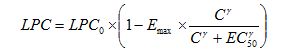Juan Eduardo Megías-Vericat1, David Martínez-Cuadrón1,2, Joaquín Martínez López3, Juan Miguel Bergua4, Mar Tormo5, Josefina Serrano6, Ataulfo González7, Jaime Pérez de Oteyza8, Susana Vives9, Belén Vidriales10, Pilar Herrera11, Juan Antonio Vera12, Aurelio López Martínez13, Adolfo de la Fuente14, Mª Lourdes Amador15, José-Ángel Hernández-Rivas16, Mª Ángeles Fernández17, Carlos Javier Cerveró18, Daniel Morillo19, Pilar Hernández Campo20, Julián Gorrochategui20, Daniel Primo20, José Luis Rojas20, Margarita Guenova21, Joan Ballesteros20, Miguel Sanz1,2 and Pau Montesinos1,2 on behalf of the Spanish PETHEMA group.
1 Hospital Universitari i Politècnic La Fe, Valencia, Spain.
2 CIBERONC, Instituto Carlos III, Madrid, Spain.
3 Hospital Universitario 12 de Octubre, UCM, CNIO, CIBERONC, Madrid, Spain.
4 Hospital San Pedro de Alcántara, Cáceres, Spain.
5 Hospital Clínico Universitario, Valencia, Spain.
6 Hospital Universitario Reina Sofía, Córdoba, Spain.
7 Hospital Universitario Clínico San Carlos, Madrid, Spain.
8 Hospital de Madrid Norte Sanchinarro, Madrid, Spain.
9
ICO-Hospital Germans Trias i Pujol, Josep Carreras Leukemia Research
Institute, Universitat Autònoma de Barcelona, Badalona, Spain.
10 Complejo Asistencial Universitario de Salamanca, Salamanca, Spain.
11 Hospital Universitario Ramón y Cajal, Madrid, Spain.
12 Hospital Universitario Virgen Macarena, Sevilla, Spain.
13 Hospital Arnau de Vilanova, Valencia, Spain.
14 MD Anderson Cancer Center, Madrid, Spain.
15 Hospital de Montecelo, Pontevedra, Spain.
16 Hospital Universitario Infanta Leonor, Universidad Complutense de Madrid, Madrid, Spain.
17 Hospital Xeral Cies, Vigo, Spain.
18 Hospital Virgen de la Luz, Cuenca, Spain.
19 Fundación Jiménez Díaz, Madrid, Spain.
20 Vivia Biotech, Tres Cantos, Madrid, Spain.
21 Specialized Hospital for Active Treatment of Hematological Diseases, Sofía, Bulgaria.
Correspondence to: Pau Montesinos. Hospital Universitari i Politècnic
La Fe and CIBERONC, Instituto Carlos III, Madrid, Spain. Tel: +34
961411966. E-mail:
montesinos_pau@gva.es
Published: March 1, 2019
Received: October 10, 2018
Accepted: January 12, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019016 DOI
10.4084/MJHID.2019.016
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Induction schedules in acute myeloid leukemia (AML) are based on
combinations of cytarabine and anthracyclines. The choice of the
anthracycline employed has been widely studied in multiple clinical
trials showing similar complete remission rates.
Materials and Methods: Using an ex vivo
test we have analyzed if a subset of AML patients may respond
differently to cytarabine combined with idarubicin, daunorubicin or
mitoxantrone. Bone marrow (BM) samples of 198 AML patients were
incubated for 48 hours in 96 well plates, each well containing
different drugs or drug combinations at different concentrations. Ex vivo
drug sensitivity analysis was made using the PharmaFlow platform
maintaining the BM microenvironment. Drug response was evaluated as
depletion of AML blast cells in each well after incubation. Annexin
V-FITC was used to quantify the ability of the drugs to induce
apoptosis, and pharmacological responses were calculated using
pharmacokinetic population models.
Results: Similar dose-respond graphs were generated for the three anthracyclines, with a slight decrease in EC50
with idarubicin (p=1.462E-06), whereas the interpatient variability of
either drug was large. To identify those cases of selective sensitivity
to anthracyclines, potency was compared, in terms of area under the
curve. Differences in anthracycline monotherapy potency greater than
30% from 3 pairwise comparisons were identified in 28.3% of samples.
Furthermore, different sensitivity was detected in 8.2% of patients
comparing combinations of cytarabine and anthracyclines.
Discussion:
A third of the patients could benefit from the use of this test in the
first line induction therapy selection, although it should be confirmed
in a clinical trial specifically designed.
|
Introduction
Induction 1st
line schedules in de novo acute myeloid leukemia (AML) are based in a
combination of an anthracycline with cytarabine (CYT) (3+7 schedule),
obtaining complete remission (CR) rates of 70-80% after 1-2 cycles.[1,2]
Daunorubicin (DNR), idarubicin (IDA), mitoxantrone (MIT, an
anthracenedione), and less frequently other anthracyclines have been
employed in these schemes. The choice of the anthracycline employed has
been widely studied in several randomized clinical trials (RCT),[3-22] showing similar CR rates, with some exceptions in which IDA reported higher CR than DNR,[4,6-8,12] finding reproduced in a Cochrane meta-analysis.[23]
Different ex vivo tests
have been employed to select the most effective drug combination from
the individualized sensitivity and resistance assays, but none of them
have been recommended in clinical practice.[24] We
are developing a Precision Medicine (PM) test based on an actionable
native environment method (PharmaFlow platform), which showed excellent
correlations with clinical responses in AML, avoiding some limitations
of other ex vivo assays.[25]
The
objective of this non-interventional study is to explore whether a
significant percentage of patients AML samples may show different ex-vivo sensitivity to IDA vs DNR vs MIT combined with CYT.
Patients and Methods
Patients and study design.
A multicenter, prospective, non-interventional cohort study was carried
out in 33 Spanish institutions of the PETHEMA group. The inclusion
period lasted five years (2012-2017), enrolling patients aged 18 years
and older with newly diagnosed AML. Diagnosis and classification of AML
were performed according to the World Health Classification (WHO)
criteria.[26] This study was approved by the Research
Ethics Board of each participating institution and was conducted
according to the Spanish law 14/2007 of biomedical research. Informed
consent was provided to all patients.
Vivia’s PharmaFlow PM Test.
• Native environment whole bone marrow sample
Ex vivo drug sensitivity analysis was made using the PharmaFlow platform (previously termed ExviTech®)[25]
maintaining the bone marrow (BM) microenvironment. A minimum BM sample
volume between 1 and 2 ml was collected by aspiration at AML diagnosis,
before starting induction chemotherapy, and was processed by an
automated method in Vivia Biotech laboratories 24 hours after
extraction. Samples were incubated for 48 hours in 96 well plates, each
well containing different drugs or drug combinations at different
concentrations, enabling calculation of dose-response curves for every
single drug (CYT, IDA, DNR, MIT) and combination used in treatments
(CYT-IDA, CYT-DNR, CYT-MIT). The number of BM samples analyzed were 289
with IDA, 333 with DNR and 274 with MIT. A more detailed description of
the procedure has been published elsewhere.[25] The concentrations assayed for each anthracycline were:
- Concentrations for IDA (µM): > 0.0002 ; 0.001 ;
0.002 ; 0.006 ; 0.01 ; 0.018 ; 0.02 ; 0.04 ; 0.05 ; 0.055 ; 0.08 ; 0.13
; 0.16 ; 0.2 ; 0.26 ; 0.4 ; 0.5 ; 0.6 ; 1.5.
