleosis.
HHV-6 has been implicated in the pathogenesis of a subset of infectious
mononucleosis (IM)-like illnesses, as indicated by serologic evidence
of active infection and increased HHV-6 specific IgG titers in the
absence of active EBV or CMV infection.[10,104-106] Coinfections of HHV-6 and EBV also occur during IM and could exacerbate the symptoms/disease course.[10]
While the absolute prevalence is unclear, HHV-6 was specified as the
etiological agent in 5% of 40 adults with IM-like illnesses in Japan,
after PCR analysis.[107,108] As is often the case
during EBV-mediated IM, HHV-6-associated IM-like conditions have been
associated with fatigue, lymphocytosis, thrombocytosis, migrating
lymphadenopathy, headaches, fever, maculopapular rash, and
hepatosplenomegaly with raised liver enzymes, increased immature
lymphoid cells, PBMC death,[109-111] and “atypical” T lymphocytosis coinciding with HHV-6 antigen expression.[111]
The effects of reactivation may persist even after resolution of active
infection; in patients with active HHV-6A and IM, the viral DNA load in
blood peaks within 4 weeks and returns to normal by 16 weeks, while
prolonged T lymphocytosis decreases to normal levels by 24-28 weeks.[112]
HHV-6B
and untyped HHV-6 DNA has been detected in the serum, lymph nodes, and
CD4+ T cells of skin tissue characterized by “atypical” infiltrating
CD4+ and CD8+ T cells, by PCR, IHC, Southern blot, and ISH.[113-115]
Examination of lymph node tissue revealed transformed lymphocytes and
immunoblast-like cells, some of which were positive for HHV-6, in
addition to some histiocytes and eosinophils. Of interest, instances of
HHV-6-associated IM have been significantly associated with Downey type
III lymphocytes (i.e. monocytoid blasts with basophilic cytoplasmic
granules,[108,116] and it appears that this type of IM may present with certain manifestations relatively unique to HHV-6.
The
resulting lymphoproliferation observed during HHV-6-associated IM-like
illnesses has been posited to stem from a response by CD8+ T cells
against HHV-6-infected CD4+ T cells;[113] analysis
of the PBMC composition has identified 35.7% of cells as CD4+ and 52.6%
as CD8+. HHV-6-specific T cells consist of CD4+ and CD8+ cells, but in
patients with acute HHV-6 infection, CD8+ cells may predominate for
several months after an initial spike in the CD4+/CD8+ ratio.[88,117]
Taking into consideration the time-dependent nature of these changes in
cellular populations, computer models have illustrated that acute HHV-6
infection can raise the CD4+/CD8+ T cell ratio initially, but there is
a subsequent sharp decrease as CD4+ cells are removed and CD8+ cells
proliferate.[117] Once HHV-6 has infected
lymphocytes, the maturation and function of the cells are altered.
During acute infection, this may manifest as an infectious
mononucleosis-like illness, while during chronic persistent infection,
chronic fatigue-type cellular changes have been observed, as has
persistent immature lymphocytosis similar to that seen in Canale-Smith
syndrome.[118]
Hemophagocytic Syndrome/Hemophagocytic Lymphohistiocytosis.
Hemophagocytic syndrome/hemophagocytic lymphohistiocytosis (HLH), a
hyperinflammatory disease marked by activation of T lymphocytes and
macrophages and driven by overproduction of cytokines, may result from
infection by herpesviruses, including EBV, CMV, and HHV-6, and at
times, more than one virus.[119,120] Systemic
manifestations during HHV-6-induced HLH, including CNS dysfunction,
respiratory distress, multiorgan failure, and disseminated
intravascular coagulation, are often severe and can be fatal.[119,121-124]
While HLH linked to reactivation of HHV-6 has occurred in patients with
underlying conditions, it has also occurred in seemingly healthy
individuals.[122] Notably, reactivation is not the
only avenue for development of hemophagocytosis and HLH, as both
localized and systemic hemophagocytosis can also occur during infancy,
and in some cases, it is likely a complication of primary infection.[125-127]
As in adults, some children who develop HHV-6-associated HLH may be
predisposed to the syndrome due to underlying disorders, including
Wiedemann-Beckwith syndrome[124] and beta-thalassemia.[128]
Hepatitis and Liver Dysfunction.
During HHV-6 reactivation post-transplant, hepatitis and liver
dysfunction may develop. Randhawa et al. reported a case of
HHV-6A-associated gastroduodenitis, pancreatitis, and hepatitis in an
adult heart transplant recipient[129] with
concomitant multinucleate giant cell transformation observed in
biopsies, and in another instance, giant cell hepatitis presumably
caused by HHV-6A was observed in a liver transplant recipient.[130]
Both species of HHV-6 have shown cell-cell fusion capabilities in vitro
in lymphocytes and other cells, although the response is more robust
for HHV-6A.[21,131,132] Cell fusion events and polyploidy are associated with malignancies and metastasis.[133]
HHV-6 infection has also been associated with an increased number of
liver allograft infiltrating lymphocytes expressing class II antigens,
LFA-1, and VLA-4,[134] and less commonly, the virus is found in liver tissue from immunocompetent patients with hepatitis[135-137] and multiple organ failure.[138]
Among patients with HHV-6-associated liver dysfunction, “atypical”
activated lymphocytes may be present in both the blood and infiltrating
lymphocytes of the liver.[139]
DIHS/DRESS.
Perhaps the deleterious effects of HHV-6-mediated lymphocytic
infiltration are exemplified best by severe DIHS/DRESS, a condition
that can mimic malignant lymphoma[140-142] characterized by erythematous rash, organ dysfunction, and occasionally organ failure.[143]
High level HHV-6 viremia is often found in these patients, and HHV-6
DNA and antigen has been detected in the CSF of patients with
encephalitis and hemophagocytosis,[143,144] the livers of patients with hepatitis and liver failure,[143,145] the kidneys of patients with renal dysfunction and failure,[146,147] and the bone marrow of patients with hemophagocytic syndrome.[148,149] HHV-6 is thought to contribute to the clinical manifestations of DIHS/DRESS, especially the atypical lymphocytosis,[150] lymphadenitis,[141]
and multiorgan involvement, and viremia is predictive of a more severe
course and flaring of symptoms, including hepatitis and fever,[151] as is hemophagocytosis.[140] Additionally, administration of valganciclovir has improved the condition of patients with severe DIHS/DRESS.[143,147,149] HHV-6 has been found in hepatocytes[143,145,152] and tubular epithelial cells of the kidney,[146,147] as well as in infiltrating lymphocytes, often atypical.[141,148,152] Notably, the detection of HHV-6 in these cells has corresponded to severe necrosis and dysfunction in the related organs.
In
addition to triggering lymphocyte infiltration into organs in
DIHS/DRESS, HHV-6 appears to disrupt the properties and functions of
lymphocytes during and after the reaction. Laboratory findings include
lymphopenia or lymphocytosis, the presence of “atypical”
hyperbasophilic lymphocytes, and sometimes hypogammaglobulinemia,
leukocytosis and thrombocytopenia.[153] Infiltrating
atypical lymphocytes may be detected in tissue samples, and atypical
lymphocytosis is present in 27-67% of DIHS/DRESS patients.[154] Strikingly, in one study[142]
HHV-6 antigen was found to be exclusively localized to the
Reed-Sternberg-like cells of the lymph node, and the cells exhibited
loss of CD3 expression. In a similar study, HHV-6 was reportedly
present in the majority of large infiltrating atypical lymphocytes with
prominent nuclear inclusions in lymph node biopsies by ISH and IHC for
p41 early antigen and DR6 and p101k late antigens, and again, there was
partial loss of CD3 expression.[141] As in cases of
persistent lymphocytosis with plasmablastoid and Downey-type cells in
generalized lymphadenopathy and IM, both cytoplasmic[152] and intranuclear (Figure 2)[148]
viral inclusions with a peripheral halo in CD3+CD4+ atypical T cells in
the lymph node have been reported in DIHS/DRESS. In the latter case,
the cells displaying positivity to HHV-6 antibodies appeared to be
Tregs, which are thought to be important in the pathogenesis of
DIHS/DRESS, as well as the development of autoimmunity after resolution
of the syndrome, when Treg activity is suppressed and Th17 cells
increase in number significantly.[153] In vitro testing indicates that HHV-6A[154] and HHV-6B[155] can induce virus-specific Tregs.
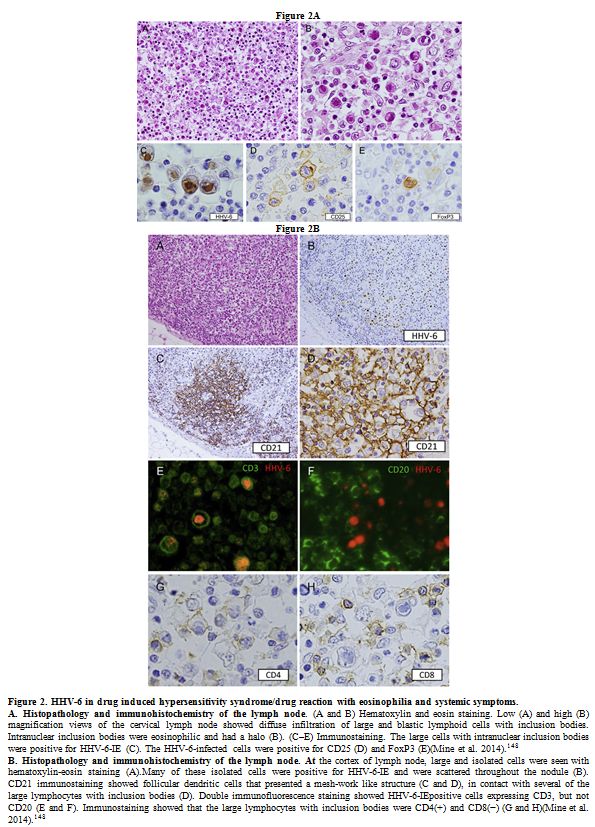 |
Figure 2. HHV-6 in drug induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms.
A.
Histopathology and immunohistochemistry of the lymph node. (A and B)
Hematoxylin and eosin staining. Low (A) and high (B) magnification
views of the cervical lymph node showed diffuse infiltration of large
and blastic lymphoid cells with inclusion bodies. Intranuclear
inclusion bodies were eosinophilic and had a halo (B). (C–E)
Immunostaining. The large cells with intranuclear inclusion bodies were
positive for HHV-6-IE (C). The HHV-6-infected cells were positive for
CD25 (D) and FoxP3 (E)(Mine et al. 2014).[ 148] B.
Histopathology and immunohistochemistry of the lymph node. At the
cortex of lymph node, large and isolated cells were seen with
hematoxylin-eosin staining (A).Many of these isolated cells were
positive for HHV-6-IE and were scattered throughout the nodule (B).
CD21 immunostaining showed follicular dendritic cells that presented a
mesh-work like structure (C and D), in contact with several of the
large lymphocytes with inclusion bodies (D). Double immunofluorescence
staining showed HHV-6-IEpositive cells expressing CD3, but not CD20 (E
and F). Immunostaining showed that the large lymphocytes with inclusion
bodies were CD4(+) and CD8(−) (G and H)(Mine et al. 2014).[ 148] |
Castleman’s Disease.
Case reports and some larger studies have found HHV-6 in the blood and
lymph nodes of patients with Castleman’s disease (CD) by PCR.[156]
When the virus identified in lymph node biopsies was typed in two
cases, it corresponded to HHV-6B, and viral loads were 5.5 and 53.7
copies/microgram DNA.[157] In a larger cohort, 2/16
(12%) CD patients, both of multicentric mixed-type CD, were HHV-6
positive by PCR in involved tissues, although they were also positive
for EBV, with a total of 9 EBV+ samples.[158] As
low-level, latent HHV-6 may be found in healthy individuals, both in
tissue and in blood, it is unclear whether these results implicate
HHV-6 in the development of CD.
Malignant Lymphoproliferation.
Hodgkin’s
Lymphoma. Interesting data has been reported on the possible
involvement of HHV-6 in the nodular sclerosis (NS) subtype of Hodgkin’s
lymphoma (HL) (Figure 3). Early
results finding HHV-6 antigens p41 and gp116/64/54 in Hodgkin’s disease
and Reed Sternberg (RS) cells in 37% of biopsies led investigators to
hypothesize that the virus might contribute to HL through dysregulation
of the cytokine network and through polyclonal stimulations of cellular
proliferation.[159] Further investigation revealed
the absence of HHV-6 DNA by Southern blot, even when positive by PCR,
absence of the virus in neoplastic cells, and no antigen expression by
IHC (for gp102, p41, and P11G1-G9C7), with the exception of 2/2 cases
of NSHL with interfollicular pattern, which were positive for HHV-6 MAb
in residual germinal centers.[160] Along the same
lines, HHV-6 early antigen p41 was not observed in viable RS cells from
lymph nodes positive by PCR in a later study, but “mummified” RS cells
did show positivity in the two cases with the highest copy numbers.[85]
Later studies, however, detected HHV-6 in lymph nodes of 41.9% of NSHL
lymph nodes at a mean viral load of 6,711.4 copies/microgram DNA, 12/13
of which were typed as HHV-6B, while tissues from 6 patients with other
HL subtypes were negative.[161] The detection of
HHV-6 DNA in NSHL cases spurred further examination of additional lymph
nodes, and in another cohort, the virus was identified in a majority
