Naser Al-Husban1, Nathir Obeidat2, Oqba Al-Kuran1, Khaled Al Oweidat2 and Faris Bakri3.
1 The
Obstetrics and Gynecology Department, School of medicine, University of
Jordan and Jordan University Hospital, Amman, Jordan.
2 The Respiratory Division, Internal Medicine Department, The School of Medicine, University of Jordan, Amman, Jordan.
3 The
Infectious Disease and Vaccine center and Department of Internal
Medicine. The School of medicine, University of Jordan, Amman, Jordan.
Correspondence to:Dr Naser Al-Husban
MD, FRCOG, Assistant Professor, Faculty of Medicine, University of
Jordan, Amman, Jordan. P O Box 2194, Amman 11941, Jordan. Mobile;
+962-772086080, Fax: +96264643217. E-mail;
Husban48@yahoo.com
Published: March 1, 2019
Received: November 11, 2018
Accepted: January 16, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019020 DOI
10.4084/MJHID.2019.020
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objective:
H1N1 infection carries an increased risk in pregnancy. Our aim was to
study the feto-maternal outcome and the effect of early initiation of
therapy.
Methods: This
is a retrospective descriptive study. Confirmed infected cases were
included. Maternal age, parity, gestational age at diagnosis,
presenting symptoms, the time between presentation and starting
therapy, ICU admission, and maternal and perinatal outcome were
evaluated.
Results:
Nineteen confirmed patients were included. Most patients are 31 years
old or more. Multiparous patients were 73.68%, and 57.89% were in the
third trimester. Most of our patients presented with cough, fever, and
chills. Two patients were admitted to the ICU. One of them was a case
of maternal mortality. 42.10% of patients were started on therapy only
one day after the clinical onset of symptoms. 26.31% delivered before
37 completed weeks. 73.68% delivered beyond term. Around one third
delivered vaginally. 45% of babies weighed more than 3 kg. Four babies
weighed less than 2 kg. Ninety percent had APGAR scores more than 8 at
1 and 5 minutes after delivery. Twenty-five percent were admitted to
the NICU with no neonatal mortalities.
Conclusions:
H1N1 influenza A infection in pregnancy is associated with adverse
maternal and perinatal outcomes. Medical and public awareness, low
threshold for testing suspected pregnant patients, very early
initiation of antiviral therapy, and a multidisciplinary approach in
our series decreased the overall adverse effects of this infection.
|
Introduction
Influenza
A viruses are classified based on the viral surface proteins,
hemagglutinin (HA or H) and neuraminidase (NA or N) such as H1N1, H5N1.
Influenza-like illness caused by a new H1N1 strain (Swine flu) was
reported from Mexico in April 2009 and rapidly spread to all the
continents. It appeared to be associated with high mortality. By May
2009, data from the USA and elsewhere showed that its virulence was
considerably less than that initially reported in Mexico.[1] There are confirmed cases in Jordan during most seasons both in pregnant and non-pregnant patients.
Data
obtained from many countries revealed that old patients seemed to be
relatively protected from getting infected. There are, however, certain
other vulnerable groups of patients.[2,3,4,5] They are
the same groups that are more vulnerable during seasonal
influenza—those with underlying heart disease, lung disease, etc. The
unexpected, much higher risk group was pregnancy. Pregnant women had a
hospitalization and death rate up to 10 times higher compared to other
females in the same age group.[3,5,6]
These data lend support to the present recommendation to promptly treat
pregnant women with H1N1 influenza virus infection with anti-influenza
drugs.[5]
Reports from the past pandemics
(1918-1919) and 2009 outbreaks showed that pregnant women are at risk
of complications from the disease.[7] Pregnancy stage
also modified the association between influenza activity and
influenza-like illness episodes. Findings estimate that 20-43
pregnant/postpartum women need to be vaccinated with an 80% effective
vaccine to prevent one influenza-like illness episode.[7]
In addition, pregnant women are prone to complications such as
pneumonia and adult respiratory distress syndrome (ARDS) because the
maternal immune system is modified to accommodate the developing fetus,
the gravid uterus elevates the diaphragm, and they have congestion and
local edema.[8]
There was a 4- times higher rate of hospital admissions in pregnant women compared to the general population.[5]
Among patients with H1N1 virus infection, pregnant women accounted for
6-9% of the intensive care unit (ICU) admissions and 6-10% of patients
who died. The risk of death is particularly increased in infected women
during the third trimester.[5,9,10]
The excess risk may be limited to women infected in the third trimester
and the first four weeks postpartum; however, available data are of low
quality.[11]
Our study is a retrospective
descriptive case series that evaluates the clinical course, the effects
of various maternal characteristics, and the impact of the timing of
antiviral therapy on feto-maternal outcome in H1N1 infected pregnant
patients.
