Tekin Aksu, Ali Fettah, İkbal
Ok Bozkaya, Mehmet Baştemur, Abdurrahman Kara, Vildan Koşan Çulha,
Namık Yaşar Özbek and Neşe Yaralı.
Pediatric
Hematology and Oncology, University of Health Sciences, Ankara Child
Health and Diseases Hematology Oncology Training and Research Hospital,
Ankara, Turkey.
Corresponding
author: Tekin Aksu, Şehit Ömer Halisdemir Cad. Kurtdereli Sok. Altındağ
/ ANKARA. Tel: 00903125969674, Fax: 00903123472330. E-mail:
tekinaksu@gmail.com
Published: July 1, 2018
Received: January 20, 2018
Accepted: June 21, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018045 DOI
10.4084/MJHID.2018.045
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and objectives:
Acute promyelocytic leukemia (APL), is a distinct subtype of acute
myeloid leukemia (AML) characterized by a tendency to hemorrhage and
excellent response to all-trans retinoic acid (ATRA). In this
retrospective study, we aimed to determine the incidence, clinical
symptoms, toxicities, and outcome of children with APL in our
center. Methods:
We retrospectively reviewed the medical records of children (age <
18 years) diagnosed with APL in our pediatric hematology department
between January 2006-December 2016.
Results:
Pediatric APL represents 20.5% of AML cases in this cohort. Most of the
cases presented as classical M3, albeit hypogranular variant was
described in 12% of the cohort. Patients with hypogranular variant APL
were differed from classical APL by co-expression of CD2 and CD34.
About ¾ of APL patients had hemorrhagic findings at admission or the
induction treatment. Severe bleeding manifested as intracranial
hemorrhage was present in three patients and intracranial arterial
thrombosis was present in one. Six patients showed side effects of ATRA
such as pseudotumor cerebri, differentiation syndrome resulting in
dilated cardiomyopathy, and pulmonary infiltrates. Five-year overall
survival (OS) and early death rate were found to be 82.5% and 12%
respectively.
Conclusions:
A high frequency (20.5%) of APL was noted among children with AML in
this single-center study. The overall mortality rate was 17.5%. Since
the induction death rate was 12% and life-threatening bleeding was the
primary problem, awareness and urgent treatment are critical factors to
reduce early losses.
|
Introduction
Acute
promyelocytic leukemia (APL) is a distinct subtype of acute myeloid
leukemia (AML) which is classified as M3 by French-American-British
(FAB) Cooperative group.[1] The incidence of APL among the AML cases in
children and adolescents vary from 2% in Switzerland to >50% in
Nicaragua.[2] However, APL incidence among eastern Mediterranean
countries is not well documented. A multicenter study from Lebanon
reported 25% APL cases among AML patients.[3] In Turkey, a study
disclosed an incidence of APL as 8.8% among 34 AML patients at
childhood.[4]
Acute promyelocytic leukemia is characterized by the
presence of reciprocal translocation between chromosomes 15 and 17
[t(15;17); promyelocytic leukemia gene (PML) - retinoic acid receptor
gene alpha (RARA) fusion].[5,6] In addition to PML, rare partner genes
such as nucleophosmin (NPM1; 5q35), nuclear mitotic apparatus protein 1
(NUMA1; 11q13), promyelocytic leukemia zinc finger (PLZF; 11q23), and
signal transducer and activator of transcription (STAT) 5β (STAT5b;
17q21) have been defined.[7] PML-RARA fusion protein impairs
differentiation of the myeloid progenitor cells and leads to arrested
maturation at the promyelocytic stage. By binding to the PML-RARA
fusion protein, ATRA induces differentiation of leukemic cells into
mature granulocytes and ultimately apoptosis.[8,9] Coagulopathy and
signs of clinical hemorrhage or thrombotic complications and an
excellent response to all-trans retinoic acid (ATRA) are distinctive
features of APL.[10] Anthracycline-based chemotherapy and ATRA
combination are curative for at least 80% of newly diagnosed APL
patients.[10,11,12] Arsenic trioxide (ATO) initially was introduced
into the treatment of relapsed APL. Subsequently, it was used as
first-line APL therapy, which can achieve remission rates of
86%.[13,14,15]
Here, we report clinical, and laboratory
findings, toxicities of ATRA treatment and outcome of APL patients,
followed in our department.
Materials and Methods
We
retrospectively reviewed the medical records of children (age < 18
years) diagnosed with AML in our pediatric hematology department
between January 2006- December 2016. Demographic, clinical, and
laboratory data (hematological and biochemical findings; bone marrow
morphology, immune phenotype, chromosomal and cytogenetic analysis;