(83.6%) of tumor samples from patients with NSHL, the predominant type
of HL in the group.[162] EBV coinfections accounted
for 49.3% of total NS cases, and in coinfected tissues, viral loads of
EBV and HHV-6 were higher than in the specimens with a single infection
(mean 47,666.5 copies HHV-6/microgram DNA in NS cases vs. 271.4
copies/microgram DNA), suggesting either an immunosuppressive
environment favoring viral reactivation,
or potentiation of viral replication through
simultaneous activity. In the same study, HHV-6 was also found among
5/10 patients with mixed-cellularity HL, one of two lymphocyte-depleted
HL, and in the only patient with lymphocyte-predominance HL. Using IHC
with antibody that appears to be reactive to both HHV-6 species, the
same team later targeted the DR7 oncoprotein, a product of the ORF-1
gene that is able to bind and inactivate the tumor suppressor p53,[163]
which was found in the Reed-Sternberg (RS) cells of 74% of EBV-negative
HL patients whose lymph nodes had previously been found HHV-6 positive
by qPCR (n=38; 36 NSHL).[164] The protein was
exclusively localized in RS cells, which were CD30+, in 61% of cases.
Similarly, exclusive staining for DR7B in RS cells was observed in 6/9
NSHL cases that were both EBV and HHV-6+. The three remaining cases
showed positivity in RS and infiltrating cells, and all nine showed
positivity for LMP-1, an EBV oncoprotein. A tenth EBV/HHV-6+ patient
with mixed-cellularity HL only stained for LMP-1 in RS cells, while
DR7B was only identified in infiltrating cells. RS cells were positive
for gp116/64/54 in 15 EBV negative patients. Notably, positive staining
of gp116/64/54 was observed significantly more frequently (p=0.0154)
among patients with stage III and IV HL compared to those at stages I
and II.
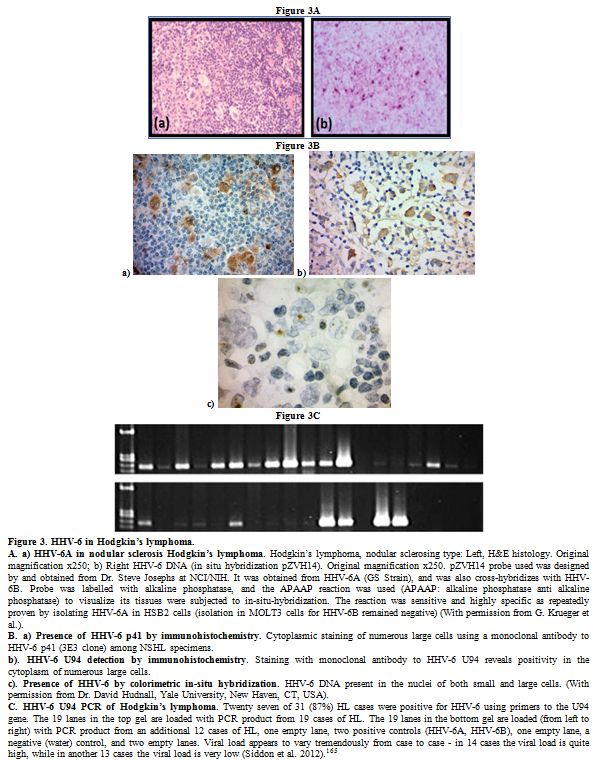 |
Figure 3. HHV-6 in Hodgkin’s lymphoma.
A.
a) HHV-6A in nodular sclerosis Hodgkin’s lymphoma. Hodgkin’s lymphoma,
nodular sclerosing type: Left, H&E histology. Original
magnification x250; b) Right HHV-6 DNA (in situ hybridization pZVH14).
Original magnification x250. pZVH14 probe used was designed by and
obtained from Dr. Steve Josephs at NCI/NIH. It was obtained from HHV-6A
(GS Strain), and was also cross-hybridizes with HHV-6B. Probe was
labelled with alkaline phosphatase, and the APAAP reaction was used
(APAAP: alkaline phosphatase anti alkaline phosphatase) to visualize
its tissues were subjected to in-situ-hybridization. The reaction was
sensitive and highly specific as repeatedly proven by isolating HHV-6A
in HSB2 cells (isolation in MOLT3 cells for HHV-6B remained negative)
(With permission from G. Krueger et al.).
B. a) Presence of HHV-6
p41 by immunohistochemistry. Cytoplasmic staining of numerous large
cells using a monoclonal antibody to HHV-6 p41 (3E3 clone) among NSHL
specimens.
b). HHV-6 U94 detection by immunohistochemistry.
Staining with monoclonal antibody to HHV-6 U94 reveals positivity in
the cytoplasm of numerous large cells.
c). Presence of HHV-6 by
colorimetric in-situ hybridization. HHV-6 DNA present in the nuclei of
both small and large cells. (With permission from Dr. David Hudnall,
Yale University, New Haven, CT, USA).
C. HHV-6 U94 PCR of
Hodgkin’s lymphoma. Twenty seven of 31 (87%) HL cases were positive for
HHV-6 using primers to the U94 gene. The 19 lanes in the top gel are
loaded with PCR product from 19 cases of HL. The 19 lanes in the bottom
gel are loaded (from left to right) with PCR product from an additional
12 cases of HL, one empty lane, two positive controls (HHV-6A, HHV-6B),
one empty lane, a negative (water) control, and two empty lanes. Viral
load appears to vary tremendously from case to case - in 14 cases the
viral load is quite high, while in another 13 cases the viral load is
very low (Siddon et al. 2012).[165]
|
Via
PCR, the prevalence of HHV-6 in 31 additional NSHL lymphoid samples was
similar to the rate of detection among tissues from patients with
reactive lymphoid hyperplasia (87% vs 83%, respectively).[165]
However, IHC analysis revealed HHV-6 whole lysate in numerous RS cells
in 48% of cases, and early and late antigen was also found in scattered
RS cells. Multiple copies of the virus were present in some RS cells,
suggesting that active replication was taking place. Of the samples
that were typed, HHV-6B was present singly in 3 cases, HHV-6A was
present in 4, and HHV-6A/B coinfection was found in 3. EBV was detected
in a total of 5 cases of 21 tested, and EBV/HHV-6 coinfections were
detected in RS cells of 3. Of interest, the mean age of patients with
HHV-6+ RS cells was 23 years, while those with EBV+ RS cells had a mean
age of 47 years (p=0.073). While examination of more cases of mixed
cellularity HL is warranted, currently, immunohistochemical findings
most strongly suggest that an association may exist between HHV-6 and
the NS subtype of HL. Non-Hodgkin’s Lymphoma.
As HHV-6 was initially isolated from HIV+ patients with malignancies,
including non-Hodgkin’s lymphoma (NHL), several early studies focused
on this population, finding the virus in 4.8-32% of tumor samples.[13,14,84,166] Direct HHV-6-mediated oncogenic activity has not been observed in NHL,[11] and the viral antigen has not been found in the neoplastic cells,[85] but rather, it has been found to be present in infiltrating cells of affected tissues as well as in blood samples.[167]
However, evidence suggests that the virus may act in conjunction with
other oncogenic agents, including other viruses, to modulate the lymph
node microenvironment and contribute to the characteristic lymphocytic
proliferation.[85] Case reports, for instance, have
documented a potential interaction between HHV-6 and HHV-8 in diffuse
large B-cell lymphoma (DLBCL). Upon analyzing affected tissues of two
patients, one with nongerminal B-cell-like DLBCL and the other with
primary cutaneous DLBCL, HHV-6 and HHV-8 were simultaneously present in
the nucleoli of lymphoma cells. Notably, HHV-6 has shown the capacity
to induce expression of HHV-8 lytic phase mRNA and proteins in BCBL-1
cells.[168] Of interest, HHV-6B was also identified
among 30.8% of a collection of DLBCL lymph nodes at a mean viral load
of 1,140.7 copies/microgram DNA.[161] In comparison,
23.1% of follicular lymphoma samples were positive, but the mean copy
number was only 39, and of the other NHL lymphomas tested, only 1 (of
4) peripheral T-cell lymphoma had over 91copies/microgram DNA, with
3,380 copies. In a similar study, 3/5 T-cell lymphoma lymph nodes were
HHV-6 (2 HHV-6B, 1 unclassified) positive, with viral loads of 9.1,
60.4, and 810 copies/microgram DNA.[157]Similarly, EBV coinfections have frequently been observed in HHV-6+ NHL lymphoid biopsies;[169-171]
one study, for example, found 57% of HHV-6+ angioimmunoblastic T cell
lymphoma/ angioimmunoblastic lymphadenopathy (AITL/AILD) lymph nodes to
be coinfected with EBV.[172] In a separate cohort,
79% of HHV-6B+ lymph nodes were EBV+, with the highest HHV-6B viral
loads in biopsies with pattern III histology (median 40 copies/1000
cells).[19] Moreover, all of the coinfected samples
showed pattern II or III histology, with the majority falling under
pattern III. However, expression of HHV-6 antigens in proliferating T
lymphocytes of AILD patients has not been observed.[85]An
inherited, chromosomally integrated form of either HHV-6A or HHV-6B,
known as ciHHV-6 or iciHHV-6, is present in about 1% of the population.
Two intriguing case reports have documented ciHHV-6+ patients with NHL:
One patient, with DLBCL and ciHHV-6 on chromosome 17p, developed marker
chromosomes that were also ciHHV-6+,[173] while the
other individual, who carried ciHHV-6A on chromosome 19q, developed a
primary effusion-like lymphoma characterized by an absence of any
integrated HHV-6.[174] It is possible that ciHHV-6 may have the potential to trigger lymphoma by affecting chromosomal stability.Lymphocytic Leukemia. Although HHV-6 has been detected in the blood[175-176]
and bone marrow of patients with lymphocytic leukemia, and heightened
titers of HHV-6 antibody have been observed in blood samples,[177-179] a role for the virus in this type of cancer is not well supported. In contrast with a later publication,[180]
one study found high rates of HHV-6A in blood and bone marrow samples-
43.8% of B-ALL samples, 52.4% of B-CLL samples, and only less than 10%
of MM.[181] HHV-6 p41 antigen has been detected in nearly half of bone marrow samples from patients with myelodysplasia by IHC,[182]
but in cases of leukemia, detection of HHV-6A/HHV-6B in the bone marrow
only by nested PCR and not via standard PCR, or at low copy numbers by
qPCR,[175] has suggested that HHV-6 present in the samples is likely latent.[183]
Moreover, when quantified, viral loads both in blood and in bone
marrow, have been found to be lower at diagnosis than at remission.[175,180,181]
As the virus can infect bone marrow progenitors and reactivate under
immunosuppressive conditions, the presence of the virus as it was
detected in these instances likely did not impact the course of
leukemia.
Discussion
The
interactions of chronic active HHV-6 infection with the immune system
may result in a variety of clinical diseases including aplasia,
autoimmune disorders (e.g. Sjögren’s syndrome, lupus erythematosus,
scleroderma), and lymphoproliferation. These manifestations reflect the
disturbed balance between viral persistence and the host’s defense
status.[184,185] The current overview focuses only on lymphoproliferative disorders in order not to further complicate the report.HHV-6
may contribute to several benign/reactive lymphoproliferative disorders
by spurring a dysfunctional immune response or through dysregulation of
cytokines and other immune mediators. It is probable that HHV-6A and
HHV-6B are two of many potential triggers for these disorders, but
immunohistochemical analysis may reveal characteristic patterns of
viral expression and cellular features in cases associated with either
virus. As more instances of HHV-6 associated lymphoproliferation are
described, clinical parameters might also be found that are indicative
of HHV-6 involvement. Both primary infection and reactivation are able
to induce “atypical” lymphocytosis, in which activated cells may appear
abnormal and may even resemble transformed, malignant cells. Under some
circumstances, HHV-6A/HHV-6B are also capable of inducing organ
dysfunction, with lymphocytic infiltration often observed in the
affected organs. Because both species of HHV-6 can infect a variety of
cells, active infection in tissues may result in an immune response
toward the infected tissues, resulting in lymphocytic infiltration, or
active infection of lymphocytes may trigger an inflammatory reaction
and drive lymphocytic infiltration directly. Although a role for HHV-6
in many lymphomatous malignancies is not strongly supported, evidence
points to a potential contribution of the virus in certain Hodgkin’s
lymphomas of the nodular sclerosis type. The wider use of quantitative
PCR and immunohistochemical techniques has enabled more accurate
interpretation of viral involvement in lymphoproliferative conditions,
and the continued utilization of these techniques will be vital to
expanding the knowledge of their association between HHV-6 and these
disorders, as well as uncovering the mechanisms behind them.
Ultimately, further research is needed to elucidate the
immunomodulatory capabilities of HHV-6A and HHV-6B as they relate to
the functionality of lymphocytes and to better define their roles in
lymphoproliferative diseases, both during acute infection and
persistent reactivation.
Aknowledgements
We would like to thank Kristin Loomis of the HHV-6 Foundation for her coordination and encouragement.
References
- Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF,
Sturzenegger S, Kaplan M, Halligan G, Biberfeld P, Wong-Staal F,
Kramarsky B, Gallo RC. Isolation of a new virus, HBLV, in patients with
lymphoproliferative disorders. Science. 1986;234(4776):596-601. https://doi.org/10.1126/science.2876520 PMid:2876520

