Alfredo Molteni1, Emanuele Ravano2, Marta Riva2, Michele Nichelatti3, Laura Bandiera4, Lara Crucitti2, Mauro Truini4 and Roberto Cairoli2.
1 Hematology, ASST Cremona, Cremona, Italy.
2 Hematology, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy.
3 Statistician - Centro Coordinamento Ricerche Cliniche, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy.
4 Anatomic Pathology, ASST Grande Ospedale Metropolitano Niguarda, Milan, Italy..
Correspondence to: Alfredo Molteni, MD. ASST Cremona, Cremona, Italy.
Viale Concordia 1, 26100, Cremona, Italy. Tel. +39 0372408105. E-mail:
alfre13667@gmail.com
Published: March 1, 2019
Received: August 20, 2018
Accepted: January 24, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019015 DOI
10.4084/MJHID.2019.015
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and objectives: Mutations of the TP53 gene have an unfavorable prognosis in Myelodysplastic Syndromes (MDS). The product of the TP53 gene is the p53 protein. Most of the TP53
mutations entail the accumulation of the protein in the nucleus of
tumor cells. The immunohistochemical (IHC) staining for p53 can be a
surrogate suggesting a mutational status and, if overexpressed, seems
to be of prognostic value by itself. The best prognostic cut-off value
of overexpression is controversial. The aim of this pilot study is to
investigate the correct value from a homogenous group of patients with
higher IPSS-R risk MDS.
Methods:
In sixty consecutive patients diagnosed with MDS and categorized as
“intermediate,” “high” and “very high” IPSS-risk, the bone marrow
biopsies performed at diagnosis were retrospectively re-examined for
IHC p53 expression. The result of p53 expression was subsequently
related to survival.
Results:
A worse overall survival was observed both in patients whose IHC p53
expression was ≥5% and ≥ 10% compared to patients with a p53 expression
below 5% (p= 0.0063) or 10% (p=0.0038) respectively.
Conclusions:
The ICH p53 expression in bone marrow biopsy in higher risk MDS was
confirmed to have prognostic value. These results indicate more than
10% expression as the best cut off value.
|
Introduction
Myelodysplastic
syndromes (MDS) are a heterogeneous group of diseases characterized by
ineffective hematopoiesis and risk of acute myeloid leukemia
progression. The prognosis in terms of overall survival (OS) and risk
of progression is now estimated by the Revised International Prognostic
Scoring System (IPSS-R),[1] developed from the previous historical system called IPSS,[2]
based on the following features: cytopenias, the percentage of bone
marrow blasts and cytogenetic aberrations. According to IPSS-R, five
prognostic categories can be distinguished with a median OS from 5.4
years (very low risk) to 0.7 years (very high risk). The biological
impact on progression and survival was recently further pointed out by
studies on recurrent gene mutation in MDS. For example, the presence of
at least one among ASXL1, RUNX1, TP53, EZH2 or ETV6 somatic point
mutations was shown to be enough to worsen the IPSS prognostic
category.[3] On the other hand, mutations of SF3B1 (a
gene encoding a core component of the RNA splicing machinery) have been
associated with a favorable prognostic impact in MDS with ring
sideroblasts.[4]
The TP53 is a gene mapped of
the locus p13.1, on chromosome 17. It is mutated in 5-10% of cases of
de novo MDS5 and about 30% of therapy-related neoplasms. In general,
this mutation is mainly observed in high-risk MDS,[6]
but it is particularly frequent both in patients with isolated Del(5q)
and those with complex karyotype associated with -5/5q-.
Its
presence is linked with an unfavorable prognosis and with a reduced OS,
regardless of prognostic or cytogenetic category. It has been shown
that the cases of complex karyotype without TP53 mutations have better
survival compared to those with mutations, at least in the transplant
setting.[7] In addition, the presence of TP53
mutations also increases the risk of progression to leukemia in MDS
patients with isolated Del(5q) and leads to poor survival in patients
with normal karyotype. [3,8,9]
The
product of the TP53 gene is the p53 protein, a tumor suppressor factor.