- Concentrations for DNR (µM): > 0.001; 0.05 ;
0.075 ; 0.093 ; 0.15 ; 0.18 ; 0.25 ; 0.3 ; 0.37 ; 0.45 ; 0.75 ; 0.85 ;
1.25 ; 1.5 ; 2.7 ; 3.
-
Concentrations for MIT (µM): > 0.001 ; 0.0016 ; 0.008 ; 0.01 ; 0.04
; 0.08 ; 0.2 ; 0.38 ; 0.6 ; 0.8 ; 1 ; 2.33 ; 3.5 ; 7.
• Modeling of ex vivo activity of CYT, IDA, DNR, MIT
Evaluation
of drug response was done by counting the number of live pathological
cells (LPC) remaining after incubation at increasing drug
concentrations. Dying cells (apoptosis) were excluded using Annexin
V-FITC. Pharmacological responses were estimated using pharmacodynamic
(PD) population-based models[27] which essentially
perform the fitting of the dependent variable (natural log of LPC) in a
non-linear mixed-effects model to derive typical population values
(fixed effects) and the magnitude of inter-patient and residual
variability (random effects). Model development was performed with the
first-order conditional estimation method using interaction option with
the software NONMEM (v7.2)[28], according to the following equation:
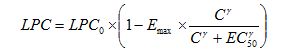 |
|
Where LPC0 parameter refers to the number of LPC after incubation in the absence of drug, Emax represents the maximum fractional decrease in LPC that the drug can elicit, EC50, is the drug concentration exerting half of Emax, and γ is the parameter governing the steepness of the LPC vs drug concentration (C) curve. Potency (EC50) and efficacy (Emax)
are PD parameters that characterize the pharmacological response and
are integrated into a single value corresponding to the measurement of
the area under the dose-response curve (Area Under the Curve, AUC).
For
data presentation, the survival index was computed, with the number of
LPC in control wells that were not exposed to any drugs being set as
100%. The number of live cells in each drug-treated well was compared
with this control value, and the survival index for each drug at each
concentration was determined as the percentage of LPC at every tested
concentration.
Interpatient variability (IPV) associated with all
parameters was described using an exponential model of the components
of variance. An additive error structure was used for the residual
variability. Population PD models were built with BM samples from 227
patients that were incubated with IDA, 271 with DNR, and 212 with MIT.
Bayesian estimation methods were then used to retrieve individual
patient parameters based on their available exposure-response
measurements in conjunction with the PD population parameters. After
several trials with different modeling strategies, we could conclude
that optimal approach, in terms of correlation with clinical output,
was achieved by forcing typical parameters to values obtained in a
different model using a dataset from samples tested at 72h. Therefore,
the typical parameter value for the maximum fractional effect (Emax)
was set to 1 for both drugs. For γ, the typical parameter value was
calculated but limited to the range 0-3. IPV for both parameters could
not be determined with this dataset.
For interaction analysis, a Surface Interaction model[29]
was used to estimate the degree of synergy, referred as α parameter,
between both drugs (R environment (v3.3.1) for statistical computing).[30]
In this analysis, a value equal to 0 is an additive effect, a value
> 0 indicates a synergistic effect, and a value < 0 reflects an
antagonistic effect.
Study endpoints.
The primary end-point was the comparison between the selective
sensitivities of the different anthracyclines individually using the
AUCs in the dose-response curve. For the comparisons between the
combinations of anthracyclines with CYT, we employed the volume under
the surface (VUS) of the dose-response curves. Besides, the differences
in either drug potency or synergism ex vivo were also calculated according to the observed and predicted response after induction.
Results
Patient Characteristics.
Overall, 332 BM samples from patients with AML suspicion were received
at the laboratory, from which 261 BM samples were completely monitored
at the end of the study. Of them, 63 (24%) were not evaluable because
of the following protocol issues: 1) incorrect informed consent form
(32 patients), 2) no available case report form (23 patients), 3)
misdiagnosis (3 patients), and 4) other unknown reasons (5 patients).
Overall, clinical data from 198 patient’s samples (60%) were available
at the end of this study. The main baseline characteristics of these
patients are displayed in Table 1.
In summary, the median age was 61 years (range, 19 to 91), all patients
were newly diagnosed AML, and 37 patients (19%) were categorized as
having high-risk cytogenetics. CR rate was obtained in 93 patients
(47%), whereas 65 patients obtained partial remission or were resistant
to induction.
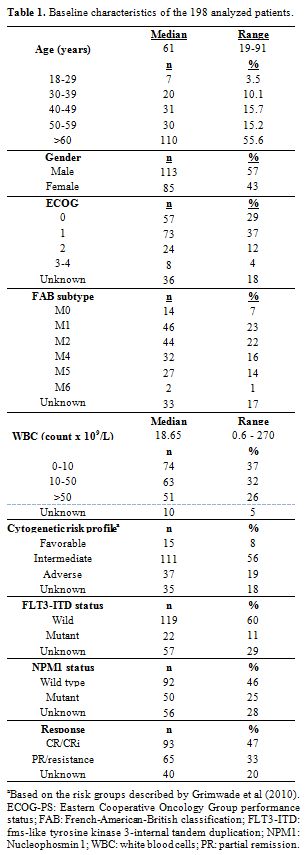 |
Table 1. Baseline characteristics of the 198 analyzed patients. |
Ex vivo PharmaFlow Test characterization of IDA, DNR and MIT models. Dose-response graphs were generated for the single drugs (IDA, DNR, and MIT) using PD models (Figure 1). Most of the observations were contained within the simulation-based 95% confidence intervals of the 5-95th
population percentiles proving good predictability of the selected
models. Pharmacological population parameters, as well as variability
and error values, are shown in Table 2.
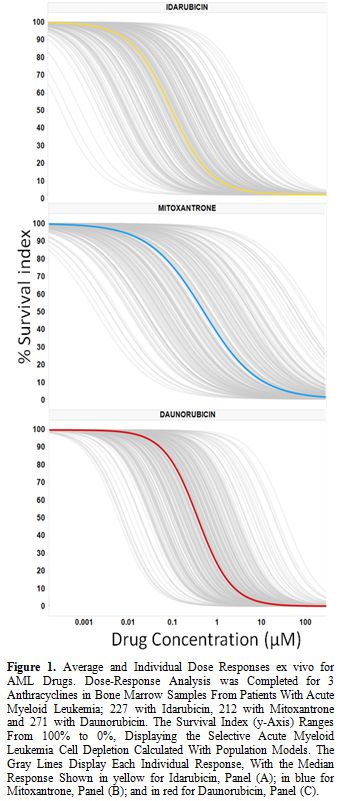 |
Figure 1.