- Salahuddin
SZ, Kelley AS, Krueger GR, Josephs SF, Gupta S, Ablashi DV. Human
herpes virus-6 (HHV-6) in diseases. Clin Diagn Virol. 1993;1(2):81-100.
https://doi.org/10.1016/0928-0197(93)90016-X

- Ablashi
D, Agut H, Alvarez-Lafuente R, Clark DA, Dewhurst S, DiLuca D, Flamand
L, Frenkel N, Gallo R, Gompels UA, Höllsberg P, Jacobson S, Luppi M,
Lusso P, Malnati M, Medveczky P, Mori Y, Pellett PE, Pritchett JC,
Yamanishi K, Yoshikawa T. Classification of HHV-6A and HHV-6B as
distinct viruses. Arch Virol. 2014;159(5):863-70. https://doi.org/10.1007/s00705-013-1902-5 PMid:24193951 PMCid:PMC4750402

- Lusso
P, Markham PD, Tschachler E, di Marzo Veronese F, Salahuddin SZ,
Ablashi DV, Pahwa S, Krohn K, Gallo RC. In vitro cellular tropism of
human B-lymphotropic virus (human herpesvirus-6). J Exp Med.
1988;167(5):1659-70. https://doi.org/10.1084/jem.167.5.1659 PMid:3259254

- Ablashi
DV, Salahuddin SZ, Josephs SF, Imam F, Lusso P, Gallo RC, Hung C, Lemp
J, Markham PD. HBLV (or HHV-6) in human cell lines. Nature.
1987;329(6136):207. https://doi.org/10.1038/329207a0 PMid:3627265

- Biberfeld
P, Kramarsky B, Salahuddin SZ, Gallo RC. Ultrastructural
characterization of a new human B lymphotropic DNA virus (human
herpesvirus 6) isolated from patients with lymphoproliferative disease.
J Natl Cancer Inst. 1987;79(5):933-41. PMid:2824914

- Ablashi
DV, Balachandran N, Josephs SF, Hung CL, Krueger GR, Kramarsky B,
Salahuddin SZ, Gallo RC. Genomic polymorphism, growth properties, and
immunologic variations in human herpesvirus-6 isolates. Virology.
1991;184(2):545-52. https://doi.org/10.1016/0042-6822(91)90424-A

- Eizuru
Y, Minematsu T, Minamishima Y, Kikuchi M, Yamanishi K, Takahashi M,
Kurata T, Borisch Chappuis B, Ellinger K, Neipel F, Kirchner T, Kujath
P, Fleckenstein B, Müller-Hermelink HK. Human herpesvirus 6 in lymph
nodes. Lancet. 1989; 333(8628):40-1. https://doi.org/10.1016/S0140-6736(89)91690-5

- Dolcetti
R, Di Luca D, Mirandola P, De Vita S, De Re V, Carbone A, Tirelli U,
Cassai E, Boiocchi M. Frequent detection of human herpesvirus 6 DNA in
HIV-associated lymphadenopathy. Lancet. 1994;344(8921):543. https://doi.org/10.1016/S0140-6736(94)91931-3

- Bertram
G, Dreiner N, Krueger GR, Ramon A, Ablashi DV, Salahuddin SZ,
Balachandram N. Frequent double infection with Epstein-Barr virus and
human herpesvirus-6 in patients with acute infectious mononucleosis. In
Vivo. 1991;5(3):271-9. PMid:1654150

- Krueger
GR, Manak M, Bourgeois N, Ablashi DV, Salahuddin SZ, Josephs SS,
Buchbinder A, Gallo RC, Berthold F, Tesch H. Persistent active herpes
virus infection associated with atypical polyclonal lymphoproliferation
(APL) and malignant lymphoma. Anticancer Res. 1989;9(6):1457-76.
PMid:2560617

- Chen T, Hudnall SD. Anatomical mapping of human herpesvirus reservoirs of infection. Mod Pathol. 2006;19(5):726-37. https://doi.org/10.1038/modpathol.3800584 PMid:16528368

- Di
Luca D, Dolcetti R, Mirandola P, De Re V, Secchiero P, Carbone A,
Boiocchi M, Cassai E. Human herpesvirus 6: a survey of presence and
variant distribution in normal peripheral lymphocytes and
lymphoproliferative disorders. J Infect Dis. 1994;170(1):211-5. https://doi.org/10.1093/infdis/170.1.211 PMid:8014502

- Dolcetti
R, Di Luca D, Carbone A, Mirandola P, De Vita S, Vaccher E, Sighinolfi
L, Gloghini A, Tirelli U, Cassai E, Boiocchi M. Human herpesvirus 6 in
human immunodeficiency virus-infected individuals: association with
early histologic phases of lymphadenopathy syndrome but not with
malignant lymphoproliferative disorders. J Med Virol.
1996;48(4):344-53. https://doi.org/10.1002/(SICI)1096-9071(199604)48:4<344::AID-JMV8>3.0.CO;2-7

- Fillet
AM, Raphael M, Visse B, Audouin J, Poirel L, Agut H. Controlled study
of human herpesvirus 6 detection in acquired immunodeficiency
syndrome-associated non-Hodgkin's lymphoma. The French Study Group for
HIV-Associated Tumors. J Med Virol. 1995;45(1):106-12. https://doi.org/10.1002/jmv.1890450119 PMid:7714485

- Lusso
P, Malnati MS, Garzino-Demo A, Crowley RW, Long EO, Gallo RC. Infection
of natural killer cells by human herpesvirus 6. Nature.
1993;362(6419):458-62. https://doi.org/10.1038/362458a0 PMid:7681936

- Kondo
K, Kondo T, Okuno T, Takahashi M, Yamanishi K. Latent human herpesvirus
6 infection of human monocytes/macrophages. J Gen Virol. 1991;72 (Pt
6):1401-8. https://doi.org/10.1099/0022-1317-72-6-1401 PMid:1646280

- Kondo
K, Kondo T, Shimada K, Amo K, Miyagawa H, Yamanishi K. Strong
interaction between human herpesvirus 6 and peripheral blood
monocytes/macrophages during acute infection. J Med Virol.
2002;67(3):364-9. https://doi.org/10.1002/jmv.10082 PMid:12116029

- Hirata
Y, Kondo K, Yamanishi K. Human herpesvirus 6 downregulates major
histocompatibility complex class I in dendritic cells. J Med Virol.
2001;65(3):576-83. https://doi.org/10.1002/jmv.2075 PMid:11596096

- Ablashi
DV, Josephs SF, Buchbinder A, Hellman K, Nakamura S, Llana T, Lusso P,
Kaplan M, Dahlberg J, Memon S, Imam F, Ablashi KL, Markham PD,
Kramarsky B, Krueger GRF, Biberfeld P, Wong-Staal F, Salahuddin SZ,
Gallo RC. Human B-lymphotropic virus (human herpesvirus-6). J Virol
Methods. 1988; 21(1-4):29-48. https://doi.org/10.1016/0166-0934(88)90050-X

- Krueger
GRF, Schneider B. Pathologic Features of HHV-6 Disease. In: Krueger
GRF, Ablashi D, eds. Perspectives in Medical Virology. Volume 12,
Elsevier. 2006; 133-48. https://doi.org/10.1016/S0168-7069(06)12010-8

- Sandhoff
T, Kleim JP, Schneweis KE. Latent human herpesvirus-6 DNA is sparsely
distributed in peripheral blood lymphocytes of healthy adults and
patients with lymphocytic disorders. Med Microbiol Immunol.
1991;180(3):127-34. https://doi.org/10.1007/BF00206116 PMid:1656178

- Yakushijin
Y, Yasukawa M, Kobayashi Y. T-cell immune response to human
herpesvirus-6 in healthy adults. Microbiol Immunol. 1991;35(8):655-60. https://doi.org/10.1111/j.1348-0421.1991.tb01597.x PMid:1661364

- Nastke
MD, Becerra A, Yin L, Dominguez-Amorocho O, Gibson L, Stern LJ,
Calvo-Calle JM. Human CD4+ T cell response to human herpesvirus 6. J
Virol. 2012;86(9):4776-92. https://doi.org/10.1128/JVI.06573-11 PMid:22357271 PMCid:PMC3347333