When p53 is activated, it has multiple antineoplastic functions in a
relationship with several transducers, including cellular growth
arrest, apoptosis, DNA repairs, and angiogenesis. Most of the TP53
mutations lead to the stabilization and the accumulation of the protein
in the nucleus of the tumor cells.[10] So, the
immunohistochemical (IHC) staining for p53 could be a surrogate
suggesting a mutation status. In other cases, a non-sense mutation may
result in a truncated, and unstable protein or the injury of both
alleles can lead to a complete loss of p53 production, with the absence
of staining.[11] As a matter of fact, TP53 mutations are associated with the overexpression of p53 protein in 75% of cases.[5]
The p53 overexpression has never been observed in cases of wild type
TP53, and that was also confirmed by another study that reported 60%
sensitivity and 100% specificity of IHC for p53 overexpression in
detecting TP53 mutations.[12]
The best way to
detect p53 mutations is certainly by molecular biology techniques.
Otherwise, considering the low diffusion and the high cost of these
procedures, the evaluation of the expression of the p53 protein as an
alternative method may be considered helpful.
The IHC p53
overexpression has been evaluated as a prognostic factor in itself and
considered as a low cost, easy diagnostic tool for assessing the
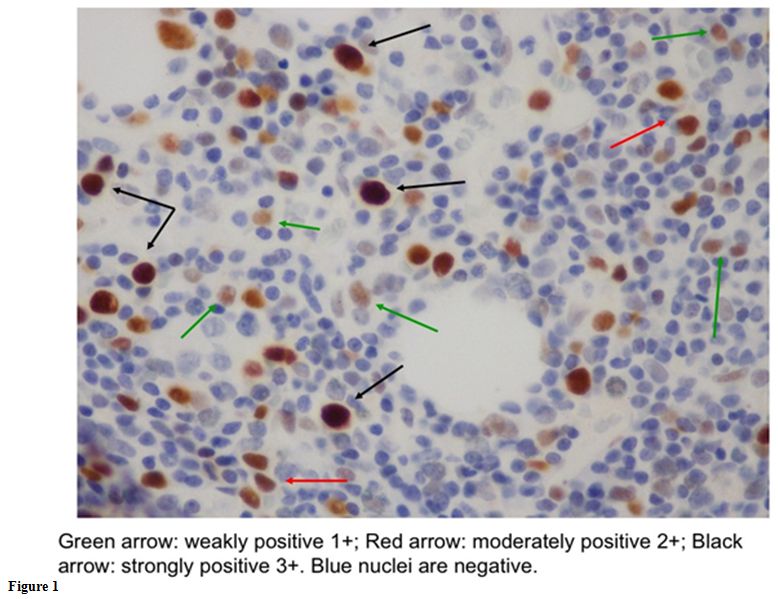
presence of TP53 mutation, especially in low-risk Del(5q) MDS.[9,13,14] Saft et al.[14] examined p53 expression in 85 Del(5q) patients enrolled in the MDS-004 trial.[15]
They also quantified the intensity of expression that identified the
positivity of the marker, using a scale in which “0” was negative; “1+”
weakly positive; “2+” moderately positive and “3+” strongly positive.
Only the cells with a strong p53 staining (3+) were regarded as
positive for the analysis. They noted that a p53 expression higher than
1% (found in 30/85 cases), was associated with higher acute myeloid
leukemia (AML) evolution risk, with shorter OS and with a lower
cytogenetic response rate to lenalidomide treatment. However, the
correct optimal p53 positivity cut-off value is still controversial. In
fact, according to the report by Jädersten et al.,[9]
there is a correlation between TP53 mutation and the presence of over
2% bone marrow progenitors with strong p53 staining. Apart from Del(5q)
setting, McGraw[12] indicated that the best p53
cut-off value for specificity and sensitivity to predict TP53 mutations
in MDS and secondary AML is 0.5%. Iwasaki et al.[6]
stated that in MDS and AML patients p53 was likely mutated when more
than 5% of cells were positively stained. In a de novo MDS cohort of
patients with moderate to severe reticulin fibrosis, higher levels of
TP53 expression (≥10% of the cells) were associated with higher BM
blast counts, poor risk karyotype, TP53 mutations and, above all, with
shorter OS.[16] In another report, overall survival was significantly lower in cases with a p53 expression in more than 50% of the cells.[17]
According
to the abovementioned works, the correlation of p53 expression with
survival seems to be confirmed. But it is evident that there is great
heterogeneity of the cut-off value above which it has to be considered
as an unfavorable prognostic parameter. In particular, few homogeneous
data are available on the possible prognostic impact of p53 IHC
expression and overall on the cut-off levels in patients with higher
risk MDS. Hence the aim of this pilot study to investigate the IHC p53
expression with OS in BM biopsies from patients with “intermediate,”
“high” and “very high” R-IPSS risk MDS.