Average and Individual Dose Responses ex vivo
for AML Drugs. Dose-Response Analysis was Completed for 3
Anthracyclines in Bone Marrow Samples From Patients With Acute Myeloid
Leukemia; 227 with Idarubicin, 212 with Mitoxantrone and 271 with
Daunorubicin. The Survival Index (y-Axis) Ranges From 100% to 0%,
Displaying the Selective Acute Myeloid Leukemia Cell Depletion
Calculated With Population Models. The Gray Lines Display Each
Individual Response, With the Median Response Shown in yellow for
Idarubicin, Panel (A); in blue for Mitoxantrone, Panel (B); and in red
for Daunorubicin, Panel (C). |
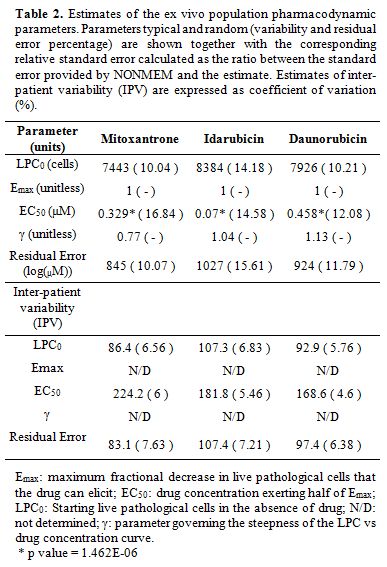 |
Table 2. Estimates of the ex vivo
population pharmacodynamic parameters. Parameters typical and random
(variability and residual error percentage) are shown together with the
corresponding relative standard error calculated as the ratio between
the standard error provided by NONMEM and the estimate. Estimates of
inter-patient variability (IPV) are expressed as coefficient of
variation (%). |
The average dose-responses of the three anthracyclines were similar, with a slight decrease in EC50 values with IDA (p-value=1.462E-06; Table 2), reproducing the results of the clinical trials.[4,6-8,12] However, the interpatient variability of either drug is quite large (Table 2, Figure 1), which could explain why some patients could show very differential sensitivities to these three drugs. As an example, Figure 2
illustrates a patient sample that is resistant to IDA and DNR (right
shifted dose-response curve) but sensitive to MIT (left shifted
dose-response curve).
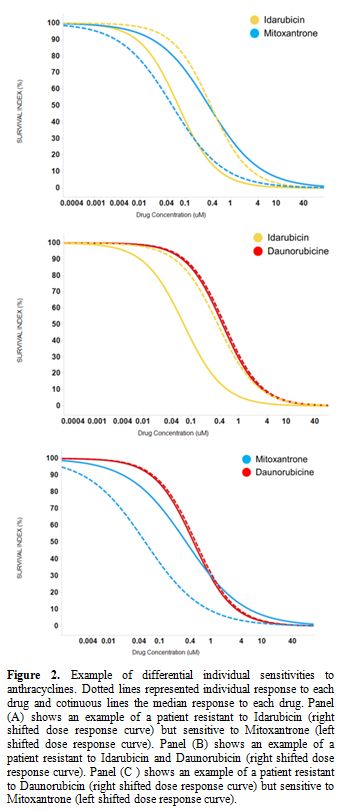 |
Figure 2. Example of
differential individual sensitivities to anthracyclines. Dotted lines
represented individual response to each drug and cotinuous lines the
median response to each drug. Panel (A) shows an example of a patient
resistant to Idarubicin (right shifted dose response curve) but
sensitive to Mitoxantrone (left shifted dose response curve). Panel (B)
shows an example of a patient resistant to Idarubicin and Daunorubicin
(right shifted dose response curve). Panel (C ) shows an example of a
patient resistant to Daunorubicin (right shifted dose response curve)
but sensitive to Mitoxantrone (left shifted dose response curve). |
To
identify these cases of selective sensitivity to anthracyclines, we
compared the potency, regarding AUC, between IDA vs. DNR, IDA vs. MIT,
and DNR vs. MIT (Figure 3, Table 3).
Most dots tend to line up, but red dots represent patient samples with
a difference in potency between these drugs >30%. Red dots from 3
pairwise comparisons identify 28.3% of patient samples with >30%
different potency among IDA-DNR-MIT (Figure 4).
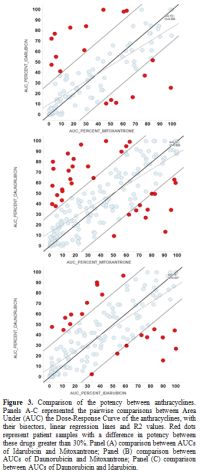 |
Figure 3.
Comparison of the potency between anthracyclines. Panels A-C
represented the pairwise comparisons between Area Under (AUC) the
Dose-Response Curve of the anthracyclines, with their bisectors, linear
regression lines and R2 values. Red dots represent patient samples with
a difference in potency between these drugs greater than 30%. Panel (A)
comparison between AUCs of Idarubicin and Mitoxantrone; Panel (B)
comparison between AUCs of Daunorubicin and Mitoxantrone; Panel (C)
comparison between AUCs of Daunorubicin and Idarubicin. |
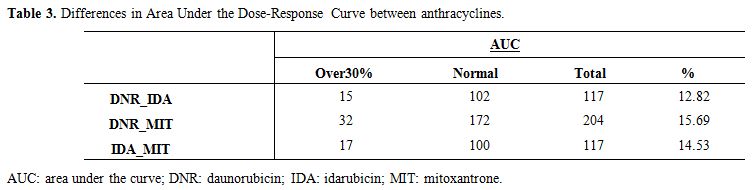 |
Table 3. Differences in Area Under the Dose-Response Curve between anthracyclines. |
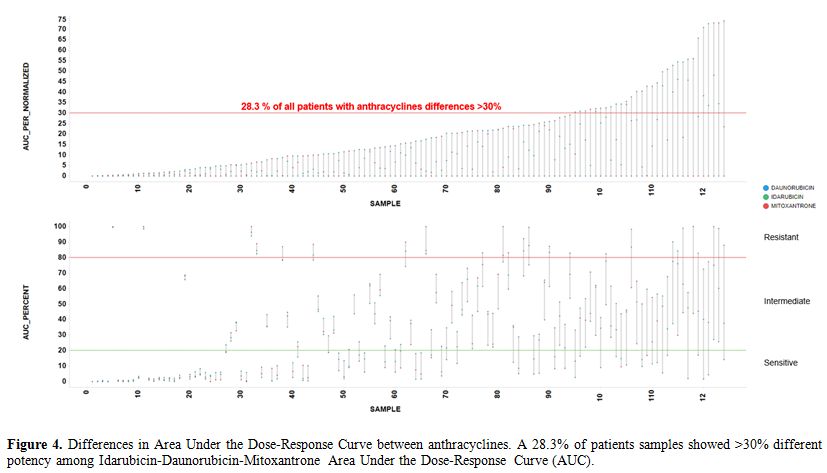 |
Figure 4. Differences in Area Under the
Dose-Response Curve between anthracyclines. A 28.3% of patients samples
showed >30% different potency among
Idarubicin-Daunorubicin-Mitoxantrone Area Under the Dose-Response Curve
(AUC). |
Ex vivo PharmaFlow Test characterization of CYT-IDA, CYT-DNR, and CYT-MIT combinations and their synergism.