- Martin
LK, Schub A, Dillinger S, Moosmann A. Specific CD8? T cells recognize
human herpesvirus 6B. Eur J Immunol. 2012;42(11):2901-12. https://doi.org/10.1002/eji.201242439 PMid:22886850

- Yamanishi
K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, Kurata T.
Identification of human herpesvirus-6 as a causal agent for exanthem
subitum. Lancet. 1988;1(8594):1065-7. https://doi.org/10.1016/S0140-6736(88)91893-4

- Knox
KK, Carrigan DR. In vitro suppression of bone marrow progenitor cell
differentiation by human herpesvirus 6 infection. J Infect Dis.
1992;165(5):925-9. https://doi.org/10.1093/infdis/165.5.925

- Carrigan
DR, Knox KK. Bone marrow suppression by human herpesvirus-6: comparison
of the A and B variants of the virus. Blood. 1995;86(2):835-6.
PMid:7606018

- Horvat
RT, Parmely MJ, Chandran B. Human herpesvirus 6 inhibits the
proliferative responses of human peripheral blood mononuclear cells. J
Infect Dis. 1993;167(6):1274-80.
https://doi.org/10.1093/infdis/167.6.1274 PMid:8388898 
- Flamand
L, Gosselin J, Stefanescu I, Ablashi D, Menezes J. Immunosuppressive
effect of human herpesvirus 6 on T-cell functions: suppression of
interleukin-2 synthesis and cell proliferation. Blood.
1995;85(5):1263-71. Erratum in: Blood 1995;86(1):418. PMid:7858257

- Yoshikawa
T, Ihira M, Asano Y, Tomitaka A, Suzuki K, Matsunaga K, Kato Y,
Hiramitsu S, Nagai T, Tanaka N, Kimura H, Nishiyama Y. Fatal adult case
of severe lymphocytopenia associated with reactivation of human
herpesvirus 6. J Med Virol. 2002;66(1):82-5. https://doi.org/10.1002/jmv.2114 PMid:11748662

- Knox
KK, Pietryga D, Harrington DJ, Franciosi R, Carrigan DR. Progressive
immunodeficiency and fatal pneumonitis associated with human
herpesvirus 6 infection in an infant. Clin Infect Dis.
1995;20(2):406-13. https://doi.org/10.1093/clinids/20.2.406 PMid:7742449

- Comar
M, D'Agaro P, Horejsh D, Galvan M, Fiorentini S, Andolina M, Caruso A,
Di Luca D, Campello C. Long-lasting CD3+ T-cell deficiency after cord
blood stem cell transplantation in a human herpesvirus 6-infected
child. J Clin Microbiol. 2005;43(4):2002-3. https://doi.org/10.1128/JCM.43.4.2002-2003.2005 PMid:15815044 PMCid:PMC1081381

- Imbert-Marcille
BM, Tang XW, Lepelletier D, Besse B, Moreau P, Billaudel S, Milpied N.
Human herpesvirus 6 infection after autologous or allogeneic stem cell
transplantation: a single-center prospective longitudinal study of 92
patients. Clin Infect Dis. 2000;31(4):881-6. https://doi.org/10.1086/318142 PMid:11049765

- Rosenfeld
CS, Rybka WB, Weinbaum D, Carrigan DR, Knox KK, Andrews DF, Shadduck
RK. Late graft failure due to dual bone marrow infection with variants
A and B of human herpesvirus-6. Exp Hematol. 1995;23(7):626-9.
PMid:7601254

- Admiraal
R, de Koning CCH, Lindemans CA, Bierings MB, Wensing AMJ, Versluys AB,
Wolfs TFW, Nierkens S, Boelens JJ. Viral reactivations and associated
outcomes in the context of immune reconstitution after pediatric
hematopoietic cell transplantation. J Allergy Clin Immunol.
2017;140(6):1643-1650.e9. Epub 2017 Apr 7. https://doi.org/10.1016/j.jaci.2016.12.992 PMid:28392330

- Greco
R, Crucitti L, Noviello M, Racca S, Mannina D, Forcina A, Lorentino F,
Valtolina V, Rolla S, Dvir R, Morelli M, Giglio F, Barbanti MC, Lupo
Stanghellini MT, Oltolini C, Vago L, Scarpellini P, Assanelli A,
Carrabba MG, Marktel S, Bernardi M, Corti C, Clementi M, Peccatori J,
Bonini C, Ciceri F. Human Herpesvirus 6 Infection Following
Haploidentical Transplantation: Immune Recovery and Outcome. Biol Blood
Marrow Transplant. 2016;22(12):2250-5. https://doi.org/10.1016/j.bbmt.2016.09.018 PMid:27697585

- Quintela
A, Escuret V, Roux S, Bonnafous P, Gilis L, Barraco F,
Labussière-Wallet H, Duscastelle-Leprêtre S, Nicolini FE, Thomas X,
Chidiac C, Ferry T, Frobert E, Morisset S, Poitevin-Later F, Monneret
G, Michallet M, Ader F; Lyon HEMINF Study Group. HHV-6 infection after
allogeneic hematopoietic stem cell transplantation: From chromosomal
integration to viral co-infections and T-cell reconstitution patterns.
J Infect. 2016;72(2):214-22. https://doi.org/10.1016/j.jinf.2015.09.039 PMid:26518057

- Wang
FZ, Linde A, Dahl H, Ljungman P. Human herpesvirus 6 infection inhibits
specific lymphocyte proliferation responses and is related to
lymphocytopenia after allogeneic stem cell transplantation. Bone Marrow
Transplant. 1999;24(11):1201-6. https://doi.org/10.1038/sj.bmt.1702058 PMid:10642809

- Illiaquer
M, Imbert-Marcille BM, Guillaume T, Planche L, Rimbert M,
Bressollette-Bodin C, Le Bourgeois A, Peterlin P, Garnier A, Le Houerou
C, Moreau P, Mohty M, Chevallier P. Impact of stem cell graft on early
viral infections and immune reconstitution after allogeneic
transplantation in adults. J Clin Virol. 2017;93:30-6. https://doi.org/10.1016/j.jcv.2017.05.019 PMid:28601677

- Smith
A, Santoro F, Di Lullo G, Dagna L, Verani A, Lusso P. Selective
suppression of IL-12 production by human herpesvirus 6. Blood.
2003;102(8):2877-84. https://doi.org/10.1182/blood-2002-10-3152 PMid:12829600

- Smith
AP, Paolucci C, Di Lullo G, Burastero SE, Santoro F, Lusso P. Viral
replication-independent blockade of dendritic cell maturation and
interleukin-12 production by human herpesvirus 6. J Virol.
2005;79(5):2807-13. https://doi.org/10.1128/JVI.79.5.2807-2813.2005 PMid:15708999 PMCid:PMC548462

- Flamand
L, Gosselin J, D'Addario M, Hiscott J, Ablashi DV, Gallo RC, Menezes J.
Human herpesvirus 6 induces interleukin-1 beta and tumor necrosis
factor alpha, but not interleukin-6, in peripheral blood mononuclear
cell cultures. J Virol. 1991;65(9):5105-10. PMid:1651426
PMCid:PMC248979

- Arena
A, Liberto MC, Iannello D, Capozza AB, Focà A. Altered cytokine
production after human herpes virus type 6 infection. New Microbiol.
1999;22(4):293-300. PMid:10555198

- Kikuta
H, Nakane A, Lu H, Taguchi Y, Minagawa T, Matsumoto S. Interferon
induction by human herpesvirus 6 in human mononuclear cells. J Infect
Dis. 1990;162(1):35-8. https://doi.org/10.1093/infdis/162.1.35 PMid:1693942

- Chi
J, Gu B, Zhang C, Peng G, Zhou F, Chen Y, Zhang G, Guo Y, Guo D, Qin J,
Wang J, Li L, Wang F, Liu G, Xie F, Feng D, Zhou H, Huang X, Lu S, Liu
Y, Hu W, Yao K. Human herpesvirus 6 latent infection in patients with
glioma. J Infect Dis. 2012;206(9):1394-8. https://doi.org/10.1093/infdis/jis513 PMid:22962688

- Yoshikawa
T, Asano Y, Akimoto S, Ozaki T, Iwasaki T, Kurata T, Goshima F,
Nishiyama Y. Latent infection of human herpesvirus 6 in astrocytoma
cell line and alteration of cytokine synthesis. J Med Virol.
2002;66(4):497-505. https://doi.org/10.1002/jmv.2172 PMid:11857528

- Meeuwsen
S, Persoon-Deen C, Bsibsi M, Bajramovic JJ, Ravid R, De Bolle L, van
Noort JM. Modulation of the cytokine network in human adult astrocytes
by human herpesvirus-6A. J Neuroimmunol. 2005; 164(1-2):37-47. https://doi.org/10.1016/j.jneuroim.2005.03.013 PMid:15904975

- Clark
DJ, Catusse J, Stacey A, Borrow P, Gompels UA. Activation of CCR2+
human proinflammatory monocytes by human herpesvirus-6B chemokine
N-terminal peptide. J Gen Virol. 2013;94(Pt 7):1624-35. https://doi.org/10.1099/vir.0.050153-0 PMid:23535574

- Caselli
E, Bortolotti D, Marci R, Rotola A, Gentili V, Soffritti I, D'Accolti
M, Lo Monte G, Sicolo M, Barao I, Di Luca D, Rizzo R. HHV-6A Infection
of Endometrial Epithelial Cells Induces Increased Endometrial NK
Cell-Mediated Cytotoxicity. Front Microbiol. 2017;8:2525. https://doi.org/10.3389/fmicb.2017.02525 PMid:29326672 PMCid:PMC5736868

- Catusse
J, Clark DJ, Gompels UA. CCR5 signalling, but not DARC or D6
regulatory, chemokine receptors are targeted by herpesvirus U83A
chemokine which delays receptor internalisation via diversion to a
caveolin-linked pathway. J Inflamm (Lond). 2009;6:22. https://doi.org/10.1186/1476-9255-6-22 PMid:19643012 PMCid:PMC2744670

- Catusse
J, Spinks J, Mattick C, Dyer A, Laing K, Fitzsimons C, Smit MJ, Gompels
UA. Immunomodulation by herpesvirus U51A chemokine receptor via CCL5
and FOG-2 down-regulation plus XCR1 and CCR7 mimicry in human
leukocytes. Eur J Immunol. 2008;38(3):763-77. https://doi.org/10.1002/eji.200737618 PMid:18286574

- Nagasaka
M, Morioka I, Kawabata A, Yamagishi Y, Iwatani S, Taniguchi-Ikeda M,
Ishida A, Iijima K, Mori Y. Comprehensive analysis of serum
cytokines/chemokines in febrile children with primary human herpes
virus-6B infection. J Infect Chemother. 2016;22(9):593-8. https://doi.org/10.1016/j.jiac.2016.05.010 PMid:27346377

- Krueger
GRF, Schonnebeck M, Braun M. Enhanced cell membrane receptor expression
following infection with human herpesvirus-6. FASEB J. 1990;4:A343.