radiologic and echocardiographic findings), chemotherapy protocols,
toxicities and the prognosis of the children were recorded for all APL
patients. Morphologic diagnosis of APL was based on FAB criteria.[1]
Leukemic cells were analyzed by flow cytometry, and the diagnosis was
confirmed by the presence of t(15;17) with fluorescence in situ
hybridization (FISH) analysis. Patients were treated according to
APL-93 trial, GIMEMA-AIEOP AIDA between 2006-2010 and AML-BFM Interim
2004 therapy protocol between 2011-2016.[11,16,17] ATRA courses were
used in all protocols with a dose of 25 to 45 mg/m2/d
from the induction to during with maintenance treatment. Maintenance
treatment was planned for 1 to 2 years in AML-BFM 2004, APL-93 and AIDA
protocols. However, none of the regimens included ATO. Complete
remission was defined according to the report of the National Cancer
Institute workshop criteria.[18] Cytogenetic remission using FISH
analysis was defined as the disappearance of the t(15;17). Early death
was defined as the death of any cause within 30 days of admission.[19]
The overall survival (OS) was calculated from the date of diagnosis to
death of any cause or last follow-up. ATRA related adverse effects such
as fever, weight gain, dyspnea, interstitial pulmonary infiltrate,
hypotension, renal insufficiency, and hyperbilirubinemia was also
recorded as differentiation syndrome (DS) which was defined according
to Frankel et al.[20]
Statistical analysis.
Statistical analysis was performed by using Statistical Package for the
Social Sciences for Windows (SPSS) version 18.0 (SPSS Inc., South
Wacker Drive, Chicago, IL, USA). The variables were investigated using
visual and analytical methods (Kolmogorov- Smirnov/Shapiro-Wilk's test)
to determine whether or not they are normally distributed. Descriptive
analyses were presented using means and standard deviations for
normally distributed and median and minimum-maximum for
non-normally distributed variables. The overall survivals of APL
patients were calculated using the Kaplan–Meier methods and the
log-rank test.
Results
Between
January 2006 - December 2016, 83 children diagnosed with AML at our
center. Among them, 17 patients (20.5%) with newly diagnosed APL were
included in the study. Eight girls and nine boys [median age 13.5 years
(range 1.5-17)] were included in the study. Pretreatment laboratory
findings and detailed characteristics of the patients are reported in Table 1 and Table 2.
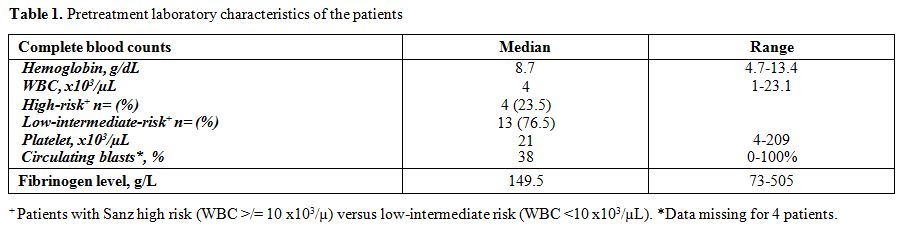 |
Table 1.
Pretreatment laboratory characteristics of the patients |
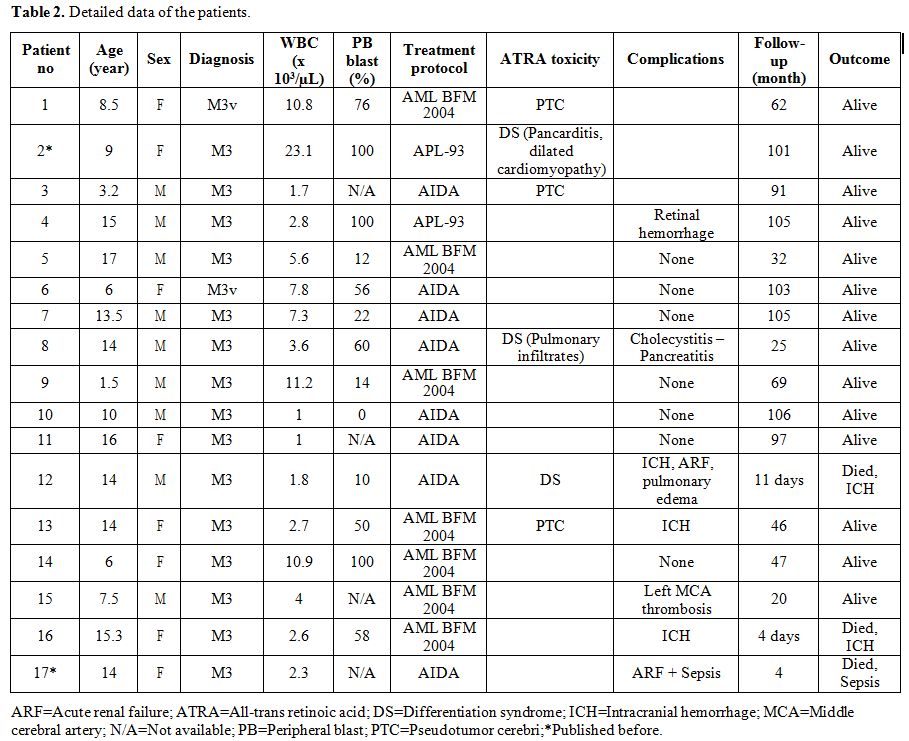 |
Table 2. Detailed data of the patients. |
Bleeding
(76.5%), fever (58.8%), and fatigue (47%) were the most common
presenting signs and symptoms. Bone pain and the headache were seen in
four and three patients. Bleeding was cutaneous in 6, and mucosal
(e.g., wet purpura, epistaxis, and gingival bleeding) in 7 patients.
Furthermore, hematuria, hemoptysis, and retinal hemorrhage were
presented, each in one patient. Intracranial hemorrhage (ICH) was
demonstrated in three patients; one of them at admission and two others
were during 11th and 23rd days of induction treatment. The patient who had ICH demonstrated 23rd
days of induction had coagulopathy at admission that recovered with
ATRA treatment. Unfortunately, subacute/chronic subdural hematoma with
midline shift was revealed while she had a neutropenic fever period
with thrombocytopenia. One patient who initially diagnosed with left
middle cerebral artery thrombosis, diagnosed with APL on the 5th day of
admission. Organomegaly was present in seven (41%) patients, including
splenomegaly in five and hepatomegaly in five patients.
Additionally, lymphadenopathy or central nervous involvement detected
in one patient each.
Median hemoglobin, white blood cell (WBC) and platelet counts were summarized in Table 1.
Patients reclassified with Sanz high-risk (WBC ≥ 10 x103/µL) versus low-intermediate-risk (WBC <10 x103/µL) in Table 1.[21]
Peripheral blasts were presented in 16 (94%) of 17 patients at
admission. Fourteen patients (82%) had coagulopathy (increased PT,
aPTT, and decreased fibrinogen levels). D-dimer levels were elevated in
the 11 of the 15 patient. Fifteen patients (88%) had classical FAB M3
type blasts at bone marrow morphology; two
patients (12%) had M3v blasts which was
estimated by morphology and then confirmed by flow cytometric findings.
Blasts of 15 patients
who had classical hypergranular APL were positive
for CD117, CD13, CD33 markers. Out of 15, three patients’ blasts were