The pairwise comparison of the combination treatments CYT-IDA, CYT-DNR,
and CYT-MIT obtained differential sensitivity to these anthracyclines
(red dots of Figure 5). In
this case, the red dots represent patient samples with a difference in
CYT + anthracyclines synergy differences >30%, and red dots from 3
pairwise comparisons identified an 8.2% of patient samples (Figure 6, Table 4).
Furthermore,
the values for the alpha parameters of the interaction models of
CYT-IDA, CYT-MIT, CYT-DNR were 0.72, 0.59 and 0.25, indicating
synergistic response in the ex vivo combination experiments.
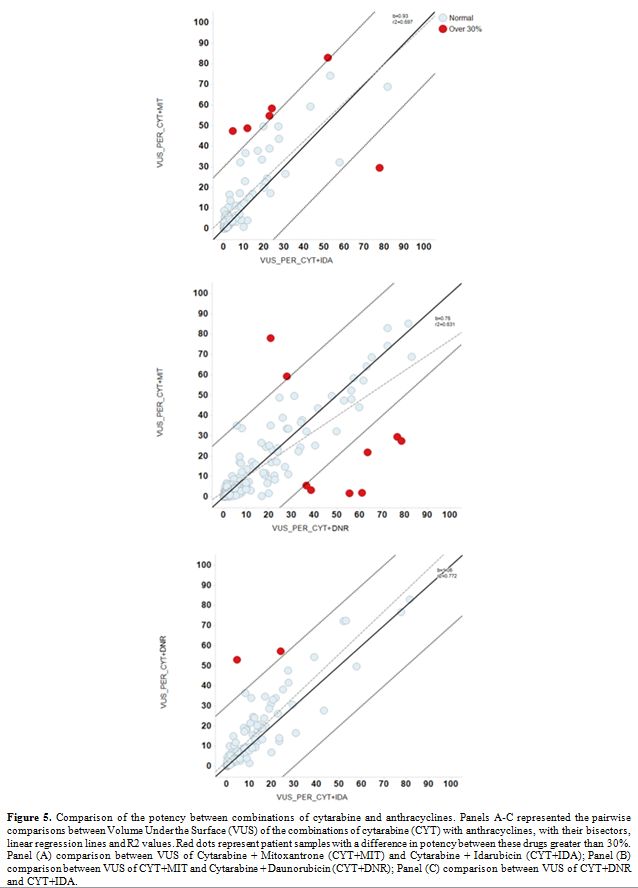 |
Figure 5.
Comparison of the potency between combinations of cytarabine and
anthracyclines. Panels A-C represented the pairwise comparisons between
Volume Under the Surface (VUS) of the combinations of cytarabine (CYT)
with anthracyclines, with their bisectors, linear regression lines and
R2 values. Red dots represent patient samples with a difference in
potency between these drugs greater than 30%. Panel (A) comparison
between VUS of Cytarabine + Mitoxantrone (CYT+MIT) and Cytarabine +
Idarubicin (CYT+IDA); Panel (B) comparison between VUS of CYT+MIT and
Cytarabine + Daunorubicin (CYT+DNR); Panel (C) comparison between VUS
of CYT+DNR and CYT+IDA. |
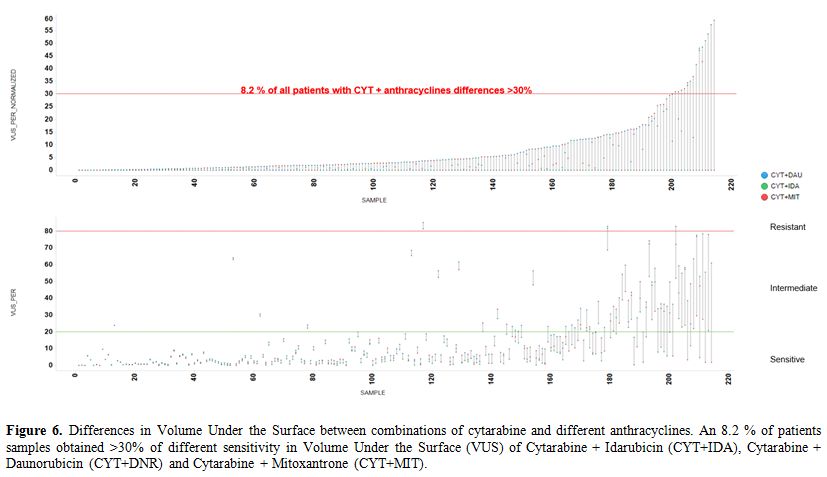 |
Figure 6. Differences in
Volume Under the Surface between combinations of cytarabine and
different anthracyclines. An 8.2 % of patients samples obtained >30%
of different sensitivity in Volume Under the Surface (VUS) of
Cytarabine + Idarubicin (CYT+IDA), Cytarabine + Daunorubicin (CYT+DNR)
and Cytarabine + Mitoxantrone (CYT+MIT). |
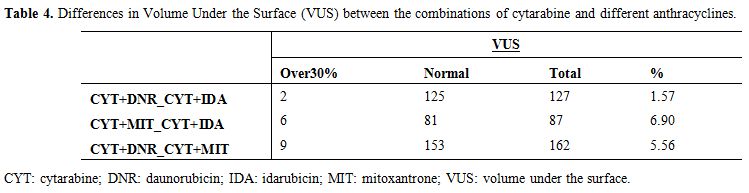 |
Table 4. Differences in Volume Under the
Surface (VUS) between the combinations of cytarabine and different
anthracyclines. |
Discussion
The
findings of this study show that PharmaFlow PM test seems able to
identify a subset of AML patients who have a significantly different ex vivo pharmacological response to anthracycline drugs. We can hypothesize that if these selective anthracycline ex vivo
responses were translated to in vivo responses, a fraction of this
28.3% subpopulation could benefit significantly from receiving a
specific anthracycline-based on the ex vivo
test sensitivity results. Furthermore, an 8.2% of patients showed a
significant difference in the synergy between CYT and anthracyclines,
in which the choice of the anthracycline could be crucial.
The first line induction therapy recommended by ELN[1] and NCCN[2] clinical guidelines includes seven days of a standard dose of CYT plus three days of an anthracycline, especially IDA (12 mg/m2) or DNR (60-90 mg/m2). The combination of CYT-MIT was not considered standard therapy, although it has been widely employed.
The influence of the anthracycline´s selection in the efficacy of induction therapy was analyzed in some RCTs.[3-22] The comparison between CYT-DNR and CYT-IDA has been studied in 13 different trials,[3-15] but only five studies reported differences in CR rates in favor of CYT-IDA.[4,6-8,12]
A meta-analysis confirmed the superiority of CYT-IDA against CYT-DNR,
obtaining higher overall survival (OS), disease-free survival (DFS),
CR, lower relapse rate, although this scheme increased induction death
and mucositis.[23] Regarding the employment of
CYT-DNR or CYT-MIT, a clinical trial reported similar CR, length of
duration of CR, OS, and toxicity.[16] No evidence of differences between CYT-IDA and CYT-MIT in CR, survival rates, and toxicity was observed in 6 RCTs[9,11,17-20] and one meta-analysis.[23] Combinations of CYT-doxorubicin showed worse outcomes than CYT-DNR[21] and CYT-IDA.[22]
According to clinical trials, in our study the average dose-responses
of IDA, DNR, and MIT were similar, with a slight decrease in EC50
with IDA, indicating a probable higher potency with IDA than DNR and
MIT. However, the anthracycline dosage of induction protocols assumed a
cumulative doses proportion of 4:1 for DNR: IDA and DNR: MIT,[31] but these proportions are not based in well-designed trials. In our cohort, according to this proportion and EC50 of DNR (0.458), the estimated EC50 of IDA and MIT was 0.115, a proportion 1.6 fold higher than IDA EC50 and three fold lower than MIT EC50 measured with ex vivo test.