- Schonnebeck
M, Krueger GRF, Braun M, Fischer M, Koch B, Ablashi DV, Balachandran N.
Human herpesvirus-6 infection may predispose to superinfection by other
viruses. In Vivo. 1991;5: 255-64. PMid:1654148

- Santoro
F, Kennedy PE, Locatelli G, Malnati MS, Berger EA, Lusso P. CD46 is a
cellular receptor for human herpesvirus 6. Cell. 1999;99(7):817-27. https://doi.org/10.1016/S0092-8674(00)81678-5

- Glosson
NL, Hudson AW. Human herpesvirus-6A and -6B encode viral immunoevasins
that downregulate class I MHC molecules. Virology. 2007;365(1):125-35.
https://doi.org/10.1016/j.virol.2007.03.048 PMid:17467766 
- Grivel
JC, Santoro F, Chen S, Fagá G, Malnati MS, Ito Y, Margolis L, Lusso P.
Pathogenic effects of human herpesvirus 6 in human lymphoid tissue ex
vivo. J Virol. 2003;77(15):8280-9.
https://doi.org/10.1128/JVI.77.15.8280-8289.2003 PMid:12857897 PMCid:PMC165251 
- Sullivan
BM, Coscoy L. Downregulation of the T-cell receptor complex and
impairment of T-cell activation by human herpesvirus 6 u24 protein. J
Virol. 2008;82(2):602-8. https://doi.org/10.1128/JVI.01571-07 PMid:17977973 PMCid:PMC2224597

- Lusso
P, Malnati M, De Maria A, Balotta C, DeRocco SE, Markham PD, Gallo RC.
Productive infection of CD4+ and CD8+ mature human T cell populations
and clones by human herpesvirus 6. Transcriptional down-regulation of
CD3. J Immunol. 1991; 147(2):685-91. PMid:1677024

- Lusso
P, Garzino-Demo A, Crowley RW, Malnati MS. Infection of gamma/delta T
lymphocytes by human herpesvirus 6: transcriptional induction of CD4
and susceptibility to HIV infection. J Exp Med. 1995;181(4):1303-10. https://doi.org/10.1084/jem.181.4.1303 PMid:7699322

- Furukawa
M, Yasukawa M, Yakushijin Y, Fujita S. Distinct effects of human
herpesvirus 6 and human herpesvirus 7 on surface molecule expression
and function of CD4+ T cells. J Immunol. 1994;152(12):5768-75.
PMid:7911490

- Zheng
X, Li S, Zang Z, Hu J, An J, Pei X, Zhu F, Zhang W, Yang H. Evidence
for possible role of toll-like receptor 3 mediating virus-induced
progression of pituitary adenomas. Mol Cell Endocrinol. 2016;426:22-32.
https://doi.org/10.1016/j.mce.2016.02.009 PMid:26891958

- Reynaud
JM, Jégou JF, Welsch JC, Horvat B. Human herpesvirus 6A infection in
CD46 transgenic mice: viral persistence in the brain and increased
production of proinflammatory chemokines via Toll-like receptor 9. J
Virol. 2014;88(10):5421-36. https://doi.org/10.1128/JVI.03763-13 PMid:24574405 PMCid:PMC4019085

- Nordström
I, Eriksson K. HHV-6B induces IFN-lambda1 responses in cord
plasmacytoid dendritic cells through TLR9. PLoS One. 2012;7(6):e38683.
Epub 2012 Jun 6. https://doi.org/10.1371/journal.pone.0038683 PMid:22701693 PMCid:PMC3368904

- Chi
J, Wang F, Li L, Feng D, Qin J, Xie F, Zhou F, Chen Y, Wang J, Yao K.
The role of MAPK in CD4(+) T cells toll-like receptor 9-mediated
signaling following HHV-6 infection. Virology. 2012;422(1):92-8. https://doi.org/10.1016/j.virol.2011.09.026 PMid:22055432

- Murakami
Y, Tanimoto K, Fujiwara H, An J, Suemori K, Ochi T, Hasegawa H,
Yasukawa M. Human herpesvirus 6 infection impairs Toll-like receptor
signaling. Virol J. 2010;7:91. https://doi.org/10.1186/1743-422X-7-91 PMid:20459723 PMCid:PMC2874541

- Krueger
GRF, Sander C. What's New in Human Herpesvirus-6? Clinical
Immunopathology of the HHV-6 Infection. Pathol Res Pract. 1989
Dec;185(6):915-29. https://doi.org/10.1016/S0344-0338(89)80299-7

- Mullins
TB, Krishnamurthy K. Roseola Infantum (Exanthema Subitum, Sixth
Disease). In: StatPearls [Internet]. Treasure Island, StatPearls
Publishing. 2018.
- Hall CB, Long CE,
Schnabel KC, Caserta MT, McIntyre KM, Costanzo MA, Knott A, Dewhurst S,
Insel RA, Epstein LG. Human herpesvirus-6 infection in children. A
prospective study of complications and reactivation. N Engl J Med.
1994;331(7):432-8. https://doi.org/10.1056/NEJM199408183310703 PMid:8035839

- Asano
Y, Yoshikawa T, Suga S, Kobayashi I, Nakashima T, Yazaki T, Kajita Y,
Ozaki T. Clinical features of infants with primary human herpesvirus 6
infection (exanthem subitum, roseola infantum). Pediatrics.
1994;93(1):104-8. PMid:8265302

- de
Freitas RB, Linhares AC, Oliveira CS, Gusmão RH, Linhares MI.
Association of human herpesvirus 6 infection with exanthem subitum in
Belem, Brazil. Rev Inst Med Trop Sao Paulo. 1995;37(6):489-92. https://doi.org/10.1590/S0036-46651995000600003 PMid:8731260

- Balachandra
K, Bowonkiratikachorn P, Poovijit B, Thattiyaphong A, Jayavasu C, Wasi
C, Takahashi M, Yamanishi K. Human herpesvirus 6 (HHV-6) infection and
exanthem subitum in Thailand. Acta Paediatr Jpn. 1991;33(4):434-9. https://doi.org/10.1111/j.1442-200X.1991.tb02567.x PMid:1665277

- Hashimoto
H, Maruyama H, Fujimoto K, Sakakura T, Seishu S, Okuda N. Hematologic
findings associated with thrombocytopenia during the acute phase of
exanthem subitum confirmed by primary human herpesvirus-6 infection. J
Pediatr Hematol Oncol. 2002;24(3):211-4. https://doi.org/10.1097/00043426-200203000-00010 PMid:11990308

- Yoshikawa
T, Suzuki K, Umemura K, Akimoto S, Miyake F, Usui C, Fujita A, Suga S,
Asano Y. Atypical clinical features of a human herpesvirus-6 infection
in a neonate. J Med Virol. 2004;74(3):463-6. https://doi.org/10.1002/jmv.20199 PMid:15368515

- Kanegane
C, Katayama K, Kyoutani S, Kanegane H, Shintani N, Miyawaki T,
Taniguchi N. Mononucleosis-like illness in an infant associated with
human herpesvirus 6 infection. Acta Paediatr Jpn. 1995;37(2):227-9. https://doi.org/10.1111/j.1442-200X.1995.tb03304.x PMid:7793262

- Hoang
MP, Ross KF, Dawson DB, Scheuermann RH, Rogers BB. Human herpesvirus-6
and sudden death in infancy: report of a case and review of the
literature. J Forensic Sci. 1999;44(2):432-7. https://doi.org/10.1520/JFS14481J PMid:10097377

- Lorenzana
A, Lyons H, Sawaf H, Higgins M, Carrigan D, Emanuel PD. Human
herpesvirus 6 infection mimicking juvenile myelomonocytic leukemia in
an infant. J Pediatr Hematol Oncol. 2002;24(2):136-41. https://doi.org/10.1097/00043426-200202000-00016 PMid:11990701

- Krueger
GRF, Ablashi DV, Whitman J, Luka J, Rojo J. Clinical pathology and
reactivation of human lymphotropic herpesviruses. Revista Med Hops Gen
Mexico. 1998;61:226-40.

- Irving WL, Cunningham AL. Serological diagnosis of infection with human herpesvirus type 6. BMJ. 1990;300(6718):156-9. https://doi.org/10.1136/bmj.300.6718.156

- Borisch
B, Ellinger K, Neipel F, Fleckenstein B, Kirchner T, Ott MM,
Müller-Hermelink HK. Lymphadenitis and lymphoproliferative lesions
associated with the human herpes virus-6 (HHV-6). Virchows Arch B Cell
Pathol Incl Mol Pathol. 1991;61(3):179-87. PMid:1685279

- Niederman
JC, Liu CR, Kaplan MH, Brown NA. Clinical and serological features of
human herpesvirus-6 infection in three adults. Lancet.
1988;2(8615):817-9. https://doi.org/10.1016/S0140-6736(88)92783-3

- Stettner-Gloning
R, Jäger G, Gloning H, Pontz BF, Emmrich P. Lymphadenopathy in
connection with human herpes virus type 6 (HHV-6) infection. Clin
Investig. 1992;70(1):59-62. https://doi.org/10.1007/BF00422942 PMid:1318124

- Asou
H, Tasaka T, Said JW, Daibata M, Kamada N, Koeffler HP. Co-infection of
HHV-6 and HHV-8 is rare in primary effusion lymphoma. Leuk Res.
2000;24(1):59-61. https://doi.org/10.1016/S0145-2126(99)00144-7

- Luppi
M, Barozzi P, Garber R, Maiorana A, Bonacorsi G, Artusi T, Trovato R,
Marasca R, Torelli G. Expression of human herpesvirus-6 antigens in
benign and malignant lymphoproliferative diseases. Am J Pathol.
1998;153(3):815-23. https://doi.org/10.1016/S0002-9440(10)65623-4

- Sandhoff
T, Kleim JP, Schneweis KE. Latent human herpesvirus-6 DNA is sparsely
distributed in peripheral blood lymphocytes of healthy adults and
patients with lymphocytic disorders. Med Microbiol Immunol.
1991;180(3):127-34. https://doi.org/10.1007/BF00206116 PMid:1656178

- Freitas
RB, Freitas MR, Linhares AC. Evidence of active herpesvirus 6
(variant-A) infection in patients with lymphadenopathy in Belém, Pará,
Brazil. Rev Inst Med Trop Sao Paulo. 2003;45(5):283-8. https://doi.org/10.1590/S0036-46652003000500008 PMid:14743669

- Forghieri
F, Luppi M, Barozzi P, Riva G, Morselli M, Bigliardi S, Quadrelli C,
Vallerini D, Maccaferri M, Coluccio V, Paolini A, Colaci E, Bonacorsi
G, Maiorana A, Tagliazucchi S, Rumpianesi F, Mattioli F, Presutti L,
Gelmini R, Cermelli C, Rossi G, Comoli P, Marasca R, Narni F, Potenza
L. Chronic and recurrent benign lymphadenopathy without constitutional
symptoms associated with human herpesvirus-6B reactivation. Br J
Haematol. 2016;172(4):561-72. https://doi.org/10.1111/bjh.13871 PMid:26684692

- Weiss LM, O'Malley D. Benign lymphadenopathies. Mod Pathol. 2013;26 Suppl 1:S88-96. https://doi.org/10.1038/modpathol.2012.176 PMid:23281438

- Levine
PH, Jahan N, Murari P, Manak M, Jaffe ES. Detection of human
herpesvirus 6 in tissues involved by sinus histiocytosis with massive
lymphadenopathy (Rosai-Dorfman disease). J Infect Dis.
1992;166(2):291-5. https://doi.org/10.1093/infdis/166.2.291 PMid:1321861

- Scheel
MM, Rady PL, Tyring SK, Pandya AG. Sinus histiocytosis with massive
lymphadenopathy: presentation as giant granuloma annulare and detection
of human herpesvirus 6. J Am Acad Dermatol. 1997;37(4):643-6. https://doi.org/10.1016/S0190-9622(97)70186-5

- Arakaki
N, Gallo G, Majluf R, Diez B, Arias E, Riudavets MA, Sevlever G.
Extranodal rosai-dorfman disease presenting as a solitary mass with
human herpesvirus 6 detection in a pediatric patient. Pediatr Dev
Pathol. 2012;15(4):324-8. https://doi.org/10.2350/11-11-1110-CR.1 PMid:22400904

- Ortonne
N, Fillet AM, Kosuge H, Bagot M, Frances C, Wechsler J. Cutaneous
Destombes-Rosai-Dorfman disease: absence of detection of HHV-6 and
HHV-8 in skin. J Cutan Pathol. 2002;29(2):113-8. https://doi.org/10.1034/j.1600-0560.2002.290209.x PMid:12150132

- Hoffmann
A, Kirn E, Kuerten A, Sander C, Krueger GR, Ablashi DV. Active human
herpesvirus-6 (HHV-6) infection associated with Kikuchi-Fujimoto
disease and systemic lupus erythematosus (SLE). In Vivo.
1991;5(3):265-9. PMid:1654149

- Rodríguez
JN, Aguayo DM, Elizalde J, Martino ML, Moreno MV, Lara C, Prados D.
Kikuchi-Fujimoto disease associated with acute infection by herpesvirus
6. Sangre (Barc). 1996;41(5):387-90.

