Nicolò Peccatori1, Roberta Ortiz2, Emanuela Rossi3, Patricia Calderon2, Valentino Conter1, Yesly García2, Andrea Biondi1, Darrel Espinoza2, Francesco Ceppi4, Luvy Mendieta5 and Maria Luisa Melzi1.
1 Centro di Emato-Oncologia Pediatrica Maria Letizia Verga, S. Gerardo Hospital, University of Milano-Bicocca, Monza, Italy.
2 Department of Hematology/Oncology, Children’s Hospital Manuel de Jesus Rivera, Managua, Nicaragua.
3
Center of Biostatistics for Clinical Epidemiology, Department of
Medicine and Surgery, University of Milano-Bicocca, Monza, Italy.
4
Pediatric Hematology-Oncology Unit Pediatric Hematology-Oncology
Research Laboratory, Division of Pediatrics, Department of
Woman-Mother-Child, University Hospital of Lausanne, Lausanne,
Switzerland.
5 Department of Nutrition, Children’s Hospital Manuel de Jesus Rivera, Managua, Nicaragua.
Corresponding
author: Valentino Conter, Centro di Emato-Oncologia Pediatrica Maria
Letizia Verga, S. Gerardo Hospital, University of Milano-Bicocca. Via
Pergolesi 33, 20900 Monza (MB), Italy. Fax: (011) 39-039-2301646.
E-mail:
valentino.conter@gmail.com
Published: June 23, 2018
Received: October 3, 2018
Accepted: May 24, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018038 DOI
10.4084/MJHID.2018.038
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Children
with cancer are particularly vulnerable to malnutrition, which can
affect their tolerance of chemotherapy and outcome. In Nicaragua
approximately two-thirds of children diagnosed with cancer present with
under-nutrition. A nutritional program for children with cancer has
been developed at “La Mascota” Hospital. Results of this oral
nutritional intervention including difficulties, benefits, and
relevance for children treated for cancer in low- and middle-income
countries are here reported and discussed.
|
Introduction
The
pediatric population diagnosed with cancer is at high risk of
malnutrition for cancer and treatment related effects. Children with
cancer tend to become malnourished during treatment because of multiple
reasons. Pain, anorexia, hormonal and inflammatory components, low
physical activity, taste aversions and chronic medications are all
factors which lead to decreased oral calories intake and can contribute
to malnutrition.[1,2] Malnutrition can affect the
tolerance of both chemotherapy and radiotherapy, may increase the risk
of comorbidities and influence overall survival.[3]
Approximately
80% of children and adolescents who are diagnosed with cancer live in
low- and middle-income countries (LMICs), where access to quality care
and chances of cure are limited. In LMICs, malnutrition represents one
of the major obstacles to effective pediatric care, together with late
diagnosis, abandonment of therapy, suboptimal supportive care, and
inefficient health-care delivery systems.[4]
In LMICs it is estimated that the prevalence of malnutrition averages 50% in children with cancer.[5]
In Nicaragua, the prevalence of malnutrition in children under 5 years
of age according to UNICEF is 30%, with 23% of children presenting
severe malnutrition and 7% moderate malnutrition.[6] A study performed in the hemato-oncology centers of the AHOPCA (Asociación de Hemato-Oncología Pediátrica Centro-Americana)[7]
countries, between 2004 and 2007, showed that nutritional status of
children with cancer at diagnosis was normal in 37% of patients,
moderately depleted in 18% and severely depleted in 45%.[8]
In Nicaragua at the Children’s Hospital Manuel de Jesus Rivera “La
Mascota” (HIMJR), 67% of patients were classified as malnourished at
diagnosis, including 47.9% with severe malnutrition.[9]
On
the basis of these evidences, a nutritional program for children
diagnosed with cancer who were inadequately nourished was developed at
HIMJR, in collaboration with the Pediatric Hemato-Oncology Center of
Monza (Milano-Bicocca University), in the context of the Monza