Other
studies analyzed the role of different anthracyclines in the AML
induction with CYT and a third component, but CR and survival rates
were similar for DNR, MIT, and aclarubicin.[32,33] Besides the selection of the anthracycline, the dose intensity is crucial in the therapy success. An RCT[34] reported significant improvements in CR, OS and event-free survival (EFS) using DNR doses of 90 mg/m2 compared to doses of 45 mg/m2.
The response-oriented individualized induction therapy is another
approach tested with IDA+CYT scheme without any advantage over the
standard scheme.[35] In addition, some specific AML
characteristics could modify the anthracycline response, such as
FLT3-ITD mutated patients which showed higher CR and survival with
high-dose DNR compared to standard-dose DNR or IDA.[36,37] These findings were reproduced in vitro in FLT3-ITD-mutated cell lines.[37] Unfortunately, we have not enough data to analyze the impact of this mutation in our cohort.
Despite the previous experiences of ex vivo drug testing with limited sensitivity[38-44], the PharmaFlow PM test aims to solve technical limitations including some novelties:[25]
a)
the use of whole BM sample, maintaining the native environment, which
has been hypothesized that it can influence the emergence of
resistance;[45-48]
b) the increase of the accuracy obtained modeling ex vivo activity with PD population models in one single step;[49]
c) the improvements in the measures performed by automated flow cytometry platform (PharmaFlow).
The correlation between in vitro and in vivo
therapy sensitivity of PharmaFlow PM test has been recently
demonstrated in a cohort of 123 AML patients after induction therapy
with CYT-IDA (most of these patients were also included in this study).[50]
This study achieved an 81% of overall accuracy in the correlations
between test predictions and hematological response, identifying with
success responders (CR/CR with incomplete recovery) in 93% of cases and
non-responders (partial remission/resistance) in 60% of cases. The
present study generates a theoretical role of PM tests in individual
anthracycline selection but does not provide enough data and critical
analyses to allow to translate their use in the routine clinical
practice.
Regarding the synergism between anthracyclines and CYT,
we observed a synergistic response with the three combinations,
especially with CYT-IDA and CYT-MIT. In a previous study, we also
reported a higher synergy with CYT-IDA and CYT-MIT combination and a
trend to an additive effect with CYT-DAU.[25]
Curiously, a novel approach in AML therapy is the use of the liposomal
formulation of CYT and DNR in a molar ratio concentration of 5:1, based
on a probable higher synergistic effect.[51,52]
Furthermore, the pairwise comparisons between combinations of CYT-IDA,
CYT-DNR, and CYT-MIT found in an 8.2% of patients synergy differences
>30%, probably associated to the interpatient variability in drug
sensibility observed in dose-response graphs.
Some limitations should be addressed in this study. First, this study analyzes the differences between ex vivo
sensitivities to three different anthracyclines combined with CYT in BM
samples of AML patients at diagnosis, but the correlation between ex vivo
responses and clinical response was not analyzed. Second, although the
incubation time was relatively short, additional transportation and
processing time could lead, in several patients, to a non-affordable
delay to start induction chemotherapy while receiving the test report.
Third, associations of the different in vitro
response of each anthracycline and specific characteristics of AML
(age, WBC, cytogenetic risk, FLT3-ITD, and NPM1 status, etc.) were not
analyzed. Finally, the findings reported are not yet validated in an
independent cohort.
Conclusions
The ex vivo
PharmaFlow PM test obtained in a 28.3% of the BM samples analyzed
overall differences in sensitivity to anthracyclines in monotherapy.
This test could allow designing a trial to explore a personalized
selection of anthracycline therapy in AML patients. A similar approach
is being tested in a clinical trial by PETHEMA group in relapsed or
refractory AML patients to select the salvage therapy based on the ex vivo
sensitivity to conventional chemotherapy agents. The role an adequate
selection in this subset of AML patients is critical because none of
the salvage regimens[53] has achieved outstanding CR rates, long-lasting remissions, and acceptable OS. Acknowledgments
We are grateful to all participating institutions and clinicians in the PETHEMA group, and all the patients included.
References
- Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum
FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL,
Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J,
Tallman MS, Tien HF, Wei AH, Löwenberg B, Bloomfield C. Diagnosis and
management of AML in adults: 2017 ELN recommendations from an
international expert panel. Blood. 2017;129(4):424-7. https://doi.org/10.1182/blood-2016-08-733196 PMid:27895058 PMCid:PMC5291965
- O'Donnell
MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA, Bhatt V,
Bixby D, Blum W, Coutre SE, De Lima M, Fathi AT, Fiorella M, Foran JM,
Gore SD, Hall AC, Kropf P, Lancet J, Maness LJ, Marcucci G, Martin MG,
Moore JO, Olin R, Peker D, Pollyea DA, Pratz K, Ravandi F, Shami PJ,
Stone RM, Strickland SA, Wang ES, Wieduwilt M, Gregory K, Ogba N. Acute
Myeloid Leukemia, Version 3.2017, NCCN Clinical Practice Guidelines in
Oncology. J Natl Compr Canc Netw 2017; 15:926-57. https://doi.org/10.6004/jnccn.2017.0116 PMid:28687581
- Petti
MC, Mandelli F. Idarubicin in acute leukemias: experience of the
Italian Cooperative Group GIMEMA. Semin Oncol 1989; 16:10-5.