- Hollingsworth
HC, Peiper SC, Weiss LM, Raffeld M, Jaffe ES. An investigation of the
viral pathogenesis of Kikuchi-Fujimoto disease. Lack of evidence for
Epstein-Barr virus or human herpesvirus type 6 as the causative agents.
Arch Pathol Lab Med. 1994;118(2):134-40. PMid:8311651

- Rosado
FG, Tang YW, Hasserjian RP, McClain CM, Wang B, Mosse CA.
Kikuchi-Fujimoto lymphadenitis: role of parvovirus B-19, Epstein-Barr
virus, human herpesvirus 6, and human herpesvirus 8. Hum Pathol.
2013;44(2):255-9. https://doi.org/10.1016/j.humpath.2012.05.016 PMid:22939574

- Sumiyoshi
Y, Kikuchi M, Ohshima K, Yoneda S, Kobari S, Takeshita M, Eizuru Y,
Minamishima Y. Human herpesvirus-6 genomes in histiocytic necrotizing
lymphadenitis (Kikuchi's disease) and other forms of lymphadenitis. Am
J Clin Pathol. 1993;99(5):609-14. https://doi.org/10.1093/ajcp/99.5.609 PMid:8388164

- Usui
Y, Rao NA, Takase H, Tsubota K, Umazume K, Diaz-Aguilar D, Kezuka T,
Mochizuki M, Goto H, Sugita S. Comprehensive polymerase chain reaction
assay for detection of pathogenic DNA in lymphoproliferative disorders
of the ocular adnexa. Sci Rep. 2016;6:36621. https://doi.org/10.1038/srep36621 PMid:27830722 PMCid:PMC5103257

- Sugita
S, Shimizu N, Kawaguchi T, Akao N, Morio T, Mochizuki M. Identification
of human herpesvirus 6 in a patient with severe unilateral panuveitis.
Arch Ophthalmol. 2007;125(10):1426-7. https://doi.org/10.1001/archopht.125.10.1426 PMid:17923557

- Sugita
S, Shimizu N, Watanabe K, Ogawa M, Maruyama K, Usui N, Mochizuki M.
Virological analysis in patients with human herpes virus 6-associated
ocular inflammatory disorders. Invest Ophthalmol Vis Sci.
2012;53(8):4692-8. PMid:22700707

- Bai
Y, Wang Z, Sun K, Yao H. HHV-6-associated acute lymphadenitis in
immunocompetent patients: a case report and review of literature. Int J
Clin Exp Pathol. 2014;7(6):3413-7. PMid:25031769 PMCid:PMC4097211

- Krueger
GR, Ablashi DV, Salahuddin SZ, Josephs SF. Diagnosis and differential
diagnosis of progressive lymphoproliferation and malignant lymphoma in
persistent active herpesvirus infection. J Virol Methods.
1988;21(1-4):255-64. https://doi.org/10.1016/0166-0934(88)90071-7

- Karino
T, Asaoka N, Sunagawa T, Ohba H, Yoneyama H, Kobashi Y, Okimoto J,
Soejima R. A case with infectious mononucleosis-like syndrome caused by
human herpes virus-6 infection. Kansenshogaku Zasshi. 2000;74(3):264-8.
https://doi.org/10.11150/kansenshogakuzasshi1970.74.264 PMid:10783582

- Buchwald
D, Freedman AS, Ablashi DV, Sullivan JL, Caligiuri M, Weinberg DS, Hall
CG, Ashley RL, Saxinger C, Balachandran N, Ritz J, Nadler LM, Komaroff
AL. A chronic "postinfectious" fatigue syndrome associated with benign
lymphoproliferation, B-cell proliferation, and active replication of
human herpesvirus-6. J Clin Immunol. 1990;10(6):335-44. https://doi.org/10.1007/BF00917479 PMid:1964694

- Steeper
TA, Horwitz CA, Ablashi DV, Salahuddin SZ, Saxinger C, Saltzman R,
Schwartz B. The spectrum of clinical and laboratory findings resulting
from human herpesvirus-6 (HHV-6) in patients with mononucleosis-like
illnesses not resulting from Epstein-Barr virus or cytomegalovirus. Am
J Clin Pathol. 1990;93(6):776-83. https://doi.org/10.1093/ajcp/93.6.776 PMid:2161178

- Naito
T, Kudo N, Inui A, Matsumoto N, Takeda N, Isonuma H, Dambara T,
Hayashida Y. Causes of infectious mononucleosis-like syndrome in adult
patients. Intern Med. 2006;45(13):833-4. https://doi.org/10.2169/internalmedicine.45.1725 PMid:16880711

- Horwitz
CA, Krueger GRF, Steeper TA, Bertram G. HHV-6-induced
mononucleosis-like illnesses. Perspectives in Medical Virology.
1992;4:159-74. https://doi.org/10.1016/S0168-7069(08)70064-8

- Ramon
A, Krueger GRF, Simoes P, Jockenhoevel F, Neumann R, Rojo J, Ablashi
DV: Immunopathology of liver diseases. Revista Med Hosp Gen Mexico.
1998;61:241-61.

- Kirchesch
H, Mertens T, Burkhardt U, Kruppenbacher JP, Höffken A, Eggers HJ.
Seroconversion against human herpesvirus-6 (and other herpesviruses)
and clinical illness. Lancet. 1988;2(8605):273-4. https://doi.org/10.1016/S0140-6736(88)92557-3

- Sobue
R, Miyazaki H, Okamoto M, Hirano M, Yoshikawa T, Suga S, Asano Y.
Fulminant hepatitis in primary human herpesvirus-6 infection. N Engl J
Med. 1991;324(18):1290. https://doi.org/10.1056/NEJM199105023241818 PMid:1849614

- Krueger
GR, Bertram G, Ramon A, Koch B, Ablashi DV, Brandt ME, Wang G, Buja LM.
Dynamics of infection with human herpesvirus-6 in EBV-negative
infectious mononucleosis: data acquisition for computer modeling. In
Vivo. 2001;15(5):373-80. PMid:11695232

- Akashi
K, Eizuru Y, Sumiyoshi Y, Minematsu T, Hara S, Harada M, Kikuchi M,
Niho Y, Minamishima Y. Brief report: severe infectious
mononucleosis-like syndrome and primary human herpesvirus 6 infection
in an adult. N Engl J Med. 1993;329(3):168-71. https://doi.org/10.1056/NEJM199307153290304 PMid:8390615

- Sumiyoshi
Y, Akashi K, Kikuchi M. Detection of human herpes virus 6 (HHV 6) in
the skin of a patient with primary HHV 6 infection and erythroderma. J
Clin Pathol. 1994;47(8):762-3. https://doi.org/10.1136/jcp.47.8.762 PMid:7962635 PMCid:PMC502155

- Sumiyoshi
Y, Kikuchi M, Ohshima K, Takeshita M, Eizuru Y, Minamishima Y. A case
of human herpesvirus-6 lymphadenitis with infectious mononucleosis-like
syndrome. Pathol Int. 1995;45(12):947-51. https://doi.org/10.1111/j.1440-1827.1995.tb03420.x PMid:8808300

- Tsaparas
YF, Brigden ML, Mathias R, Thomas E, Raboud J, Doyle PW. Proportion
positive for Epstein-Barr virus, cytomegalovirus, human herpesvirus 6,
Toxoplasma, and human immunodeficiency virus types 1 and 2 in
heterophile-negative patients with an absolute lymphocytosis or an
instrument-generated atypical lymphocyte flag. Arch Pathol Lab Med.
2000;124(9):1324-30. PMid:10975931

- Wang
G, Krueger GR, Buja LM. Mathematical model to simulate the cellular
dynamics of infection with human herpesvirus-6 in EBV-negative
infectious mononucleosis. J Med Virol. 2003;71(4):569-77. https://doi.org/10.1002/jmv.10522 PMid:14556271

- Krueger
GR, Brandt ME, Wang G, Berthold F, Buja LM. A computational analysis of
Canale-Smith syndrome: chronic lymphadenopathy simulating malignant
lymphoma. Anticancer Res. 2002;22(4):2365-71. PMid:12174928

- Hoang
MP, Dawson DB, Rogers ZR, Scheuermann RH, Rogers BB. Polymerase chain
reaction amplification of archival material for Epstein-Barr virus,
cytomegalovirus, human herpesvirus 6, and parvovirus B19 in children
with bone marrow hemophagocytosis. Hum Pathol. 1998;29(10):1074-7. https://doi.org/10.1016/S0046-8177(98)90416-6

- Syrucková
Z, Starý J, Sedlácek P, Smísek P, Vavrinec J, Komrska V, Roubalová K,
Vandasová J, Sintáková B, Housková J, Hassan M. Infection-associated
hemophagocytic syndrome complicated by infectious lymphoproliferation:
a case report. Pediatr Hematol Oncol. 1996;13(2):143-50. https://doi.org/10.3109/08880019609030804 PMid:8721028

- Tadokoro
R, Okumura A, Nakazawa T, Hara S, Yamakawa Y, Kamata A, Kinoshita K,
Obinata K, Shimizu T. Acute encephalopathy with biphasic seizures and
late reduced diffusion associated with hemophagocytic syndrome. Brain
Dev. 2010;32(6):477-81. https://doi.org/10.1016/j.braindev.2009.05.007 PMid:19556082

- Tanaka
H, Nishimura T, Hakui M, Sugimoto H, Tanaka-Taya K, Yamanishi K. Human
herpesvirus 6-associated hemophagocytic syndrome in a healthy adult.
Emerg Infect Dis. 2002;8(1):87-8. https://doi.org/10.3201/eid0801.010149 PMCid:PMC2730277

- Dharancy
S, Crombe V, Copin MC, Boleslawski E, Bocket L, Declerck N, Canva V,
Dewilde A, Mathurin P, Pruvot FR. Fatal hemophagocytic syndrome related
to human herpesvirus-6 reinfection following liver transplantation: a
case report. Transplant Proc. 2008;40(10):3791-3. https://doi.org/10.1016/j.transproceed.2008.05.083 PMid:19100492

- Marabelle
A, Bergeron C, Billaud G, Mekki Y, Girard S. Hemophagocytic syndrome
revealing primary HHV-6 infection. J Pediatr. 2010;157(3):511. https://doi.org/10.1016/j.jpeds.2010.02.064 PMid:20400100

- Takagi
M, Unno A, Maruyama T, Kaneko K, Obinata K. Human herpesvirus-6
(HHV-6)-associated hemophagocytic syndrome. Pediatr Hematol Oncol.
1996;13(5):451-6. https://doi.org/10.3109/08880019609030857 PMid:10897817