Material and Methods
We
performed a retrospective analysis considering a cohort of higher risk
(“intermediate,” “high” and “very high” risk according to IPSS-R) MDS
patients. Since survival was the main endpoint of the study, we
selected, from our records, patients with at least three years follow
up (or who died before three years from diagnosis) and with an
available bone marrow biopsy performed at the time of diagnosis. No
data about TP53 mutation were available, so TP53 could not be
considered. The purpose was to verify if p53 expression maintains a
prognostic value in itself in higher MDS patients and investigate the
best cut-off value. A cohort of 60 patients was considered. We
extracted from the archives all the bone marrow samples performed at
the diagnosis; IHC p53 expression was performed and evaluated by two
independent pathologists (L.B.; M.T.). They evaluated p53 positivity in
all hemopoietic mononucleated cells (megakaryocytes and mature
granulocyte were excluded from the count). Staining was quantified, as
in the work of Saft and colleagues,[14] in an
intensity scale as follows: “0” if negative; “1+” if weakly positive;
“2+” if moderately positive; “3+” if strongly positive (Figure 1).
Only cells with strong p53 staining (3+) were considered as
positive for the analysis. The two pathologists worked blindly and
independently. The few cases (less than 5%) without full concordance
were jointly reviewed and a shared conclusion was obtained for each of
them. Fibrosis was also evaluated according to the European
clinicopathological criteria[18] which define “MF0”
the normal bone marrow fibrosis, “MF1” a slight reticulin fibrosis,
“MF2” an advanced reticulin and initial collagen fibrosis and “MF3” an
advanced collagen fibrosis. The number of medullar blasts for each
patient was reconsidered too and reviewed on the cytological staining
performed at diagnosis by A.M and M.R. The result of p53 expression was
subsequently related to survival. Survival was considered globally,
regardless of treatment. In fact, since a monocentric study, the best
possible treatment was chosen homogeneously, according to established
criteria based on the expertise of the center. In particular,
transplanted patients were not censored at the time of the transplant.
Lastly, we assessed a possible correlation between p53 expression and
the presence of fibrosis, the amount of BM blasts and the cytogenetic
risk according to the IPSS-R classification. The statistical
evaluations were carried out with logistic analysis. Influence of p53
expression – as a continuous variable – on survival was analyzed by Cox
proportional hazard regression, verifying the assumptions by Schoenfeld
residuals. A ROC analysis was performed as a tool to evaluate the
possible cut-off values suitable to dichotomize the p53 continuous
variable and to individuate an optimum on the basis of their positive
predictive value (PPV), negative predictive value (NPV), sensitivity
and specificity, which were evaluated together with the respective 95%
confidence interval (95%CI). Survivorships after dichotomizations were
estimated with the Kaplan-Meier product limit method, followed by the
log-rank test. The search for possible association between categorical
variables was carried out by the Fisher exact test.
 |
Figure
1 |
Results
The
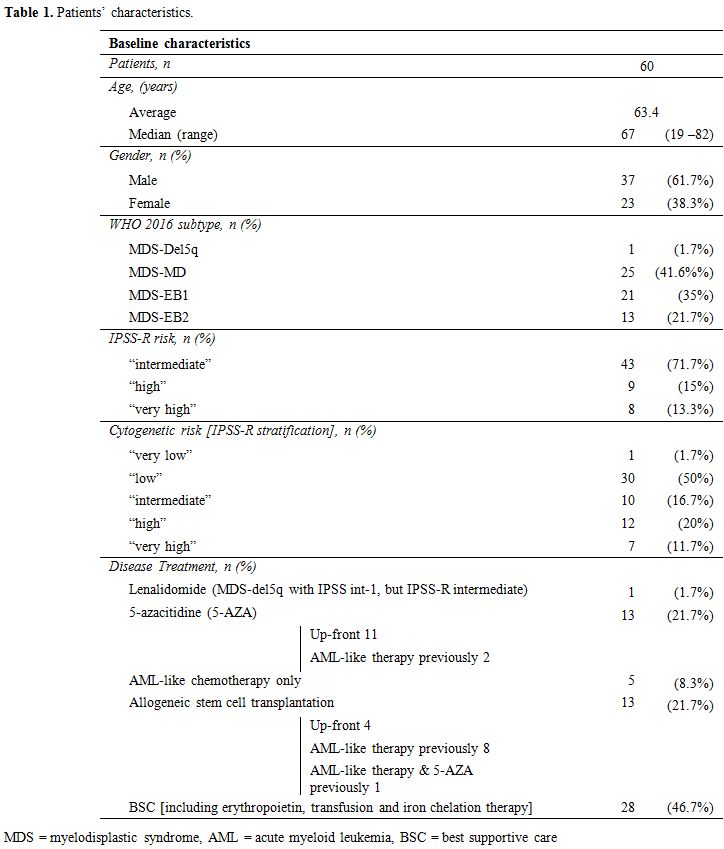
median age of patients was 67 (range 19 – 82). Diagnosis, according to
the WHO 2016 nomenclature, was MDS-Del5q (MDS with deletion of long arm
of chromosome 5) in 1/60 cases (1.6%), MDS-MLD (MDS with multilinear
dysplasia) in 25/60 cases (41.6%); MDS-EB1 (MDS with excess of blast
type 1) in 21/60 cases (35%); MDS-EB2 (MDS with excess of blast type 2)
in 13/60 cases (21.7%). The IPSS-R was “intermediate” in 43 cases
(71.7%); “high” in 9 cases (15%) and “very high” in 8 cases (13.3%).