PMid:2928805
- Berman E, Heller G, Santorsa
J, McKenzie S, Gee T, Kempin S, Gulati S, Andreeff M, Kolitz J,
Gabrilove J, et al. Results of a randomized trial comparing idarubicin
and cytosine arabinoside with daunorubicinand cytosine arabinoside in
adult patients with newly diagnosed acute myelogenous leukemia. Blood
1991; 77:1666-74. PMid:2015395
- Mandelli
F, Petti MC, Ardia A, Di Pietro N, Di Raimondo F, Ganzina F, Falconi E,
Geraci E, Ladogana S, Latagliata R, et al. A randomised clinical trial
comparing idarubicin and cytarabine to daunorubicin and cytarabine in
the treatment of acute non-lymphoid leukaemia. A multicentric study
from the Italian Co-operative Group GIMEMA. Eur J Cancer 1991;
27:750–5. https://doi.org/10.1016/0277-5379(91)90181-C
- Vogler
WR, Velez-Garcia E, Weiner RS, Flaum MA, Bartolucci AA, Omura GA,
Gerber MC, Banks PL. A phase III trial comparing idarubicin and
daunorubicin in combination with cytarabine in acute myelogenous
leukemia: A Southeastern Cancer Study Group study. J Clin Oncol 1992;
10:1103–11. https://doi.org/10.1200/JCO.1992.10.7.1103 PMid:1607916
- Wiernik
PH, Banks PLC, Case Jr DC, Arlin ZA, Periman PO, Todd MB, Ritch PS,
Enck RE, Weitberg AB. Cytarabine plus idarubicin or daunorubicin as
induction and consolidation therapy for previously untreated adult
patients with acute myeloid leukemia. Blood 1992; 79:313-9. PMid:1730080
- Reiffers
J, Huguet F, Stoppa AM, Molina L, Marit G, Attal M, Gastaut JA,
Michallet M, Lepeu G, Broustet A, Pris J, Maraninchi D, Hollard D,
Fabères C, Mercier M, Hurteloup P, Danel P, Tellier Z, Berthaud P. A
prospective randomized trial of idarubicin vs daunorubicin in
combination chemotherapy for acute myelogenous leukemia of the age
group 55 to 75. Leukemia 1996; 10(3):389-95. PMid:8642852
- Rowe
JM, Neuberg D, Friedenberg W, Bennett JM, Paietta E, Makary AZ,
Liesveld JL, Abboud CN, Dewald G, Hayes FA, Tallman MS, Wiernik PH;
Eastern Cooperative Oncology. A phase 3 study of three induction
regimens and of priming with GM-CSF in older adults with acute myeloid
leukemia: A trial by the Eastern Cooperative Oncology Group. Blood
2004; 103:479–85. https://doi.org/10.1182/blood-2003-05-1686 PMid:14512295
- Gardin
C, Turlure P, Fagot T, Thomas X, Terre C, Contentin N, Raffoux E, de
Botton S, Pautas C, Reman O, Bourhis JH, Fenaux P, Castaigne S,
Michallet M, Preudhomme C, de Revel T, Bordessoule D, Dombret H.
Postremission treatment of elderly patients with acute myeloid leukemia
in first complete remission after intensive induction chemotherapy:
Results of the multicenter randomized Acute Leukemia French Association
(ALFA) 9803 trial. Blood 2007; 109:5129–35. https://doi.org/10.1182/blood-2007-02-069666 PMid:17341661
- Mandelli
F, Vignetti M, Suciu S, Stasi R, Petti MC, Meloni G, Muus P, Marmont F,
Marie JP, Labar B, Thomas X, Di Raimondo F, Willemze R, Liso V, Ferrara
F, Baila L, Fazi P, Zittoun R, Amadori S, de Witte T. Daunorubicin
versus mitoxantrone versus idarubicin as induction and consolidation
chemotherapy for adults with acute myeloid leukemia: The EORTC and
GIMEMA groups study AML-10. J Clin Oncol 2009; 27:5397–403. https://doi.org/10.1200/JCO.2008.20.6490 PMid:19826132 PMCid:PMC2773224
- Pautas
C, Merabet F, Thomas X, Raffoux E, Gardin C, Corm S, Bourhis JH, Reman
O, Turlure P, Contentin N, de Revel T, Rousselot P, Preudhomme C,
Bordessoule D, Fenaux P, Terré C, Michallet M, Dombret H, Chevret S,
Castaigne S. Randomized study of intensified anthracycline doses for
induction and recombinant interleukin-2 for maintenance in patients
with acute myeloid leukemia age 50 to 70 years: Results of the ALFA-
9801 study. J Clin Oncol. 2010; 28:808–14. https://doi.org/10.1200/JCO.2009.23.2652 PMid:20048183
- Ohtake
S, Miyawaki S, Fujita H, Kiyoi H, Shinagawa K, Usui N, Okumura H,
Miyamura K, Nakaseko C, Miyazaki Y, Fujieda A, Nagai T, Yamane T,
Taniwaki M, Takahashi M, Yagasaki F, Kimura Y, Asou N, Sakamaki H,
Handa H, Honda S, Ohnishi K, Naoe T, Ohno R. Randomized study of
induction therapy comparing standard-dose idarubicin with high-dose
daunorubicin in adult patients with previously untreated acute myeloid
leukemia: The JALSGAML201 study. Blood 2011; 117:2358–65. https://doi.org/10.1182/blood-2010-03-273243 PMid:20693429
- Creutzig
U, Zimmermann M, Bourquin J-P, Dworzak MN, Fleischhack G, Graf N,
Klingebiel T, Kremens B, Lehrnbecher T, von Neuhoff C, Ritter J, Sander
A, Schrauder A, von Stackelberg A, Starý J, Reinhardt D. Randomized
trial comparing liposomal daunorubicin with idarubicin as induction for
pediatric acute myeloid leukemia: results from Study AML-BFM 2004.
Blood 2013; 122:37–43. https://doi.org/10.1182/blood-2013-02-484097 PMid:23704089
- Récher
C, Béné MC, Lioure B, Pigneux A, Vey N, Delaunay J, Luquet I, Hunault
M, Guyotat D, Bouscary D, Fegueux N, Jourdan E, Lissandre S,
Escoffre-Barbe M, Bonmati C, Randriamalala E, Guièze R, Ojeda-Uribe M,
Dreyfus F, Harousseau JL, Cahn JY, Ifrah N, Guardiola P; Groupe
Ouest-Est d' étude des Leucé mies Aiguës et autres. Long-term results
of a randomized phase 3 trial comparing idarubicin and daunorubicin in
younger patients with acute myeloid leukaemia. Leukemia 2014; 28:440–3.
https://doi.org/10.1038/leu.2013.290 PMid:24166215
- Pavlovsky
S, Gonzalez Llaven J, Sobrevilla P, Eppinger-Helft M, Marin A,
López-Hernández M, Fernandez I, Rubio ME, Ibarra S, et al. A randomized
study of mitoxantrone plus cytarabine versus daunomycin plus cytarabine
in the treatment of previously untreated adult patients with acute
nonlymphocytic leukemia. Ann Hematol 1994; 69:11-5. https://doi.org/10.1007/BF01757342 PMid:8061102
- Beksac
M, Arslan O, Koc H, Akan H, Ilhan O, Arat M, Ozcan M, Gürman G, Konuk
N, Uysal A. Randomised unicenter trial for comparison of three regimens
in de novo adult acute nonlymphoblastic leukaemia. Med Oncol 1998;
15:183–90. https://doi.org/10.1007/BF02821937 PMid:9819795
- Archimbaud
E, Jehn U, Thomas X, De Cataldo F, Fillet G, Belhabri A, Peaud PY,
Martin C, Amadori S, Willemze R. Multicenter randomized phase II trial
of idarubicin vs mitoxantrone, combined with VP-16 and cytarabine for
induction/consolidation therapy, followed by a feasibility study of
autologous peripheral blood stem cell transplantation in elderly
patients with acute myeloid leukemia. Leukemia 1999; 13:843–9. https://doi.org/10.1038/sj.leu.2401445 PMid:10360370
- Indrak
K, Hubacek J, Mayer J, Voglová J, Jarosová M, Krahulová M, Malý J,
Faber E, Penka M, Kmonícek M, Jebavý L, Szotkowski T, Knotková R, Hlusí
A, Zapletalová J. Comparison of the effectiveness of idarubicin
(Zavedos) and mitoxantrone (Refador) in induction therapy of acute
myeloid leukemia in elderly patients (55-75) (a prospective multicenter
randomized study conducted 1998-2000. Vnitr Lek 2001; 47:48–56.