positive for CD34 and/or HLA DR. But, flow cytometric analysis of 2
patients with M3v APL differed from classical APL by co-expression of
CD2 and CD34 in addition to CD117, CD13. Meanwhile, CD2 expression was
present at a low level (24%) in only one patient with classical M3. All
of the patients had t(15;17) by FISH analysis, and three patients (18%)
had hypodiploid karyotype as well.
Fifteen patients (88%) achieved complete
remission. Mean morphologic remission and complete
cytogenetic remission intervals were 30.4 ± 9.1 days (15-45 days) and
51.7 ± 19.6 days (26-98 days), respectively. There were no relapses
during the entire follow-up period through June 2017 (follow-up range:
10-106 months). Two patients died at the induction before hematological
response achieved. The induction death rate and the overall mortality
were 12% and 17.5%, respectively. One of them, a 15-year old girl who
admitted in a coma with massive ICH. Her history revealed that she had
been followed 72 hours in a local hospital before diagnosis.
Unfortunately, despite ATRA and supportive treatment, she died at day 4
of admission to our hospital. We suggested that delay in the ATRA
treatment that caused ICH was the main cause of death. Another patient,
a 14-year old boy died due to acute renal failure, pulmonary edema, and
ICH at day 11 of induction treatment. Though DS was suggested, sepsis
and DIC were the additional causative factors that ultimately caused
death. Additionally, a 14-year-old girl died due to sepsis four months
after the diagnosis. After excluding these three patients, median
follow up period of the patients was 69 months (range 10 – 106).
Estimated 5-year overall survival rate was 82.5 ± 9.1 (95 CI: 64.7 –
100.4).
Several complications were detected during APL treatment (Table 2).
Three patients (18%) developed pseudotumor cerebri (PTC); one of them
diagnosed at the fifth month, at the early phase of maintenance
therapy. She treated with topiramate and repeated lumbar punctures. The
second patient developed PTC 10 months after APL diagnosis while
receiving maintenance treatment. He was treated with acetazolamide and
serial lumbar punctures. The last patient developed PTC 45 days after
diagnosis of APL and treated with acetazolamide, serial lumbar
punctures, and dexamethasone. A 14-y-old boy developed pulmonary
infiltrates, tinnitus and hypotension on the sixth day of induction
treatment, diagnosed with DS, responded to dexamethasone. Additionally,
he suffered from cholecystitis and pancreatitis at the second month of
APL treatment. A previously described 9-year old girl from our
department who developed endocarditis and myocarditis at the induction
of the APL treatment, recovered after cessation of ATRA who has been
reported elsewhere.[22] However, readministration of
ATRA at the maintenance therapy caused pancarditis and severe pulmonary
edema that might have been part of DS, which recovered with
corticosteroids treatment and discontinuation of ATRA. Unfortunately,
she developed dilated cardiomyopathy and still ongoing with digitalis
treatment. The clinical picture strongly suggested the ATRA treatment
as the causative factor even if anthracyclines were an additional risk
factor. Febrile neutropenia has been observed during induction
treatment in 15 patients (88%), including septicemia and
typhilitis. Median febrile neutropenia attack rate was 3.5 (range 1-7)
during the treatment period.
Discussion
Pediatric
APL represents 20.5% of AML cases in our cohort. Even if our center is
a reference hospital in Ankara, this high incidence of APL needs to be
confirmed in larger pediatric series among Turkey. Early diagnosis and
immediate treatment with ATRA may reduce hemorrhagic complications that
lead to early morbidity and mortality, and significant concern is
discrimination of APL from other subtypes of AML. In our patients whose
presenting, symptoms are bleeding and/or coagulopathy, expeditious
immunophenotypic analysis to exclude M3 or M3v is performed. We started
ATRA as soon as possible, although two patients experienced ICH after
the first week of ATRA. Unfortunately, delays in diagnosis contributed
to mortality in one of the patient. However, favorable response to ATRA
has been achieved in the rest of the patients. Morphology and
immunophenotypic analysis are still essential tools for rapid
recognition of APL. Most of our APL cases presented as hypergranular or
classical M3, albeit morphological hypogranular or microgranular
variant type, M3v, was also described in 2 patients (12%). Hypogranular
variant type accounts for 15-20% of APL cases which is characterized by