Cytogenetic risk according to the IPSS-R stratification was “very low”
in one case (1.6%); “low” in 30 cases (50%); “intermediate” in 10 cases
(16.7%); “high” in 12 cases (20%); “very high” in 7 cases (11.7%) (Table 1).
Regarding the disease treatment, 1 patient was treated with
Lenalidomide (MDS-del5q with IPSS int-1, but IPSS-R intermediate), 13
patients with 5-azacitidine, 2 of them also with AML-like previous
therapy, 5 solely with AML-like chemotherapy and 13 patients underwent
allogeneic stem cell transplantation (among them 8 with a previous
AML-like therapy, 1 with both previous AML-like therapy and
5-azacitidine). In the remaining 28 cases, the best supportive care
(including erythropoietin, transfusion, and iron chelation therapy) was
employed (Table 1). The median OS considering all the patients was 41 months.
 |
Table
1. Patients’ characteristics. |
The
p53 expression was < 1% in 39 cases (65.0%), 1% in 5 cases (8.3%),
2% in 6 cases (10.0%), 3% in 2 cases (3.3%), 5% in 3 cases (5.0%), ≥
10% in 5 cases (8.3%). Average MF grading was 2 (17% of patients had MF
grading >2). The average number of marrow blasts was 7.3% (in 28% of
patients it was ≥ 10%).
Upon univariate analysis, a significant
association between the percentage of p53 expression and survival was
found (p=0.013; Hazard Ratio 1.067; 95% CI: 1.014 - 1.124). The better
cut-off value predicting a shorter survival was therefore investigated.
Cut-off values of 1%, 2%, 3%, 5% and 10% were examined (Table 2). The 5% and 10% cut-off values showed a significant PPV compared to the other values in predicting the outcome (see table 2
for a synoptic comparison regarding PPV, NPV, and also sensitivity and
specificity – here given as additional info – in relation to the event
of death). Therefore, as shown in figure 2,
a better OS was observed in patients whose BM p53 expression was lower
than 5% or 10% compared to patients with a BM p53 expression equal or
above 5% (p=0.0063) and 10% (p=0.0038), respectively. The 10% cut-off
value had the best statistical significance and therefore was
considered as the best candidate to be the cut-off of reference. A
different probability of outcome was not found for the lower cut-off
values of 1%, 2% and 3% (p> 0.05).