Materials and Methods
This
is a retrospective descriptive study at the maternal unit in Jordan
University Hospital, which is a tertiary referral hospital in Amman,
Jordan. We studied positive cases of H1N1 infection in pregnancy and up
to 6 weeks postpartum in the period January 2017 to January 2018. All
patients who presented to the emergency obstetric unit or the antenatal
clinics suspected of having the H1N1 infection were tested by taking
throat (pharyngeal) or nasal swabs (special H1N1 swabs), or tracheal
aspirate for intubated patients and sent to the laboratory. The
indications for H1N1 testing were fever (oral temperature of more than
38°C), cough, sore throat, and shortness of breath. Our laboratory used
real time reverse transcriptase polymerase chain reaction (RT PCR) for
testing H1N1. During the study period, 68 pregnant patients were
tested. 19 patients were confirmed to have H1N1 infection (wherein a
confirmed case was defined as an acute respiratory illness with
laboratory-confirmed H1N1 virus infection by RT PCR). Once a positive
diagnosis was obtained, patients were admitted and isolated. All
persons who enter the patients’ room put on effective masks, protective
surgical gowns, gloves, head caps and shoe covers. The number of
visitors allowed was restricted. Wasteproducts were considered
biohazards and were disposed of as per hospital policy. All infected
patients were further investigated with complete blood count (CBC),
kidney function test (KFT), urinalysis and culture, blood culture, and
liver function test (LFT). All patients were evaluated for fetal
wellbeing using an ultrasound scan (U/S) &/or cardiotocography
(CTG) according to their gestational age. All patients were evaluated
by the respiratory and infection teams at our hospital. Chest
radiograph (CXR) was only performed in selected critical cases as
judged by the respiratory consultants. Patients were then closely
monitored by vital signs and pulse oximeters. All but one confirmed
cases were given neuraminidase inhibitor, Oseltamivir, 75 mg twice
daily for 5 days in addition to several other supportive medications
(bronchodilators, nasal decongestants, and oxygen therapy). All
patients were also started on antibiotics, oral or intra-venous, as
dictated by their clinical condition. Data was collected using our
hospital’s electronic and paper-based systems. The demographic data
were obtained for these patients. The following details were noted and
studied: maternal age, parity, gestational age at presentation and
diagnosis, coexisting medical diseases, presenting symptoms, signs,
chest radiographs, time between presentation and starting therapy, need
for oxygen, ICU admission, gestational age at delivery, mode of
delivery, birth weight, APGAR score, premature delivery, stillbirth,
neonatal ICU (NICU) admission and maternal mortality.
Results
Jordan
University Hospital is a tertiary teaching hospital in the capital of
Jordan, Amman. It has an annual delivery rate of 4,600. These cases
were collected between January 2017 and January 2018.
A total of
243 non-pregnant patients and a total of 68 pregnant patients were
tested for H1N1 influenza A based on their presenting symptoms. Among
the pregnant population, 27 patients (39.70%) were found to be positive
for the H1N1.