- Chen
RL, Lin KH, Lin DT, Su IJ, Huang LM, Lee PI, Hseih KH, Lin KS, Lee CY.
Immunomodulation treatment for childhood virus-associated
haemophagocytic lymphohistiocytosis. Br J Haematol. 1995;89(2):282-90. https://doi.org/10.1111/j.1365-2141.1995.tb03302.x PMid:7873378

- Huang
LM, Lee CY, Lin KH, Chuu WM, Lee PI, Chen RL, Chen JM, Lin DT. Human
herpesvirus-6 associated with fatal haemophagocytic syndrome. Lancet.
1990;336(8706):60-1. https://doi.org/10.1016/0140-6736(90)91580-4

- Liu
DL, Teng RJ, Ho MM, Hwang KC, Hwang LM. Human herpesvirus-6 associated
hemophagocytic syndrome in beta-thalassemia: report of one case.
Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1995;36(5):373-5.
PMid:8607365

- Randhawa
PS, Jenkins FJ, Nalesnik MA, Martens J, Williams PA, Ries A, Pham S,
Demetris AJ. Herpesvirus 6 variant A infection after heart
transplantation with giant cell transformation in bile ductular and
gastroduodenal epithelium. Am J Surg Pathol. 1997;21(7):847-53. https://doi.org/10.1097/00000478-199707000-00014 PMid:9236842

- Potenza
L, Luppi M, Barozzi P, Rossi G, Cocchi S, Codeluppi M, Pecorari M,
Masetti M, Di Benedetto F, Gennari W, Portolani M, Gerunda GE,
Lazzarotto T, Landini MP, Schulz TF, Torelli G, Guaraldi G. HHV-6A in
syncytial giant-cell hepatitis. N Engl J Med. 2008;359(6):593-602. https://doi.org/10.1056/NEJMoa074479 PMid:18687640

- Mori
Y, Seya T, Huang HL, Akkapaiboon P, Dhepakson P, Yamanishi K. Human
herpesvirus 6 variant A but not variant B induces fusion from without
in a variety of human cells through a human herpesvirus 6 entry
receptor, CD46. J Virol. 2002;76(13):6750-61. https://doi.org/10.1128/JVI.76.13.6750-6761.2002 PMid:12050388 PMCid:PMC136280

- Pedersen
SM, Oster B, Bundgaard B, Höllsberg P. Induction of cell-cell fusion
from without by human herpesvirus 6B. J Virol. 2006;80(19):9916-20. https://doi.org/10.1128/JVI.02693-05 PMid:16973598 PMCid:PMC1617257

- Bastida-Ruiz D, Van Hoesen K, Cohen M. The Dark Side of Cell Fusion. Int J Mol Sci. 2016;17(5):638. https://doi.org/10.3390/ijms17050638 PMid:27136533 PMCid:PMC4881464

- Lautenschlager
I, Härmä M, Höckerstedt K, Linnavuori K, Loginov R, Taskinen E. Human
herpesvirus-6 infection is associated with adhesion molecule induction
and lymphocyte infiltration in liver allografts. J Hepatol.
2002;37(5):648-54. https://doi.org/10.1016/S0168-8278(02)00246-5

- Schmitt
K, Deutsch J, Tulzer G, Meindi R, Aberle S. Autoimmune hepatitis and
adrenal insufficiency in an infant with human herpesvirus-6 infection.
Lancet. 1996;348(9032):966. https://doi.org/10.1016/S0140-6736(05)65385-8

- Charnot-Katsikas
A, Baewer D, Cook L, David MZ. Fulminant hepatic failure attributed to
infection with human herpesvirus 6 (HHV-6) in an immunocompetent woman:
A case report and review of the literature. J Clin Virol.
2016;75:27-32. https://doi.org/10.1016/j.jcv.2015.12.002 PMid:26745830

- Asano
Y, Yoshikawa T, Suga S, Yazaki T, Kondo K, Yamanishi K. Fatal fulminant
hepatitis in an infant with human herpesvirus-6 infection. Lancet.
1990;335(8693):862-3. https://doi.org/10.1016/0140-6736(90)90983-C

- Chang
YL, Parker ME, Nuovo G, Miller JB. Human herpesvirus 6-related
fulminant myocarditis and hepatitis in an immunocompetent adult with
fatal outcome. Hum Pathol. 2009;40(5):740-5. https://doi.org/10.1016/j.humpath.2008.08.017 PMid:19144379

- Grima
P, Chiavaroli R, Calabrese P, Tundo P, Grima P. Severe hepatitis with
autoimmune features following a HHV-6: a case report. Cases J.
2008;1(1):110. https://doi.org/10.1186/1757-1626-1-110 PMid:18710564 PMCid:PMC2531091

- Descamps
V, Bouscarat F, Laglenne S, Aslangul E, Veber B, Descamps D, Saraux JL,
Grange MJ, Grossin M, Navratil E, Crickx B, Belaich S. Human
herpesvirus 6 infection associated with anticonvulsant hypersensitivity
syndrome and reactive haemophagocytic syndrome. Br J Dermatol.
1997;137(4):605-8. https://doi.org/10.1111/j.1365-2133.1997.tb03795.x PMid:9390340

- Johnson
S, Mathews S, Hudnall SD. Human herpesvirus 6 lymphadenitis in drug
rash with eosinophilia and systemic symptoms syndrome: a lymphoma
mimic. Histopathology. 2017;70(7):1166-70. https://doi.org/10.1111/his.13157 PMid:28008656

- Saraya
T, Mikoshiba M, Kamiyama H, Yoshizumi M, Tsuchida S, Tsukagoshi H,
Ishioka T, Terada M, Tanabe E, Tomioka C, Ishii H, Kimura H, Kozawa K,
Shiohara T, Takizawa H, Goto H. Evidence for reactivation of human
herpesvirus 6 in generalized lymphadenopathy in a patient with
drug-induced hypersensitivity syndrome. J Clin Microbiol.
2013;51(6):1979-82. https://doi.org/10.1128/JCM.00097-13 PMid:23536404 PMCid:PMC3716082

- Eshki
M, Allanore L, Musette P, Milpied B, Grange A, Guillaume JC, Chosidow
O, Guillot I, Paradis V, Joly P, Crickx B, Ranger-Rogez S, Descamps V.
Twelve-year analysis of severe cases of drug reaction with eosinophilia
and systemic symptoms: a cause of unpredictable multiorgan failure.
Arch Dermatol. 2009;145(1):67-72. https://doi.org/10.1001/archderm.145.1.67 PMid:19153346

- Fujino
Y, Nakajima M, Inoue H, Kusuhara T, Yamada T. Human herpesvirus 6
encephalitis associated with hypersensitivity syndrome. Ann Neurol.
2002;51(6):771-4. https://doi.org/10.1002/ana.10194 PMid:12112085

- Mennicke
M, Zawodniak A, Keller M, Wilkens L, Yawalkar N, Stickel F, Keogh A,
Inderbitzin D, Candinas D, Pichler WJ. Fulminant liver failure after
vancomycin in a sulfasalazine-induced DRESS syndrome: fatal recurrence
after liver transplantation. Am J Transplant. 2009;9(9):2197-202. https://doi.org/10.1111/j.1600-6143.2009.02788.x PMid: 19706026

- Miyashita
K, Shobatake C, Miyagawa F, Kobayashi N, Onmori R, Yonekawa S, Tanabe
K, Kawate K, Morita K, Asada H. Involvement of Human Herpesvirus 6
Infection in Renal Dysfunction Associated with DIHS/DRESS. Acta Derm
Venereol. 2016;96(1):114-5. https://doi.org/10.2340/00015555-2149 PMid:26039936

- Hagiya
H, Iwamuro M, Tanaka T, Hasegawa K, Hanayama Y, Kimura M, Otsuka F.
Reactivation of Human Herpes Virus-6 in the Renal Tissue of a Patient
with Drug-induced Hypersensitivity Syndrome/Drug Rash with Eosinophilia
and Systemic Symptoms (DIHS/DRESS). Intern Med. 2016;55(13):1769-74. https://doi.org/10.2169/internalmedicine.55.6287 PMid:27374681

- Mine
S, Suzuki K, Sato Y, Fukumoto H, Kataoka M, Inoue N, Ohbayashi C,
Hasegawa H, Sata T, Fukayama M, Katano H. Evidence for human
herpesvirus-6B infection of regulatory T-cells in acute systemic
lymphadenitis in an immunocompetent adult with the drug reaction with
eosinophilia and systemic symptoms syndrome: a case report. J Clin
Virol. 2014;61(3):448-52. https://doi.org/10.1016/j.jcv.2014.08.025 PMid:25249343

- Descamps V, Ranger-Rogez S. DRESS syndrome. Joint Bone Spine. 2014;81(1):15-21. https://doi.org/10.1016/j.jbspin.2013.05.002 PMid:23816504

- Nakazato
T, Suzuki K, Mihara A, Sanada Y, Aisa Y, Kakimoto T. ATL-like marked
atypical lymphocytosis associated with drug-induced hypersensitivity
syndrome and human herpesvirus-6 reactivation. Int J Hematol.
2009;90(5):648-50. https://doi.org/10.1007/s12185-009-0449-4 PMid:19937484

- Tohyama
M, Hashimoto K, Yasukawa M, Kimura H, Horikawa T, Nakajima K, Urano Y,
Matsumoto K, Iijima M, Shear NH. Association of human herpesvirus 6
reactivation with the flaring and severity of drug-induced
hypersensitivity syndrome. Br J Dermatol. 2007;157(5):934-40. https://doi.org/10.1111/j.1365-2133.2007.08167.x PMid:17854362

- Maric
I, Bryant R, Abu-Asab M, Cohen JI, Vivero A, Jaffe ES, Raffeld M,
Tsokos M, Banks PM, Pittaluga S. Human herpesvirus-6-associated acute
lymphadenitis in immunocompetent adults. Mod Pathol.
2004;17(11):1427-33. https://doi.org/10.1038/modpathol.3800179 PMid:15494709 PMCid:PMC2288737

- Takahashi
R, Kano Y, Yamazaki Y, Kimishima M, Mizukawa Y, Shiohara T. Defective
regulatory T cells in patients with severe drug eruptions: timing of
the dysfunction is associated with the pathological phenotype and
outcome. J Immunol. 2009;182(12):8071-9. https://doi.org/10.4049/jimmunol.0804002 PMid:19494333

- Wang
F, Chi J, Peng G, Zhou F, Wang J, Li L, Feng D, Xie F, Gu B, Qin J,
Chen Y, Yao K. Development of virus-specific CD4+ and CD8+ regulatory T
cells induced by human herpesvirus 6 infection. J Virol.
2014;88(2):1011-24. https://doi.org/10.1128/JVI.02586-13 PMid:24198406 PMCid:PMC3911638

- Wang
F, Yao K, Yin QZ, Zhou F, Ding CL, Peng GY, Xu J, Chen Y, Feng DJ, Ma
CL, Xu WR. Human herpesvirus-6-specific interleukin 10-producing CD4+ T
cells suppress the CD4+ T-cell response in infected individuals.
Microbiol Immunol. 2006;50(10):787-803. https://doi.org/10.1111/j.1348-0421.2006.tb03855.x PMid:17053315

- Fazakas
A, Csire M, Berencsi G, Szepesi A, Matolcsy A, Jakab L, Karádi I,
Várkonyi J. Multicentric plasmocytic Castleman's disease with
polyneuropathy, organomegaly, endocrinopathy, M protein, skin changes
syndrome and coexistent human herpes virus-6 infection--a possible
relationship. Leuk Lymphoma. 2009;50(10):1661-5. https://doi.org/10.1080/10428190903162743 PMid:19863339