promyelocytes with bilobed-multilobed or angel wing shaped nucleus look
as if monoblastic leukemia.[8,23] On
both occasions, identification of the cytogenetic abnormality, t(15;17)
or PML/RARA translocation has utmost importance. M3v morphology is not
diagnostic; however, co- expression of CD2 and CD34 markers are
remarkable and useful for early diagnosis.[24,25] The
absence of HLA-DR, low expression or absence of CD34, and positivity
for CD13 and/or CD33 markers has been reported on both forms.[24,25]
In our cohort, fifteen patients (88%) expressed CD117, CD13, CD33
markers. They did not express CD34 and/or HLA-DR except for three cases
(17.5%) who diagnosed with APL ultimately. Two patients with
hypogranular variant were differed from classical APL by co-expression
of CD2 and CD34 (100%) in this study.
APL cases were frequently presented with consumptive coagulopathy that may cause life- threatening hemorrhages.[26] Furthermore, thrombotic complications may also be seen infrequently.[26]
About ¾ of our APL patients had hemorrhagic findings at admission or
induction treatment. Severe bleeding manifested as intracranial
hemorrhage was present in three patients. One of them admitted with
severe ICH, but we demonstrated ICH in two patients after the first
week of ATRA treatment. The other patient who had bleeding on day 11 of
induction, had been diagnosed with sepsis and DIC, and also possible
DS. Patients with ICH has been supported with aggressive platelet and
fibrinogen replacement along with ATRA therapy guided by numerous
coagulation studies. ATRA has dramatically enhanced survival rates and
diminished relapse rates in APL patients. In the present study,
five-year overall survival (OS) and early death rate were found to be
82.5% and 12%, respectively. ATRA resistance and relapse were not
observed in any patient. Our results were comparable to those obtained
in population-based studies and also to early death rates for APL.[27,28] Nevertheless, Abla et al.[19] reported the incidence of early death as 4.7%, recently.
High
WBC, high peripheral blast count, M3v and black ethnicity were
independent predictors of early hemorrhagic death in several studies.[19,29] However, our patients who died due to early ICH had low WBC counts (1.8 and 2.6 x103/µL), and their peripheral blast percentages were also low (10 and 58%, Table 2).
Hypogranular APL patients of our cohort did not have severe hemorrhagic
complications. The patients who relieved from early hemorrhagic
complications have an excellent OS after ATRA era, as is our patients.
In
our study, mean morphologic and cytogenetic remission by FISH analysis
has been obtained at days 30.4 (15-45 days) and 51.7 (26-98 days),
respectively. One may speculate that mean cytogenetic remission times
were early because the FISH analysis is not sensitive to polymerase
chain reaction (PCR) based methods to detect PML/RARA. We were not able
to analyze PML/RARA translocation during treatment for all patients.
Zhou et al.[14] reported that PML/RARA disappeared within 3 to 9 months after complete hematological response using PCR.
Although
excellent remission rates, different from other AML types, might be
attributable to ATRA, six patients in this study have experienced
severe side effects such as PTC, pancarditis, and pulmonary
infiltrates. Two patients suffered from DS while they were receiving
AIDA protocol, but no DS was seen with AML BFM 2004 protocol.
Otherwise, there was no difference in toxicity (e.g., heart) and
efficacy between these protocols in this study. Pseudotumor cerebri
incidence was reported to be 1.7 - 16% in patients on ATRA therapy.[30,31]
In our study, PTC incidence was 17.6%, but clear definitions and
incidence of this complication were not established. Botton et al.[31] recommended lower ATRA (25mg/m2) doses to avoid from PTC. In contrast to that study, our patients were receiving low dose ATRA (25mg/m2) courses when they developed PTC.
Conclusions
A
high frequency (20.5%) of APL was noted among children with AML in this
single-center study. The overall mortality rate was 17.5%. Since the
induction death rate was 12% and life-threatening bleeding was the
primary problem, awareness and urgent treatment are critical factors to
reduce early losses.
References
- Bennett JM, Catovsky D, Daniel MT, Flandrin G,
Galton DA, Gralnick HR, Sultan C. Proposed revised criteria for the
classification of acute myeloid leukemia. A report of the
French-American-British Cooperative Group. Ann Intern Med. 1985;
103:620-5. https://doi.org/10.7326/0003-4819-103-4-620 PMid:3862359