International School of Pediatric Hematology/Oncology (MISPHO)
initiative.[10]
HIMJR is the only hospital of
Nicaragua where children with cancer can be treated; approximately 250
children with cancer per year are referred from the whole country.
The
nutritional program started in February 2016 with the objectives to
reduce the adverse effect of malnutrition on treatment related
morbidity and improve clinical outcome.
The aim of this study is
to assess the role of oral/enteral supplementation in children treated
for oncological diseases in the context of LMICs, where this
intervention still needs to be properly investigated.
Material and Methods
Patients
with cancer, aged one month to 17 years, diagnosed between February
2016 and March 2017 at the HIMJR were screened for nutritional status
by a qualified nutritionist at diagnosis. The nutritional status
assessment was based on weight, height or length and the anthropometric
measures of mid upper arm circumference (MUAC) and triceps skin fold
thickness (TSFT), as already reported.[8]
TSFT and MUAC percentiles were estimated for age and gender using the LMS procedure according to the CDC and WHO growth charts.[11] Patients were considered adequately nourished (AN) if both TSFT and MUAC were >10th percentile, severely depleted (SD) if TSFT or MUAC <5th percentile, and moderately depleted (MD) in all other cases.
Eligible
for the study, after obtaining written informed consent from parents or
legal guardians, were: 1) patients inadequately nourished at diagnosis,
2) patients with borderline nutritional status at diagnosis undergoing
intensive chemotherapy, considered at high risk of developing
under-nutrition by the nutritionist and treating physician, and 3)
patients developing under-nutrition during the treatment. Children with
advanced disease at diagnosis and eligible only for palliative care
were excluded.
Patients entered in the study were given oral
polymeric hyper-caloric formulas containing a balanced mixture of
proteins, fats, and carbohydrates according to the indication by age
for patients up to 10 years or older (up to 17 years). Parents were
instructed to reconstitute formulas with water and to administer them
to their children at home after discharge from the hospital. The
adherence to the nutritional treatment was ascertained by the
nutritionist from parents’ interviews.
Only when oral feeding
was not considered possible or safe, formulas were administered through
enteral feeding. Nutritionists and treating physicians decided the
schedule of nutritional reassessments on the basis of the clinical
conditions and the treatment plan for each patient.
Gender, date
of birth, date of diagnosis, dates of nutritional assessment and type
of cancer were recorded. The data were collected in the
Pediatric-Oncology-Network-Database (POND).[12]
This
study has made a comparison with a historical cohort of patients with
comparable characteristics diagnosed from 2004 to 2007 at HIMJR,[8]
to evaluate the impact on event-free-survival (EFS) of the addition of
nutritional support. Their nutritional status has been here
re-classified according to the schema adopted in the current study.
Statistical analysis.
Patients’ characteristics were described using frequency, percentages,
medians and interquartile range (IQR). The 1-year EFS was estimated in
the overall cohort of 104 patients, and a comparison between the cohort
in the study and the historical one was made using Kaplan Meier
survival curves and tested using Andersen pseudo-values regression,
adjusting for type of tumor and nutritional status at first evaluation.
This test was adopted due to the extremely different follow-up duration
of the two cohorts.
P-values were considered statistically
significant if lower than 0.05. Statistical analyses and figure were
done with SAS v9.4, STATA and R.
Results
A
total of 259 children were diagnosed with cancer at HIMJR in the period