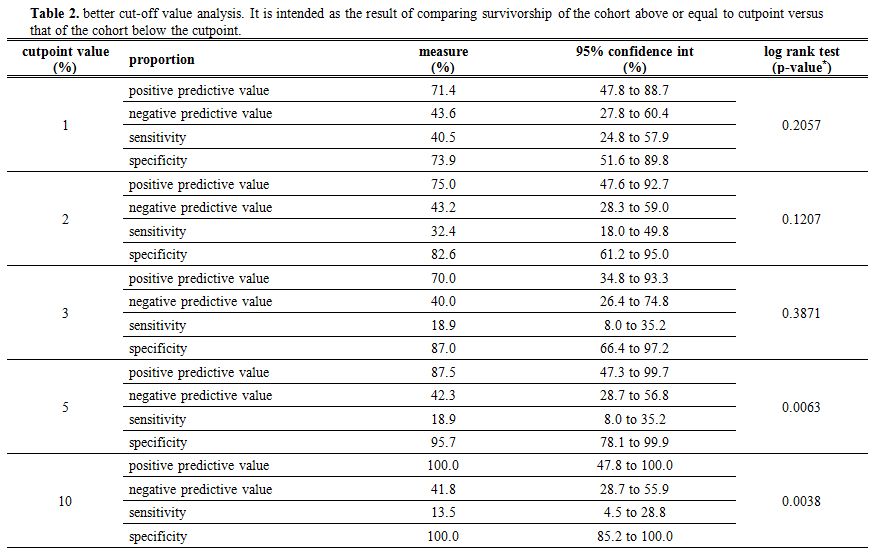 |
Table 2 . Better cut-off
value analysis. It is intended as the result of comparing survivorship
of the cohort above or equal to cutpoint versus that of the cohort
below the cutpoint. |
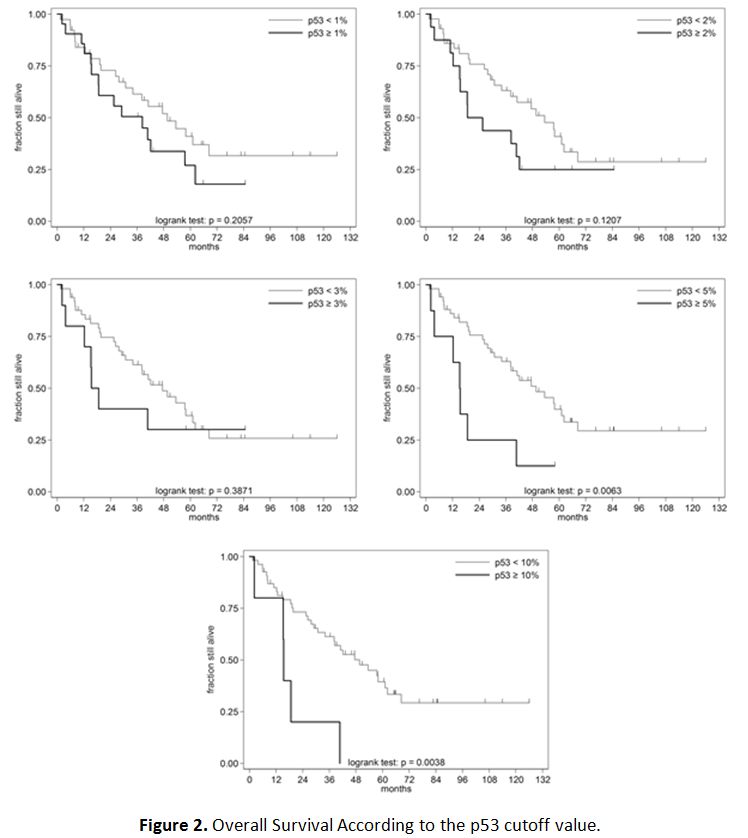 |
Figure 2. Overall Survival According to the p53 cutoff value. |
Notably,
considering the 8 patients with p53 expression ≥ 5%, 6 of them (75%)
were treated with drugs that could potentially modify the natural
course of the disease (3 with 5-azacitidine, 1 with AML-like
chemotherapy, 1 with allogeneic bone marrow transplantation preceded by
AML-like chemotherapy and 1 with upfront allogeneic bone marrow
transplantation).
Considering p53 expression ≥ 10%, in 4 of 5
cases (80%) we administrated a therapy able to modify the natural
course of the disease: two with 5-azacitidine, one with AML-like
chemotherapy and one with allogeneic bone marrow transplantation
preceded by AML-like chemotherapy. As a matter of fact, the best
supportive care was offered only to 25% of patients with a BM p53
expression ≥ 5% and 20% of patients with a BM p53 expression ≥ 10%,
compared to 46.1% (24/52) of patients with a BM p53 expression < 5%,
and 45.4% (25/55) of patients with a BM p53 expression < 10%.