- Zhang
L, Samad A, Pombo-de-Oliveira MS, Scelo G, Smith MT, Feusner J, Wiemels
JL, Metayer C. Global characteristics of childhood acute promyelocytic
leukemia. Blood Rev. 2015;29:101-25. https://doi.org/10.1016/j.blre.2014.09.013 PMid:25445717 PMCid:PMC4379131

- Farah
RA, Horkos JG, Bustros YD, Farhat HZ, Abla O. A Multicenter Experience
from Lebanon in Childhood and Adolescent Acute Myeloid Leukemia: High
rate of Early Death in Childhood Acute Promyelocytic Leukemia. Mediterr
J Hematol Infect Dis. 2015;7(1):e2015012. https://doi.org/10.4084/mjhid.2015.012 PMid:25574371 PMCid:PMC4283923

- Kömür
M, Erbey F, Bayram I, Tanyeli A. Incidence and prognostic importance of
molecular genetic defects in children with acute myeloblastic leukemia.
Asian Pac J Cancer Prev. 2010;11:1393-5. PMid:21198299

- Longo
L, Pandolfi PP, Biondi A, Rambaldi A, Mencarelli A, Lo Coco F, Diverio
D, Pegoraro L, Avanzi G, Tabilio A, Zangrilli D, Alcalay M, Donti E,
Grignani F, Pelicci PG. Rearrangements and aberrant expression of the
retinoic acid receptor alpha gene in acute promyelocytic leukemias. J
Exp Med. 1990;172:1571-5. https://doi.org/10.1084/jem.172.6.1571 PMid:2175343