of the study; of them, 104 entered the study and received nutritional
supplementation. Nutritional formulas were given free of charge and
were accepted by all families.
Patients’ characteristics at the onset of disease are described in table 1.
Forty-four were female (42.3%), and 60 were male (57.7%); 34 were
affected by acute lymphoblastic leukemia (ALL), 5 by acute myeloid
leukemia, 13 by lymphomas and 52 by solid tumors, including brain
tumors (n=20), retinoblastoma (n=3), bone and soft-tissue sarcoma
(n=15), Wilms’ tumor (n=7) and others (n=7). Diseases were clustered in
two groups -leukemia/lymphomas and solid tumors- for further analyses.
At
the start of the nutritional support, the median age was 7.0 years
(IQR: 3.4-11.5). Almost all patients (n=99) were exclusively orally
supplemented; in only three patients the nutritional formula was
administered via nasogastric tube and in two via gastrostomy. The
nutritional supplementation was started within one month from diagnosis
in 63 cases (60.6%), between 1 and 2 months in 9 patients (8.6%) and
later than 2 months in 32 cases (30.8%). At first work-up (before
supplementation) according to their anthropometric measurements
patients were overall classified as follows: 65.4% severely depleted,
13.5% moderately depleted and 21.1% borderline/adequately nourished
(considered at risk of developing under-nutrition during treatment) (Table 1).
Seventeen
patients died of progressive disease, and 23 did not have a nutritional
follow-up. Thus, nutritional reassessment after supplementation was
performed in 64 patients at a median time from the first assessment of
2.7 months (IQR 2.0-5.4); 29 of them were severely depleted (45.3%), 12
moderately depleted (18.8%) and 23 adequately nourished (35.9%) (Table 1).
Compared with the first evaluation there was a decrease in the
percentage of severely depleted patients in both leukemia/lymphoma
groups (from 63.2 to 36.8%) and the solid tumor group (from 73.1 to
57.7%). Furthermore, in leukemia/lymphoma group, we observed an
increase in the percentage of adequately nourished patients from 28.9%
to 47.4% (Table 2)
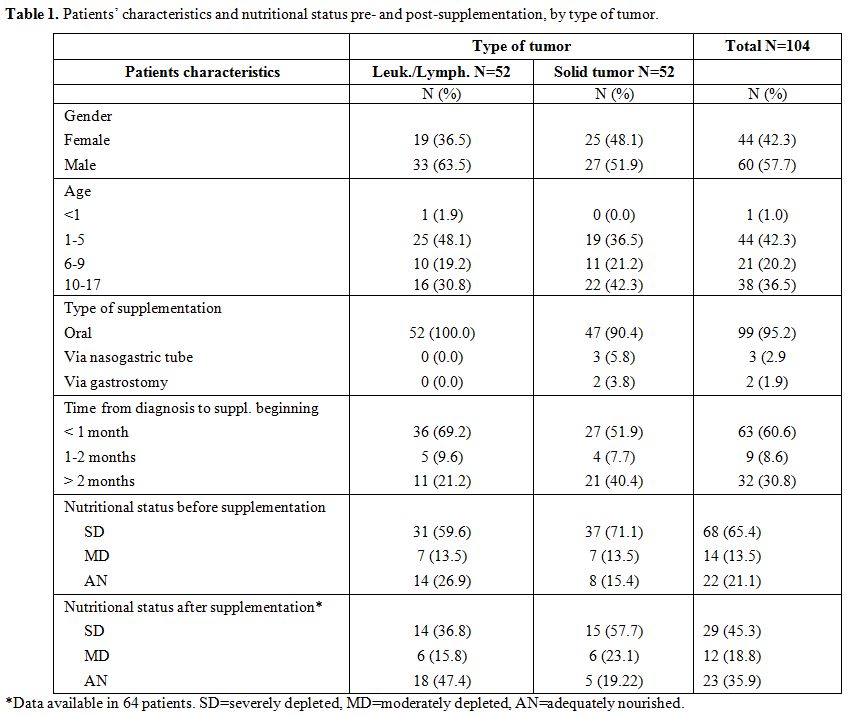 |
Table 1.
Patients’ characteristics and nutritional status pre- and post-supplementation, by type of tumor. |
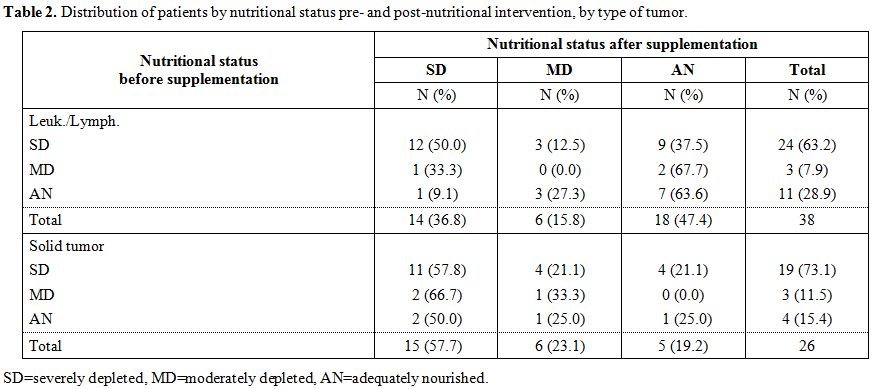 |
Table 2. Distribution of patients by nutritional status pre- and post-nutritional intervention, by type of tumor. |
Overall, 55% of
patients in leukemia/lymphoma group and 35% in the solid tumor group
improved their condition or remained in an adequately nourished status.
There was not a significant statistical difference between the
proportions of patients improved by type of tumor (p-value=0.104).
The
1-year EFS of the overall cohort of patients recruited in this study
was 70.1%, which compares favorably with that of a historical cohort of
patients treated at HIMJR and not supplemented, who had a 1-year EFS of
60% (SE=2.8), p-value=0.022 (Figure 1).
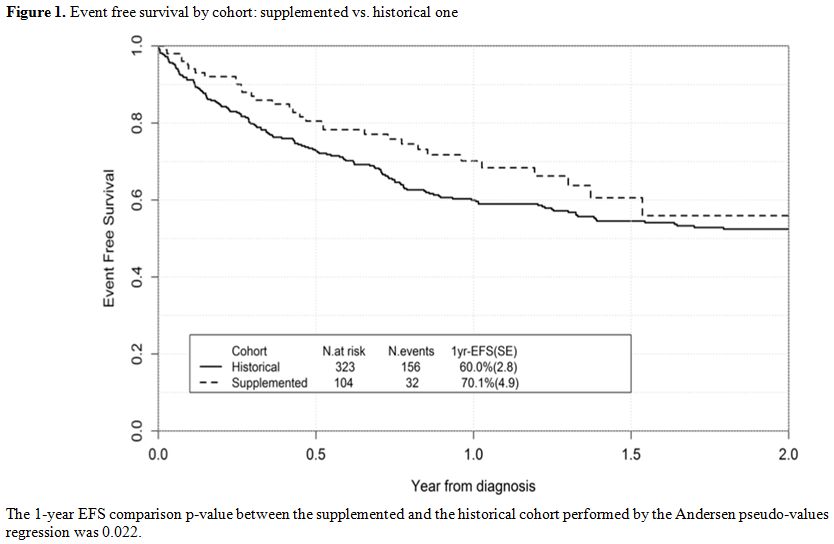 |
Figure 1. Event free survival by cohort: supplemented vs. historical one |
Adjusting
for the type of tumor and nutritional status at diagnosis the
supplemented cohort seemed to have statistically significant 1-year EFS
higher than the historical cohort (p-value=0.013, gain in EFS of about
13%, 95% CI=[2.9%-24.0%]).
Discussion
Malnutrition
remains a critical health issue for pediatric oncology in LMICs, where
the nutritional status of children with cancer has an important effect
on the outcome, being associated with the risk of abandonment and low
tolerance of therapy.[8,9,13] Thus,
children with oncological diseases should be regularly assessed for
nutritional status and supported, as recommended by the SIOP PODC
(Nutrition Working Group of the Society of Pediatric Oncology), that
presented a framework for establishing and monitoring nutritional care,