These
observations reveal that a treatment that could potentially modify the
natural course of the disease was employed to a greater extent in
patients with p53 expression over 5% and to an even greater extent with
p53 over 10%, apparently with no influence on the prognostic impact of
high p53 expression. The low number of patients having p53 expression
equal to or higher than 5 or 10% did not warrant performing a
multivariate analysis to evaluate the impact of therapy on the outcome
better. No association between p53 expression either with fibrosis or
BM blast count was found (p> 0.05 for both the variables). On the
contrary, we observed a significant association between p53 expression
and cytogenetic risk according to IPSS-R stratification. Note that
seven of the eight patients with p53 expression of at least 5%, had a
complex karyotype; none of them showed a 17p alteration. For any single
arbitrary unitary increase in the cytogenetic risk score according to
the R-IPSS stratification, the odds of a BM p53 expression > 10%
rise by 1600% (p=0.015).
Discussion
This
pilot study confirmed the unfavorable prognostic significance of BM p53
expression also in a population of intermediate, high and very high
IPSS-R risk patients. These results were expected since overexpression
is never observed in cases of wild type TP53, meanwhile not all TP53
mutations lead to p53 overexpression. In other terms, IHC p53
overexpression is always a sign of a molecular alteration with negative
prognostic impact, even though it underestimates the real frequency of
TP53 mutations.
The only cohort of patients with homogeneity
regarding the prognostic risk and in which ICH p53 expression was
related to survival was analyzed in the Saft work on low-risk Del(5q).[13]
There are some differences in comparison to our higher risk group of
patients. First of all, the prevalence of p53 overexpression was more
evident in our data: we found 35% patients with p53 expression ≥ 1%,
27% cases with p53 expression ≥ 2% and 13% higher or equal to 5%. Our
rate was higher than that found in the cohort analyzed by Saft et al.
(30%, 19%, and 6% respectively). Evidently, higher risk MDS are
characterized by a more frequent occurrence of TP53 mutations compared
with the low-risk category Del(5q). The other important difference was
the cut-off value which has to be considered significant for the
prognostic impact of IHC p53 overexpression. In our cohort of higher
risk MDS patients, the cut-off levels were considerably higher (5-10%)
than those reported by Saft et al. (1%). This discrepancy may be due to
the fact that different factors from the p53 expression, could strongly
affect survival in patients with higher-risk MDS. Thus, the negative
prognostic value of p53 overexpression emerges only at higher levels of
its expression. This hypothesis can only work if based on the
assumption that a greater accumulation of the protein in tumor nucleus
is linked to a TP53 mutation with a more severe impact on cellular
homeostasis. In other terms, we may suppose that the higher the p53
cellular accumulation is, the higher the impairment of the protein is
in its anti-neoplastic functions, especially inducing apoptosis.
In
this pilot study, 10% cut-off value appears to be the best to identify
a poor prognosis, according to statistical analysis, and that must be
considered only a preliminary finding and needs to be confirmed in a
larger series.
Another interesting issue is the association
between p53 expression and the IPSS-R cytogenetic risk score. We
speculate a correlation between the presence of a TP53 mutation with a
severe injury of p53 function and the presence of further DNA damage.
If we consider that a properly functioning p53 protein is related to
different DNA repair mechanisms, this hypothesis appears appropriate
from a biological point of view. However, this theory has to be
confirmed in a larger group of patients too.
Overall, IHC
detectable p53 cellular accumulation may be considered as an
unfavorable prognostic marker in MDS; whereas, the absence of this
protein in the IHC assessment is not evidence of TP53 mutation absence.
In higher-risk MDS, IHC identification of p53 expression seems to be an
unfavorable prognostic factor only when largely overexpressed (best
cut-off value seems to be 10%), contrary to the lower risk, at least in
the setting of patients with Del(5q). The IHC for p53 is a low-cost
test if compared to molecular detection of TP53 mutations by PCR or NGS
techniques. Furthermore, it should be routinely employed in the MDS
diagnostic workup, independently from the IPSS-R risk, and used as a
tool to help clinical decisions.
References
- Greenberg PL, Tuechler H, Schanz J, Sanz G,
Garcia-Manero G, Solé F, Bennet JM, Bowen D, Fenaux P, Dreyfus F,
Kantarjian H, Kuendgen A, Levis A, Malcovati L, Cazzola M, Cermak J,
Fonatsch C, Le Beau MM, Slovak ML, Krieger O, Luebbert M, Maciejewski
J, Magalhaes SMM, Miyazaki Y, Pfeilstöcker M, Sekeres M, Sperr WR,
Stauder R, Tauro S, Valent P, Vallespi T, van de Loosdrecht AA, Germing
U, Haase D. Revised international prognostic scoring system for
myelodysplastic syndromes, Blood 2012;120:2454-65. https://doi.org/10.1182/blood-2012-03-420489 PMid:22740453 PMCid:PMC4425443
- Greenberg
P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T,
Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J.