PMid:11693063
- De Moerloose B, Suciu S,
Munzer, Piette C, Yakouben K, Margueritte G, Lutz P, Uyttebroeck A,
Rohrlich P, Ferster A, Boutard P, Dresse MF, Rialland X, Norton L,
Sirvent N, Karrasch M, Benoit Y, Bertrand Y. Similar efficacy and
toxicity profile for idarubicin and mitoxantrone in induction and
intensification treatment of children with acute myeloid leukemia (AML)
or myelodysplasia (MDS): Long-term results of the EORTC-CLG randomized
phase III trial 58921. Blood 2011; 118:Abstract 2615.
- Yates
J, Glidewell O, Wiernik P, Cooper MR, Steinberg D, Dosik H, Levy R,
Hoagland C, Henry P, Gottlieb A, Cornell C, Berenberg J, Hutchison JL,
Raich P, Nissen N, Ellison RR, Frelick R, James GW, Falkson G, Silver
RT, Haurani F, Green M, Henderson E, Leone L, Holland JF. Cytosine
arabinoside with daunorubicin or adriamycin for therapy of acute
myelocytic leukemia: a CALGB study. Blood 1982; 60:454-62. PMid:6953986
- Bezwoda
WR, Dansey RD. Idarubicin plus cytarabine versus doxorubicin plus
cytarabine in induction therapy for acute non-lymphoid leukaemia: A
randomized trial. Leuk Lymphoma 1990; 1:221–5. https://doi.org/10.3109/10428199009042483 PMid:27463989
- Li
X, Xu S, Tan Y, Chen J. The effects of idarubicin versus other
anthracyclines for induction therapy of patients with newly diagnosed
leukaemia. Cochrane Database Syst Rev 2015; (6):CD010432. https://doi.org/10.1002/14651858.CD010432.pub2
- Schrag
D, Garewal HS, Burstein HJ, Samson DJ, Von Hoff DD, Somerfield MR; ASCO
Working Group on Chemotherapy Sensitivity and Resistance Assays.
American Society of Clinical Oncology Technology Assessment:
chemotherapy sensitivity and resistance assays. J Clin Oncol 2004;
22:3631-8 https://doi.org/10.1200/JCO.2004.05.065 PMid:15289488
- Bennett
TA, Montesinos P, Moscardo F, Martinez-Cuadron D, Martinez J, Sierra J,
García R, de Oteyza JP, Fernandez P, Serrano J, Fernandez A, Herrera P,
Gonzalez A, Bethancourt C, Rodriguez-Macias G, Alonso A, Vera JA, Navas
B, Lavilla E, Lopez JA, Jimenez S, Simiele A, Vidriales B, Gonzalez BJ,
Burgaleta C, Hernandez Rivas JA, Mascu-ano RC, Bautista G, Perez Simon
JA, Fuente Ade L, Rayón C, Troconiz IF, Janda A, Bosanquet AG,
Hernandez-Campo P, Primo D, Lopez R, Liebana B, Rojas JL, Gorrochategui
J, Sanz MA, Ballesteros J. Pharmacological profiles of acute myeloid
leukemia treatments in patient samples by automated flow cytometry: a
bridge to individualized medicine. Clin Lymphoma Myeloma Leuk 2014;
14:305-318. https://doi.org/10.1016/j.clml.2013.11.006 PMid:24468131
- Vardiman
JW, Harris NL, Brunning RD. The World Health Organization (WHO)
classification of the myeloid neoplasms. Blood 2002; 100:2292-302. https://doi.org/10.1182/blood-2002-04-1199 PMid:12239137
- Upton
RN, Mould DR. Basic concepts in population modeling, simulation, and
model-based drug development: part 3-introduction to pharmacodynamic
modeling methods. CPT Pharmacometrics Syst Pharmacol 2014; 3:e88. https://doi.org/10.1038/psp.2013.71 PMid:24384783 PMCid:PMC3917320
- Beal SL, Sheiner LB, Boeckmann AJ, et al. NONMEM Users Guides. Ellicot City, Maryland, Icon Development Solutions, 1989-2001
- Greco
WR, Bravo G, Parsons JC. The search for synergy: a critical review from
a response surface perspective. Pharmacol Rev 1995; 47:331-385.
PMid:7568331
- Wood SN. Generalized Additive Models. An Introduction with R. Boca Raton, Florida, Chapman & Hall/CRC, 2006 https://doi.org/10.1201/9781420010404
- Cheesman S, Shields A; London Cancer North and East. Maximum Anthracycline Doses Guidance. 2016. Available: http://www.londoncancer.org/media/75901/140214-Maximum-Anthracycline-doses-Guideline-v1.pdfm
- Labar
B, Nemet D, Minigo H, Bogdanić V, Jaksić B, Malesević M, Mrsić M.
Aclarubicin in the treatment of de-novo acute myelocytic leukaemia.
Bone Marrow Transplant 1989; 4 Suppl 3:45-6. PMid:2697400
- Büchner
T, Hiddemann W, Blasius S, Koch P, Maschmeyer G, Tirier C, Sodomann H,
Kuse R, Thiel E, Ludwig WD, et al. Adult AML: the role of chemotherapy
intensity and duration. Two studies of the AML Cooperative Group.
Haematol Blood Transfus. 1990; 33:261-6. https://doi.org/10.1007/978-3-642-74643-7_47
- Lee
JH, Joo YD, Kim H, Bae SH, Kim MK, Zang DY, Lee JL, Lee GW, Lee JH,
Park JH, Kim DY, Lee WS, Ryoo HM, Hyun MS, Kim HJ, Min YJ, Jang YE, Lee
KH; Cooperative Study Group A for Hematology. A randomized trial
comparing standard versus high-dose daunorubicin induction in patients
with acute myeloid leukemia. Blood 2011; 118:3832-41. https://doi.org/10.1182/blood-2011-06-361410 PMid:21828126
- Ohtake
S, Miyawaki S, Kiyoi H, Miyazaki Y, Okumura H, Matsuda S, Nagai T,
Kishimoto Y, Okada M, Takahashi M, Handa H, Takeuchi J, Kageyama S,
Asou N, Yagasaki F, Maeda Y, Ohnishi K, Naoe T, Ohno R. Randomized
trial of response-oriented individualized versus fixed-schedule
induction chemotherapy with idarubicin and cytarabine in adult acute
myeloid leukemia: the JALSG AML95 study. Int J Hematol 2010; 91:276-83.
https://doi.org/10.1007/s12185-009-0480-5 PMid:20054669
- Lee
JH, Kim H, Joo YD, Lee WS, Bae SH, Zang DY, Kwon J, Kim MK, Lee J, Lee
GW, Lee JH, Choi Y, Kim DY, Hur EH, Lim SN, Lee SM, Ryoo HM, Kim HJ,
Hyun MS, Lee KH; Cooperative Study Group A for Hematology. Prospective
Randomized Comparison of Idarubicin and High-Dose Daunorubicin in
Induction Chemotherapy for Newly Diagnosed Acute Myeloid Leukemia. J
Clin Oncol. 2017; 35(24):2754-63. https://doi.org/10.1200/JCO.2017.72.8618 PMid:28632487
- Choi
EJ, Lee JH, Lee JH, Park HS, Ko SH, Hur EH, Moon J, Goo BK, Kim Y, Seol
M, Lee YS, Kang YA, Jeon M, Woo JM, Lee KH. Comparison of
anthracyclines used for induction chemotherapy in patients with
FLT3-ITD-mutated acute myeloid leukemia. Leuk Res. 2018; 68:51-6. https://doi.org/10.1016/j.leukres.2018.03.006 PMid:29544132
- Staib
P, Staltmeier E, Neurohr K, Cornely O, Reiser M, Schinköthe T.