- Ohyashiki
JH, Abe K, Ojima T, Wang P, Zhou CF, Suzuki A, Ohyashiki K, Yamamoto K.
Quantification of human herpesvirus 6 in healthy volunteers and
patients with lymphoproliferative disorders by PCR-ELISA. Leuk Res.
1999;23(7):625-30. https://doi.org/10.1016/S0145-2126(99)00079-X

- Barozzi
P, Luppi M, Masini L, Marasca R, Savarino M, Morselli M, Ferrari MG,
Bevini M, Bonacorsi G, Torelli G. Lymphotropic herpes virus (EBV,
HHV-6, HHV-8) DNA sequences in HIV negative Castleman's disease. Clin
Mol Pathol. 1996;49(4):M232-5. https://doi.org/10.1136/mp.49.4.M232 PMid:16696081 PMCid:PMC408065

- Krueger
GR, Guenther A, Knuefermann R, Kluppelberg U, Luka J, Pearson GR,
Ablashi DV, Juecker M, Tesch H. Human herpesvirus-6 (HHV-6) in
Hodgkin's disease: cellular expression of viral antigens as compared to
oncogenes met and fes, tumor suppressor gene product p53, and
interleukins 2 and 6. In Vivo. 1994;8(4):501-16. PMid:7893977

- Valente
G, Secchiero P, Lusso P, Abete MC, Jemma C, Reato G, Kerim S, Gallo RC,
Palestro G. Human herpesvirus 6 and Epstein-Barr virus in Hodgkin's
disease: a controlled study by polymerase chain reaction and in situ
hybridization. Am J Pathol. 1996;149(5):1501-10. PMid:8909240
PMCid:PMC1865277

- Collot
S, Petit B, Bordessoule D, Alain S, Touati M, Denis F, Ranger-Rogez S.
Real-time PCR for quantification of human herpesvirus 6 DNA from lymph
nodes and saliva. J Clin Microbiol. 2002;40(7):2445-51. https://doi.org/10.1128/JCM.40.7.2445-2451.2002 PMid:12089260 PMCid:PMC120581

- Lacroix
A, Jaccard A, Rouzioux C, Piguet C, Petit B, Bordessoule D,
Ranger-Rogez S. HHV-6 and EBV DNA quantitation in lymph nodes of 86
patients with Hodgkin's lymphoma. J Med Virol. 2007;79(9):1349-56. https://doi.org/10.1002/jmv.20868 PMid:17607791

- Kashanchi
F, Araujo J, Doniger J, Muralidhar S, Hoch R, Khleif S, Mendelson E,
Thompson J, Azumi N, Brady JN, Luppi M, Torelli G, Rosenthal LJ. Human
herpesvirus 6 (HHV-6) ORF-1 transactivating gene exhibits malignant
transforming activity and its protein binds to p53. Oncogene.
1997;14(3):359-67. https://doi.org/10.1038/sj.onc.1200840 PMid:9018122

- Lacroix
A, Collot-Teixeira S, Mardivirin L, Jaccard A, Petit B, Piguet C,
Sturtz F, Preux PM, Bordessoule D, Ranger-Rogez S. Involvement of human
herpesvirus-6 variant B in classic Hodgkin's lymphoma via DR7
oncoprotein. Clin Cancer Res. 2010;16(19):4711-21. https://doi.org/10.1158/1078-0432.CCR-10-0470 PMid:20858841

- Siddon
A, Lozovatsky L, Mohamed A, Hudnall SD. Human herpesvirus 6 positive
Reed-Sternberg cells in nodular sclerosis Hodgkin lymphoma. Br J
Haematol. 2012;158(5):635-43. https://doi.org/10.1111/j.1365-2141.2012.09206.x PMid:22757777

- Carbone
A, Dolcetti R, Gloghini A, Maestro R, Vaccher E, di Luca D, Tirelli U,
Boiocchi M. Immunophenotypic and molecular analyses of acquired immune
deficiency syndrome-related and Epstein-Barr virus-associated
lymphomas: a comparative study. Hum Pathol. 1996;27(2):133-46. https://doi.org/10.1016/S0046-8177(96)90366-4

- Braun
DK, Pellett PE, Hanson CA. Presence and expression of human herpesvirus
6 in peripheral blood mononuclear cells of S100-positive, T cell
chronic lymphoproliferative disease. J Infect Dis. 1995;171(5):1351-5. https://doi.org/10.1093/infdis/171.5.1351 PMid:7751716

- Lu
C, Zeng Y, Huang Z, Huang L, Qian C, Tang G, Qin D. Human herpesvirus 6
activates lytic cycle replication of Kaposi's sarcoma-associated
herpesvirus. Am J Pathol. 2005;166(1):173-83. https://doi.org/10.1016/S0002-9440(10)62242-0

- Josephs
SF, Buchbinder A, Streicher HZ, Ablashi DV, Salahuddin SZ, Guo HG,
Wong-Staal F, Cossman J, Raffeld M, Sundeen J. Detection of human
B-lymphotropic virus (human herpesvirus 6) sequences in B cell lymphoma
tissues of three patients. Leukemia. 1988;2(3):132-5. PMid:3258048

- Zhou
Y, Attygalle AD, Chuang SS, Diss T, Ye H, Liu H, Hamoudi RA, Munson P,
Bacon CM, Dogan A, Du MQ. Angioimmunoblastic T-cell lymphoma:
histological progression associates with EBV and HHV6B viral load. Br J
Haematol. 2007;138(1):44-53. https://doi.org/10.1111/j.1365-2141.2007.06620.x PMid:17555446

- Strong
MJ, O'Grady T, Lin Z, Xu G, Baddoo M, Parsons C, Zhang K, Taylor CM,
Flemington EK. Epstein-Barr virus and human herpesvirus 6 detection in
a non-Hodgkin's diffuse large B-cell lymphoma cohort by using RNA
sequencing. J Virol. 2013;87(23):13059-62. https://doi.org/10.1128/JVI.02380-13 PMid:24049168 PMCid:PMC3838131

- Luppi
M, Marasca R, Barozzi P, Artusi T, Torelli G. Frequent detection of
human herpesvirus-6 sequences by polymerase chain reaction in
paraffin-embedded lymph nodes from patients with angioimmunoblastic
lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma.
Leuk Res. 1993;17(11):1003-11. https://doi.org/10.1016/0145-2126(93)90049-Q

- Campioni
D, Gentili V, Cavazzini F, Bortolotti D, Nacheva EP, Cuneo A, Di Luca
D, Rizzo R. Detection of inherited chromosomally integrated HHV-6
(ciHHV-6) in a marker chromosome. Eur J Haematol. 2017;98(6):635-7. https://doi.org/10.1111/ejh.12872 PMid:28244148

- Zhang
E, Cotton VE, Hidalgo-Bravo A, Huang Y, Bell AJ, Jarrett RF, Wilkie GS,
Davison AJ, Nacheva EP, Siebert R, Majid A, Kelpanides I, Jayne S, Dyer
MJ, Royle NJ. HHV-8-unrelated primary effusion-like lymphoma associated
with clonal loss of inherited chromosomally-integrated human
herpesvirus-6A from the telomere of chromosome 19q. Sci Rep.
2016;6:22730. https://doi.org/10.1038/srep22730 PMid:26947392 PMCid:PMC4779988

- Seror
E, Coquerel B, Gautheret-Dejean A, Ballerini P, Landman-Parker J,
Leverger G, Schneider P, Vannier JP. Quantitation of Human herpes virus
6 genome in children with acute lymphoblastic leukemia. J Med Virol.
2008;80(4):689-93. https://doi.org/10.1002/jmv.21118 PMid:18297709

- Mori
S, Sugahara K, Uemura A, Akamatsu N, Hirakata Y, Murata K, Hasegawa H,
Yamada Y, Kamihira S. Usefulness of a comprehensive PCR-based assay for
human herpes viral DNA in blood mononuclear cell samples. Lab Hematol.
2005;11(3):163-70. https://doi.org/10.1532/LH96.05027 PMid:16174601

- Levine
PH, Ablashi DV, Saxinger WC, Connelly RR. Antibodies to human herpes
virus-6 in patients with acute lymphocytic leukemia. Leukemia.
1992;6(11):1229-31. PMid:1331626

- Schlehofer
B, Blettner M, Geletneky K, Haaf HG, Kaatsch P, Michaelis J,
Mueller-Lantzsch N, Niehoff D, Winkelspecht B, Wahrendorf J, Schlehofer
JR. Sero-epidemiological analysis of the risk of virus infections for
childhood leukaemia. Int J Cancer. 1996;65(5):584-90. https://doi.org/10.1002/(SICI)1097-0215(19960301)65:5<584::AID-IJC5>3.0.CO;2-Z

- Steininger
C, Rassenti LZ, Vanura K, Eigenberger K, Jäger U, Kipps TJ, Mannhalter
C, Stilgenbauer S, Popow-Kraupp T. Relative seroprevalence of human
herpes viruses in patients with chronic lymphocytic leukaemia. Eur J
Clin Invest. 2009;39(6):497-506. https://doi.org/10.1111/j.1365-2362.2009.02131.x PMid:19490058 PMCid:PMC3709071

- Faten
N, Agnès GD, Nadia BF, Nabil AB, Monia Z, Abderrahim K, Henri A, Salma
F, Mahjoub A. Quantitative analysis of human herpesvirus-6 genome in
blood and bone marrow samples from Tunisian patients with acute
leukemia: a follow-up study. Infect Agent Cancer. 2012;7(1):31. https://doi.org/10.1186/1750-9378-7-31 PMid:23146098 PMCid:PMC3527176

- Hermouet
S, Sutton CA, Rose TM, Greenblatt RJ, Corre I, Garand R, Neves AM,
Bataille R, Casey JW. Qualitative and quantitative analysis of human
herpesviruses in chronic and acute B cell lymphocytic leukemia and in
multiple myeloma. Leukemia. 2003;17(1):185-95. https://doi.org/10.1038/sj.leu.2402748 PMid:12529677

- Krueger
GR, Kudlimay D, Ramon A, Klueppelberg U, Schumacher K. Demonstration of
active and latent Epstein-Barr virus and human herpevirus-6 infections
in bone marrow cells of patients with myelodysplasia and chronic
myeloproliferative diseases. In Vivo. 1994;8(4):533-42. PMid:7893980

- Morales-Sánchez
A, Pompa-Mera EN, Fajardo-Gutiérrez A, Alvarez-Rodríguez FJ,
Bekker-Méndez VC, Flores-Chapa Jde D, Flores-Lujano J,
Jiménez-Hernández E, Pe-aloza-González JG, Rodríguez-Zepeda MC,
Torres-Nava JR, Velázquez-Avi-a MM, Amador-Sánchez R, Alvarado-Ibarra
M, Reyes-Zepeda N, Espinosa-Elizondo RM, Pérez-Saldivar ML,
Nú-ez-Enríquez JC, Mejía-Aranguré JM, Fuentes-Pananá EM. EBV, HCMV,
HHV6, and HHV7 screening in bone marrow samples from children with
acute lymphoblastic leukemia. Biomed Res Int. 2014;2014:548097. https://doi.org/10.1155/2014/548097 PMid:25309913 PMCid:PMC4189769

- Krueger
GR, Klueppelberg U, Hoffmann A, Ablashi DV. Clinical correlates of
infection with human herpesvirus-6. In Vivo. 1994;8(4):457-85.
PMid:7893974

- Krueger
GR, Ferrer Argote V. A unifying concept of viral immunopathogenesis of
proliferative and aproliferative diseases (working hypothesis). In
Vivo. 1994;8(4):493-9. PMid:7893976

[TOP]




























































































































































