International scoring system for evaluating prognosis in
myelodysplastic syndromes. Blood 1997; 89:2079–88. PMid:9058730
- Bejar
R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G,
Kantarjian H, Raza A, Levine RL, Neuberg D, Ebert BL. Clinical effect
of point mutations in myelodysplastic syndromes. N Engl J Med.
2011;364:2496-2506. https://doi.org/10.1056/NEJMoa1013343 PMid:21714648 PMCid:PMC3159042
- Malcovati
L, Papaemmanuil E, Bowen DT, Boultwood J, Della Porta MG, Pascutto C,
Travaglino E, Groves MJ, Godfrey AL, Ambaglio I, Gallì A, Da Vià MC,
Conte S, Tauro S, Keenan N, Hyslop A, Hinton J, Mudie LJ, Wainscoat JS,
Futreal PA, Stratton MR, Campbell PJ, Hellström-Lindberg E, Cazzola M.
Chronic Myeloid Disorders Working Group of the International Cancer
Genome Consortium and of the Associazione Italiana per la Ricerca sul
Cancro Gruppo Italiano Malattie Mieloproliferative. Clinical
significance of SF3B1 mutations in myelodysplastic syndromes and
myelodysplastic/myeloproliferative neoplasms. Blood. 2011;118:6239-46. https://doi.org/10.1182/blood-2011-09-377275 PMid:21998214 PMCid:PMC3236114
- Kulasekararaj
AG, Smith AE, Mian SA, Mohamedali AM, Krishnamurthy P, Lea NC, Gäken J,
Pennaneach C, Ireland R, Czepulkowski B, Pomplun S, Marsh JC, Mufti GJ.
TP53 mutations in myelodysplastic syndrome are strongly correlated with
aberrations of chromosome 5 and correlate with adverse prognosis. Br J
Haematol. 2013;160:660-72. https://doi.org/10.1111/bjh.12203 PMid:23297687
- Iwasaki
T, Murakami M, Sugisaki C, Sobue S, Ohashi H, Asano H, Suzuki M,
Nakamura S, Ito M, Murate T. Characterization of myelodysplastic
syndrome and aplastic anemia by immunostaining of p53 and hemoglobin F
and karyotype analysis: Differential diagnosis between refractory
anemia and aplastic anemia. Pathology International. 2008;58:353-360. https://doi.org/10.1111/j.1440-1827.2008.02236.x PMid:18477214
- Yoshizato
T, Nannya Y, Atsuta Y, Shiozawa Y, Iijima-Yamashita Y, Yoshida K,
Shiraishi Y, Suzuki H, Nagata Y, Sato Y, Kakiuchi N, Matsuo K, Onizuka
M, Kataoka K, Chiba K, Tanaka H, Ueno H, Nakagawa MM, Przychodzen B,
Haferlach C, Kern W, Aoki K, Itonaga H, Kanda Y, Sekeres MA,
Maciejewski JP, Haferlach T, Miyazaki Y, Horibe K, Sanada M, Miyano S,
Makishima H, Ogawa S. Genetic abnormalities in myelodysplasia and
secondary acute myeloid leukemia: impact on outcome of stem cell
transplantation. Blood. 2017;129:2347-58. https://doi.org/10.1182/blood-2016-12-754796 PMid:28223278 PMCid:PMC5409449
- Bejar
R, Stevenson KE, Caughey BA, Abdel-Wahab O, Steensma DP, Galili N, Raza
A, Kantarjian H, Levine RL, Neuberg D, Garcia-Manero G, Ebert BL.
Validation of a prognostic model and the impact of mutations in
patients with lower-risk myelodysplastic syndromes. J Clin Oncol.
2012;30:3376-82. https://doi.org/10.1200/JCO.2011.40.7379 PMid:22869879 PMCid:PMC3438234
- Jädersten
M, Saft L, Smith A, Kulasekararaj A, Pomplun S, Göhring G, Hedlund A,
Hast R, Schlegelberger B, Porwit A, Hellström-Lindberg E, Mufti GJ.