- Lo-Coco
F, Ammatuna E, Montesinos P, Sanz MA. Acute promyelocytic leukemia:
recent advances in diagnosis and management. Semin Oncol.
2008;35:401-9. https://doi.org/10.1053/j.seminoncol.2008.04.010 PMid:18692690

- Yan
W, Zhang G. Molecular Characteristics and Clinical Significance of 12
Fusion Genes in Acute Promyelocytic Leukemia: A Systematic
Review. Acta
Haematol. 2016;136:1-15. https://doi.org/10.1159/000444514 PMid:27089249

- Calleja
EM, Warrell RP Jr. Differentiating agents in pediatric malignancies:
all-trans-retinoic acid and arsenic in acute promyelocytic leukemia.
Curr Oncol Rep. 2000; 2(6):519-23. https://doi.org/10.1007/s11912-000-0105-x

- Huang
ME, Ye YC, Chen SR, Chai JR, Lu JX, Zhoa L, Gu LJ, Wanq ZY. Use of
all-trans retinoic acid in the treatment of acute promyelocytic
leukemia. Blood 1988;72:567-72. PMid:3165295

- Abla O, Ribeiro RC. How I treat children and adolescents with acute promyelocytic leukaemia. Br J Haematol. 2014;164:24-38. https://doi.org/10.1111/bjh.12584 PMid:24117210 PMCid:PMC5127390

- Testi
AM, Biondi A, Lo Coco F, Moleti ML, Giona F, Vignetti M, Menna G,
Locatelli F, Pession A, Barisone E, De Rossi G, Diverio D, Micalizzi C,
Aricò M, Basso G, Foa R, Mandelli F. GIMEMA- AIEOPAIDA protocol for the
treatment of newly diagnosed acute promyelocytic leukemia (APL) in
children. Blood. 2005;106:447-53. https://doi.org/10.1182/blood-2004-05-1971 PMid:15677559

- Creutzig
U, Zimmermann M, Dworzak M, Urban C, Henze G, Kremens B, Lakomek M,
Bourquin JP, Stary J, Reinhardt D. Favourable outcome of patients with
childhood acute promyelocytic leukaemia after treatment with reduced
cumulative anthracycline
doses. Br J Haematol. 2010;149(3):399-409. https://doi.org/10.1111/j.1365-2141.2010.08107.x PMid:20230404

- Mathews
V, George B, Lakshmi KM, Viswabandya A, Bajel A, Balasubramanian P,
Shaji RV, Srivastava VM, Chandy M. Single- agent arsenic trioxide in
the treatment of newly diagnosed acute promyelocytic leukemia: durable
remissions with minimal toxicity. Blood. 2006;107:2627-32. https://doi.org/10.1182/blood-2005-08- 3532 PMid:16352810

- Zhou
J, Zhang Y, Li J, Li X, Hou J, Zhao Y, Liu X, Han X, Hu L, Wang S, Zhao
Y, Zhang Y, Fan S, Lv C, Li L, Zhu L. Single-agent arsenic trioxide in
the treatment of children with newly diagnosed acute promyelocytic
leukemia. Blood. 2010;115:1697-702. https://doi.org/10.1182/blood-2009-07-230805 PMid:20029047