Prediction of individual response to chemotherapy in patients with
acute myeloid leukaemia using the chemosensitivity index Ci. Br J
Haematol 2005; 128:783-91. https://doi.org/10.1111/j.1365-2141.2005.05402.x PMid:15755281
- Pemovska
T, Kontro M, Yadav B, Edgren H, Eldfors S, Szwajda A, Almusa H,
Bespalov MM, Ellonen P, Elonen E, Gjertsen BT, Karjalainen R, Kulesskiy
E, Lagström S, Lehto A, Lepistö M, Lundán T, Majumder MM, Marti JM,
Mattila P, Murumägi A, Mustjoki S, Palva A, Parsons A, Pirttinen T,
Rämet ME, Suvela M, Turunen L, Västrik I, Wolf M, Knowles J,
Aittokallio T, Heckman CA, Porkka K, Kallioniemi O, Wennerberg K.
Individualized systems medicine strategy to tailor treatments for
patients with chemorefractory acute myeloid leukemia. Cancer Discov
2013; 3:1416-29. https://doi.org/10.1158/2159-8290.CD-13-0350 PMid:24056683
- Jun
KR, Jang S, Chi HS, Lee KH, Lee JH, Choi SJ, Seo JJ, Moon HN, Im HJ,
Park CJ. Relationship between in vitro chemosensitivity assessed with
MTT assay and clinical outcomes in 103 patients with acute leukemia.
Korean J Lab Med 2007; 27:89-95. https://doi.org/10.3343/kjlm.2007.27.2.89 PMid:18094557
- Pierceall
WE, Kornblau SM, Carlson NE, Huang X, Blake N, Lena R, Elashoff M,
Konopleva M, Cardone MH, Andreeff M. BH3 profiling discriminates
response to cytarabine-based treatment of acute myelogenous leukemia.
Mol Cancer Ther 2013; 12:2940-9. https://doi.org/10.1158/1535-7163.MCT-13-0692 PMid:24092807 PMCid:PMC3881173
- Yamada
S, Hongo T, Okada S, Watanabe C, Fujii Y, Ohzeki T. Clinical relevance
of in vitro chemoresistance in childhood acute myeloid leukemia.
Leukemia 2001; 15:1892-7. https://doi.org/10.1038/sj.leu.2402305 PMid:11753610
- Bosanquet
AG, Nygren P, Weisenthal LM, et al. Individualized tumor response
testing in leukemia and lymphoma., in Kaspers GJ, Coiffier B, Heinrich
MC, et al. editors: Innovative leukemia and lymphoma therapy. New York
(NY) Informa Healthcare, 2008:23-44.
- Norgaard
JM, Langkjer ST, Palshof T, Pedersen B, Hokland P. Pretreatment
leukaemia cell drug resistance is correlated to clinical outcome in
acute myeloid leukaemia. Eur J Haematol 2001; 66:160-7. https://doi.org/10.1034/j.1600-0609.2001.00361.x PMid:11350484
- Sison
EA, Brown P. The bone marrow microenvironment and leukemia: biology and
therapeutic targeting. Expert Rev Hematol 2011; 4:271-83. https://doi.org/10.1586/ehm.11.30 PMid:21668393 PMCid:PMC3131221
- Tabe Y, Konopleva M. Role of Microenvironment in Resistance to Therapy in AML. Curr Hematol Malig Rep 2015; 10:96-103. https://doi.org/10.1007/s11899-015-0253-6 PMid:25921386 PMCid:PMC4447522
- Zahreddine H, Borden KL. Mechanisms and insights into drug resistance in cancer. Front Pharmacol 2013; 4:28. https://doi.org/10.3389/fphar.2013.00028 PMid:23504227 PMCid:PMC3596793
- Li ZW, Dalton WS. Tumor microenvironment and drug resistance in hematologic malignancies. Blood Rev 2006; 20:333-42. https://doi.org/10.1016/j.blre.2005.08.003 PMid:16920238
- Quartino
A, Karlsson MO, Freijs A, Jonsson N, Nygren P, Kristensen J, Lindhagen
E, Larsson R. Modeling of in vitro drug activity and prediction of
clinical outcome in acute myeloid leukemia. J Clin Pharmacol 2007;
47:1014-21. https://doi.org/10.1177/0091270007302563 PMid:17660484
- Martínez-Cuadrón
D, Gil C, Serrano J, Rodríguez G, Pérez-Oteyza J, García-Boyero R,
Jiménez-Bravo S, Vives S, Vidriales MB, Lavilla E, Pérez-Simón JA,
Tormo M, Colorado M, Bergua J, López JA, Herrera P, Hernández-Campo P,
Gorrochategui J, Primo D, Rojas JL, Villoria J, Moscardó F, Troconiz I,
Linares Gómez M, Martínez-López J, Ballesteros J, Sanz M, Montesinos P;
Spanish PETHEMA group. A precision medicine test predicts clinical
response after idarubicin and cytarabine induction therapy in AML
patients. Leuk Res. 2018;76:1-10. https://doi.org/10.1016/j.leukres.2018.11.006 PMid:30468991
- Kim
HP, Gerhard B, Harasym TO, Mayer LD, Hogge DE. Liposomal encapsulation
of a synergistic molar ratio of cytarabine and daunorubicin enhances
selective toxicity for acute myeloid leukemia progenitors as compared
to analogous normal hematopoietic cells. Exp Hematol 2011; 39:741-50. https://doi.org/10.1016/j.exphem.2011.04.001 PMid:21530609
- Lancet
JE, Cortes JE, Hogge DE, Tallman MS, Kovacsovics TJ, Damon LE, Komrokji
R, Solomon SR, Kolitz JE, Cooper M, Yeager AM, Louie AC, Feldman EJ.
Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of
cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults
with untreated AML. Blood 2014; 123:3239-46. https://doi.org/10.1182/blood-2013-12-540971 PMid:24687088 PMCid:PMC4624448
- Megías-Vericat
JE, Martínez-Cuadrón D, Sanz MA, Montesinos P. Salvage regimens using
conventional chemotherapy agents for relapsed/refractory adult AML
patients: a systematic literature review. Ann Hematol 2018; 97:1115-53.
https://doi.org/10.1007/s00277-018-3304-y PMid:29680875
[TOP]