based on the infrastructure of institutions in LMICs.[14]
A
recent study performed in Guatemala in childhood ALL showed that
establishing an appropriate nutritional program may overcome the
adverse prognostic impact of malnutrition.[15] Data on effects of oral/enteral nutritional support programs in pediatric cancer patients are, however, quite limited.[16]
Thus, there is still a need for further investigations to assess
feasibility, costs and efficacy of nutritional interventions in
children with cancer treated in LIMICs.
Our experience
demonstrates that oral supplementation with polymeric formulas has a
favorable impact on the nutritional status in a relevant number of
cases. Overall, in this study after the supplementation, 55% of
patients in leukemia/lymphoma group and 35% in the solid tumor group
improved their condition or remained in an adequately nourished status
despite the toxicity of treatment. This intervention also appears to
have contributed to improving the 1-year EFS as also reported in the
Guatemala experience.[15]
However, since a
significant fraction of patients with severe malnutrition (23/43) did
not improve their nutritional status, it may be inferred that a more
intensive nutritional support with enteral feeding could have been more
beneficial for these patients. In this study enteral-tube-feeding (ETF)
was administered only in very few cases (5/104), due to fear of
complications, particularly in patients with neutropenia,
thrombocytopenia, and mucositis. These reasons may have played a role
in inducing reluctance in both physicians and families to use ETF. The
use of ETF may however be feasible also in these circumstances as
suggested by a study published in 2001, which showed that tube feeding
was safe and cost-effective in children treated with intensive
chemotherapy.[17] Data in this field remain however extremely limited.[18]
The risk-benefit of the use of ETF should thus be carefully
investigated, in order to define the most appropriate strategies of
nutritional support in these patients, particularly in LMICs where the
need of enteral nutrition but also the risk of complications may be
higher compared to high-income countries.
Another aspect of
interest regards the use of commercial formulas. In our experience,
these formulas, which are expensive and not easily available in LMICs,
were often not well accepted by the patients and their parents, who
would have instead favored homemade preparations. It should thus be
considered the appropriateness of homemade oral supplements as a viable
alternative, as described by a study conducted in Brazil.[19]
Although
the weaknesses related to the small number of patients, the shortage of
personnel, the lack of standardization between first and second
nutritional evaluation and the heterogeneity of disease population,
this study in our opinion provides important information. Our
experience indicates that oral nutritional support in children treated
for cancer in LMICs may be helpful to improve outcome and that homemade
formulas should be considered according to the local contexts. Large
prospective studies should be conducted to establish the best
cost-effective methodologies in LMICs and the role of enteral nutrition
in the patients not responding to oral supplementation.
Malnutrition in children with cancer should not be tolerated as an inevitable process,[3]
also and above all in LMICs, where nutritional interventions with the
aim to prevent or reverse malnutrition should be part of routine
supportive care in the treatment plan for childhood cancer.
.
Acknowledgments
The authors wish to thank “Chiesa Valdese Italiana” and “Comitato Maria Letizia Verga” for their support.
References
- Schoeman J. Nutritional assessment and intervention in a pediatric oncology unit. Indian J Cancer 2015;52:186-90. http://www.indianjcancer.com/text.asp?2015/52/2/186/175832