- Kutny
MA, Alonzo TA, Gerbing RB, Wang YC, Raimondi SC, Hirsch BA, Fu CH,
Meshinchi S, Gamis AS, Feusner JH, Gregory JJ Jr. Arsenic Trioxide
Consolidation Allows Anthracycline Dose Reduction for Pediatric
Patients With Acute Promyelocytic Leukemia: Report From the Children's
Oncology Group Phase III Historically Controlled Trial AAML0631. J Clin
Oncol. 2017;35(26):3021-3029. https://doi.org/10.1200/JCO.2016.71.6183 PMid:28767288

- Kelaidi
C, Chevret S, De Botton S, Raffoux E, Guerci A, Thomas X, Pigneux A,
Lamy T, Rigal-Huguet F, Meyer-Monard S, Chevallier P, Maloisel F,
Deconinck E, Ferrant A, Fegueux N, Ifrah N, Sanz M, Dombret H, Fenaux
P, Adès L. Improved outcome of acute promyelocytic leukemia with high
WBC counts over the last 15 years: the European APL Group experience. J
Clin Oncol. 2009;27:2668-76. https://doi.org/10.1200/JCO.2008.18.4119 PMid:19414681

- Creutzig
U, Zimmermann M, Bourquin JP, Dworzak MN, Fleischhack G, Graf N,
Klingebiel T, Kremens B, Lehrnbecher T, von Neuhoff C, Ritter J, Sander
A, Schrauder A, von Stackelberg A, Starý J, Reinhardt D.
Randomized trial comparing liposomal daunorubicin with idarubicin as
induction for pediatric acute myeloid leukemia: results from Study
AML-BFM 2004. Blood. 2013;122:37-43. https://doi.org/10.1182/blood-2013-02-484097 PMid:23704089

- Cheson
BD, Cassileth PA, Head DR, Schiffer CA, Bennett JM, Bloomfield CD,
Brunning R, Gale RP, Grever MR, Keating MJ. Report of the National
Cancer Institute-sponsored workshop on definitions of diagnosis and
response in acute myeloid leukemia. J Clin Oncol. 1990;8:813-9. https://doi.org/10.1200/JCO.1990.8.5.813 PMid:2185339

- Abla
O, Ribeiro RC, Testi AM, Montesinos P, Creutzig U, Sung L, Di Giuseppe
G, Stephens D, Feusner JH, Powell BL, Hasle H, Kaspers GJL, Dalla-Pozza
L, Lassaletta A, Tallman MS, Locatelli F, Reinhardt D, Lo-Coco F,
Hitzler J, Sanz MA. Predictors of thrombohemorrhagic early death in
children and adolescents with t(15;17)-positive acute promyelocytic
leukemia treated with ATRA and chemotherapy. Ann Hematol.
2017;96(9):1449-1456. https://doi.org/10.1007/s00277-017- 3042-6 PMid:28597167

- Frankel
SR, Eardley A, Lauwers G, Weiss M, Warrell RP Jr. The "retinoic acid
syndrome" in acute promyelocytic leukemia. Ann Intern Med.
1992;117:292-6. https://doi.org/10.7326/0003-4819-117-4-292 PMid:1637024

- Sanz
MA, Lo Coco F, Martín G, Avvisati G, Rayón C, Barbui T, Díaz-Mediavilla
J, Fioritoni G, González JD, Liso V, Esteve J, Ferrara F, Bolufer P,
Bernasconi C, Gonzalez M, Rodeghiero F, Colomer D, Petti MC, Ribera JM,
Mandelli F. Definition of relapse risk and role of nonanthracycline
drugs for consolidation in patients with acute promyelocytic leukemia:
a joint study of the PETHEMA and GIMEMA cooperative groups. Blood.
2000;96(4):1247-53 PMid:10942364

- Işık
P, Çetin I, Tavil B, Azik F, Kara A, Yarali N, Tunc B. All-
transretinoic acid (ATRA) treatment-related pancarditis and severe
pulmonary edema in a child with acute promyelocytic leukemia. J
Pediatr Hematol
Oncol. 2010;32(8):e346-8. https://doi.org/10.1097/MPH.0b013e3181e75731 PMid:20881874