TP53 mutations in low-risk myelodysplastic syndromes with del(5q)
predict disease progression. J Clin Oncol. 2011;29:1971-79. https://doi.org/10.1200/JCO.2010.31.8576 PMid:21519010
- Bártek
J, Bártková J, Lukás J, Stasková Z, Vojtĕsek B, Lane DP.
Immunohistochemical analysis of the p53 oncoprotein on paraffin
sections using a series of novel monoclonal antibodies. J Pathol.
1993;169:27-34. https://doi.org/10.1002/path.1711690106 PMid:8433213
- Nenutil
R, Smardova J, Pavlova S, Hanzelkova Z, Muller P, Fabian P, Hrstka R,
Janotova P, Radina M, Lane DP, Coates PJ, Vojtesek B. Discriminating
functional and non-functional p53 in human tumors by p53 and MDM2
immunohistochemistry. J Pathol. 2005;207:251-9. https://doi.org/10.1002/path.1838 PMid:16161005
- McGraw
KL, Nguyen J, Komrokji RS, Sallman D, Al Ali NH, Padron E, Lancet JE,
Moscinski LC, List AF, Zhang L. Immunohistochemical pattern of p53 is a
measure of TP53 mutation burden and adverse clinical outcome in
myelodysplastic syndromes and secondary acute myeloid leukemia.
Haematologica. 2016;101:e320-e323. https://doi.org/10.3324/haematol.2016.143214 PMid:27081179 PMCid:PMC4967580
- Jädersten
M, Saft L, Pellagatti A, Göhring G, Wainscoat JS, Boultwood J, Porwit
A, Schlegelberger B, Hellström-Lindberg E. Clonal heterogeneity in the
5q- syndrome: p53 expressing progenitors prevail during lenalidomide
treatment and expand at disease progression. Haematologica.
2009;94:1762-66. https://doi.org/10.3324/haematol.2009.011528 PMid:19797731 PMCid:PMC2791931
- Saft
L, Karimi M, Ghaderi M, Matolcsy A, Mufti GJ, Kulasekararaj A, Göhring
G, Giagounidis A, Selleslag D, Muus P, Sanz G, Mittelman M, Bowen D,
Porwit A, Fu T, Backstrom J, Fenaux P, MacBeth KJ, Hellström-Lindberg
E. p53 protein expression independently predicts outcome in patients
with lower-risk myelodysplastic syndromes with del(5q). Haematologica.
2014;99:1041-49. https://doi.org/10.3324/haematol.2013.098103 PMid:24682512 PMCid:PMC4040908
- Fenaux
P, Giagounidis A, Selleslag D, Beyne-Rauzy O, Mufti G, Mittelman M,
Muus P, Te Boekhorst P, Sanz G, Del Ca-izo C, Guerci-Bresler A, Nilsson
L, Platzbecker U, Lübbert M, Quesnel B, Cazzola M, Ganser A, Bowen D,
Schlegelberger B, Aul C, Knight R, Francis J, Fu T, Hellström-Lindberg
E. MDS-004 Lenalidomide del5q Study Group. A randomized phase 3 study
of lenalidomide versus placebo in RBC transfusion-dependent patients
with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q.
Blood. 2011;118:3765-76. https://doi.org/10.1182/blood-2011-01-330126 PMid:21753188
- Loghavi
S, Al-Ibraheemi A, Zuo Z, Garcia-Manero G, Yabe M, Wang SA, Kantarjian
HM, Yin CC, Miranda RN, Luthra R, Medeiros LJ, Bueso-Ramos CE, Khoury
JD. TP53 Overexpression is an Independent Adverse Prognostic Factor in
de novo Myelodysplastic Syndromes with Fibrosis. Br J Haematol.
2015;171:91-99. https://doi.org/10.1111/bjh.13529 PMid:26123119 PMCid:PMC5577911
- Bektas
O, Uner A, Buyukasik Y, Uz B, Bozkurt S, Eliacik E, Işik A,
Haznedaroglu IC, Goker H, Demiroglu H, Aksu S, Ozcebe OI, Sayinalp N.
Clinical and pathological correlations of marrow PUMA and P53
expressions in myelodysplastic syndromes. AMPIS. 2015;123:445-51. https://doi.org/10.1111/apm.12369
- Thiele
J, Kvasnicka HM, Facchetti F, Vito F, van der Walt J, Orazi A. European
Consensus on grading of bone marrow fibrosis and assessment of
cellularity. Haematologica. 2005;90:1128–32. PMid:16079113
[TOP]