- Sala, A., Pencharz, P. and Barr, R. D. Children, cancer, and nutrition—A dynamic triangle in review. Cancer. 2004;100: 677–687. http://dx.doi.org/10.1002/cncr.11833

- Jacqueline
Bauer, Heribert Jürgens, Michael C. Frühwald; Important Aspects of
Nutrition in Children with Cancer, Advances in Nutrition, Volume 2,
Issue 2, 1 March 2011, Pages 67–77. https://doi.org/10.3945/an.110.000141

- Rodriguez-Galindo
C, Friedrich P, Alcasabas P, et al. Toward the Cure of All Children
With Cancer Through Collaborative Efforts: Pediatric Oncology As a
Global Challenge. Journal of Clinical Oncology. 2015;33(27):3065-3073. http://ascopubs.org/doi/10.1200/JCO.2014.60.6376

- Barr
RD, Ribeiro RC, Agarwal BR, Masera G, Hesseling PB, Magrath IT.
Pediatric oncology in countries with limited resources. In: Pizzo PA,
Poplack DG, editors. Principles and practice of pediatric oncology, 4th ed. Philadelphia: Lippincott, Williams and Wilkins, 2002:1541–1552.
- UNICEF. The state of the world’s children 2012. https://www.unicef.org/sowc2012/
- Barr,
R. D., Klussmann, F. A., Baez, F., Bonilla, M., Moreno, B., Navarrete,
M., Nieves, R., Peña, A., Conter, V., De Alarcón, P., Howard, S. C.,
Ribeiro, R. C., Rodriguez-Galindo, C., Valsecchi, M. G., Biondi, A.,
Velez, G., Tognoni, G., Cavalli, F. and Masera, G. Asociación de
Hemato-Oncología Pediátrica de Centro América (AHOPCA): A model for
sustainable development in pediatric oncology. Pediatr Blood Cancer.
2014;61: 345–354. http://dx.doi.org/10.1002/pbc.24802