- Sainty
D, Liso V, Cantù-Rajnoldi A, Head D, Mozziconacci MJ, Arnoulet C,
Benattar L, Fenu S, Mancini M, Duchayne E, Mahon FX, Gutierrez N, Birg
F, Biondi A, Grimwade D, Lafage-Pochitaloff M, Hagemeijer A, Flandrin
G; Groupe Français d'Hématologie Cellulaire; Groupe Français de
Cytogénétique Hématologique; UK Cancer Cytogenetics Group; BIOMED 1
European Community-Concerted Action "Molecular Cytogenetic Diagnosis in
Haematological Malignancies". A new morphologic classification system
for acute promyelocytic leukemia distinguishes cases with underlying
PLZF/RARA gene rearrangements. Blood. 2000;96:1287-96. PMid:10942370

- Gorczyca
W. Acute promyelocytic leukemia: four distinct patterns by flow
cytometry immunophenotyping. Pol J Pathol. 2012;63:8-17. PMid:22535601

- Albano
F, Mestice A, Pannunzio A, Lanza F, Martino B, Pastore D, Ferrara F,
Carluccio P, Nobile F, Castoldi G, Liso V, Specchia G. The biological
characteristics of CD34+ CD2+ adult acute promyelocytic leukemia and
the CD34 CD2 hypergranular (M3) and microgranular (M3v) phenotypes.
Haematologica. 2006;91:311-6. PMid:16531253

- Cicconi L, Lo-Coco F. Current management of newly diagnosed acute promyelocytic leukemia. Ann Oncol. 2016;27:1474-81. https://doi.org/10.1093/annonc/mdw171 PMid:27084953

- Stein EM, Tallman MS. Acute promyelocytic leukemia in children and adolescents. Acta Haematol. 2014;132:307-12. https://doi.org/10.1159/000365117 PMid:25228556

- Takahashi
H, Watanabe T, Kinoshita A, Yuza Y, Moritake H, Terui K, Iwamoto S,
Nakayama H, Shimada A, Kudo K, Taki T, Yabe M, Matsushita H, Yamashita
Y, Koike K, Ogawa A, Kosaka Y, Tomizawa D, Taga T, Saito AM, Horibe K,
Nakahata T, Miyachi H, Tawa A, Adachi S. High event-free survival rate
with minimum-dose- anthracycline treatment in childhood acute
promyelocytic leukaemia: a nationwide prospective study by the Japanese
Paediatric Leukaemia/Lymphoma Study Group. Br J Haematol. 2016;174:437-
43. https://doi.org/10.1111/bjh.14068 PMid:27029412

- Mantha
S, Goldman DA, Devlin SM, Lee JW, Zannino D, Collins M, Douer D, Iland
HJ, Litzow MR, Stein EM, Appelbaum FR, Larson RA, Stone R, Powell BL,
Geyer S, Laumann K, Rowe JM, Erba H, Coutre S, Othus M, Park JH,
Wiernik PH, Tallman MS. Determinants Of fatal bleeding during induction
therapy for acute promyelocytic leukemia in the ATRA era. Blood.
2017;129:1763-1767. https://doi.org/10.1182/blood-2016-10-747170 PMid:28082441 PMCid:PMC5374291

- Coombs
CC, DeAngelis LM, Feusner JH, Rowe JM, Tallman MS. Pseudotumor Cerebri
in Acute Promyelocytic Leukemia Patients on Intergroup Protocol 0129:
Clinical Description and Recommendations for New Diagnostic Criteria.
Clin Lymphoma Myeloma Leuk. 2016;16:146-51. https://doi.org/10.1016/j.clml.2015.11.018 PMid:26724834 PMCid:PMC5028896

- de
Botton S, Coiteux V, Chevret S, Rayon C, Vilmer E, Sanz M, de La
Serna J, Philippe N, Baruchel A, Leverger G, Robert A, San Miguel J,
Conde E, Sotto JJ, Bordessoule D, Fegueux N, Fey M, Parry A, Chomienne
C, Degos L, Fenaux P. Outcome of childhood acute promyelocytic leukemia
with all-trans-retinoic acid and chemotherapy. J Clin Oncol.
2004;22:1404-12. https://doi.org/10.1200/JCO.2004.09.008 PMid:15084614

[TOP]


