- Sala
A, Rossi E, Antillon F, et al. Nutritional status at diagnosis is
related to clinical outcomes in children and adolescents with cancer: a
perspective from Central America. Eur J Cancer. 2012;48(2):243–252. https://doi.org/10.1016/j.ejca.2011.06.006

- Pribnow
AK, Ortiz R, Báez LF, Mendieta, L, Luna-Fineman S. Effects of
malnutrition on treatment-related morbidity and survival of children
with cancer in Nicaragua. Pediatr Blood Cancer. 2017;64:e26590. http://dx.doi.org/10.1002/pbc.26590

- Howard
SC, Marinoni M, Castillo L, et al. Improving outcomes for children with
cancer in low-income countries in Latin America: A report on the recent
meetings of the Monza International School of Pediatric
Hematology/Oncology (MISPHO)—Part I. Pediatr Blood Cancer
2007;48:364–369. http://dx.doi.org/10.1002/pbc.21003

- https://www.cdc.gov/growthcharts/
- Quintana
Y, Patel AN, Arreola M, et al. POND4Kids: A global web-based database
for pediatric hematology and oncology outcome evaluation and
collaboration. Stud Health Technol Inform, 2013;183:251–256.

- Antillon,
F., Rossi, E., Molina, A. L., Sala, A., Pencharz, P., Valsecchi, M. G.
and Barr R.. Nutritional status of children during treatment for acute
lymphoblastic leukemia in Guatemala. Pediatr Blood Cancer 2013;60:
911–915. http://dx.doi.org/10.1002/pbc.24377

- Ladas,
E. J., Arora, B., Howard, S. C., Rogers, P. C., Mosby, T. T. and Barr,
R. D.. A Framework for Adapted Nutritional Therapy for Children With
Cancer in Low- and Middle-Income Countries: A Report From the SIOP PODC
Nutrition Working Group. Pediatr Blood Cancer 2016;63: 1339–1348. http://dx.doi.org/10.1002/pbc.26016

- Antillón,
F. G., Blanco, J. G., Valverde, P. D., Castellanos, M., Garrido, C. P.,
Girón, V., Letona, T. R., Osorio, E. J., Borrayo, D. A., Mack, R. A.,
Melgar, M. A., Lorenzana, R., Ribeiro, R. C., Metzger, M., Conter, V.,
Rossi, E. and Valsecchi, M. G.. The treatment of childhood acute
lymphoblastic leukemia in Guatemala: Biologic features, treatment
hurdles, and results. Cancer. 2017;123: 436–448. http://dx.doi.org/10.1002/cncr.30257

- Ward
EJ, Henry LM, Friend AJ, Wilkins S, Phillips RS. Nutritional support in
children and young people with cancer undergoing chemotherapy. Cochrane
Database of Systematic Reviews 2015, Issue 8. Art. No.: CD003298. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003298.pub3/abstract

- DeSwarte-Wallace
J, Firouzbakhsh S, Finklestein JZ, Using research to change practice:
enteral feedings for pediatric oncology patients. J Pediatr Oncol Nurs.
2001 Sep-Oct;18(5):217-23. https://doi.org/10.1053/jpon.2001.26875

- Trimpe
K, Shaw MR, Wilson M, Haberman MR. Review of the effectiveness of
enteral feeding in Pediatric oncology patients. J Pediatr Oncol Nurs.
2017 Nov/Dec;34(6):439-445.

- Garófolo,
Adriana, Alves, Fernanda Rodrigues, & Rezende, Maria Aurélia do
Carmo. Suplementos orais artesanais desenvolvidos para pacientes com
câncer: análise descritiva. Revista de Nutrição, 2010;23(4), 523-533. http://dx.doi.org/10.1590/S1415-52732010000400003

[TOP]




