None of the confirmed cases received the seasonal
flu vaccine or specific A/H1N1 2009 influenza vaccine that season
(starting September 2017). There were five patients who missed their
antenatal care and/or did not deliver at our unit. Every effort was
done to obtain further information about their antenatal course,
delivery and neonatal outcome, including telephone calls. Besides,
there were three patients who, after obtaining the positive result for
H1N1 infection in the second trimester, denied and refused admission
and treatment. We could not obtain any further data concerning their
pregnancy and delivery.
A total of nine of our patients (47.37%)
are of age 31 years or older. Only one patient was less than 20 years
of age. The vast majority (14 patients, 73.68%) were multiparous
patients. There was one case of twin pregnancy. Eleven patients
(57.89%) were infected in the third trimester (57.89%), six patients
(31.6%) in the second trimester, one patient (5.3%) in the first
trimester and one (5.3%) in the postpartum period. (Table 1).
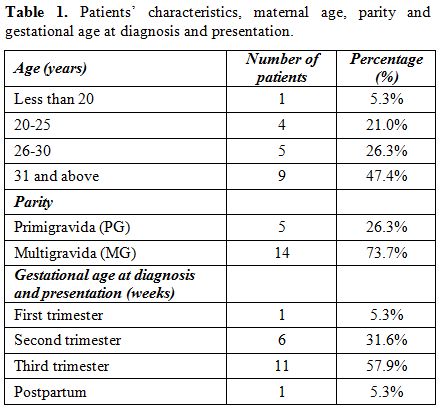 |
Table 1. Patients’ characteristics, maternal age, parity and gestational age at diagnosis and presentation. |
Most
of our patients presented with cough, fever, and chills; 17, 16 and 13
patients, respectively. Other causes of fever were excluded by
urinalysis and urine and blood cultures. There were also a variety of
symptoms including a runny nose, generalized fatigue, shortness of
breath, sore throat, sputum, headache, myalgia, and vomiting.
Documented fever at presentation was a prominent sign in our patients
(14 patients, 73.68%). There are also other physical signs including
tachycardia, pharyngitis, wheezes, hypoxia and decreased air entry. One
patient had sinus bradycardia (at a rate of 36-40 beats/minute with
normal echocardiogram and thyroid function) which was relieved two days
after starting antiviral therapy, and another patient had hemoptysis (Table 2).
Chest radiographs were deemed necessary in 3 patients dictated by their
clinical situation. The first of them had mild atelectasis, the second
had diffuse bilateral ground-glass opacification and pleural effusion,
and was admitted to the ICU. She later underwent an urgent cesarean
section due to severe respiratory distress and hypoxia. The third
patient had severe infiltration and opacification, and she passed away
in the ICU.
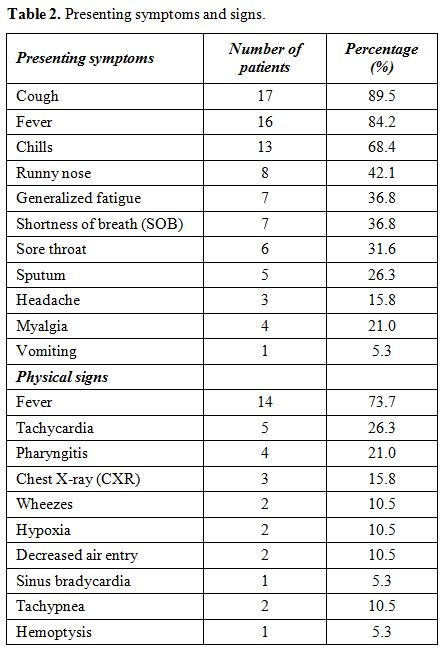 |
Table
2. Presenting symptoms and signs. |
Eight
patients (42.10%) were started on Oseltamivir therapy, 75 mg twice
daily, only one day after the clinical onset of symptoms and they were
the patients with no feto-maternal complications. Two patients started
antiviral therapy five days after the onset of symptoms (Table 3).
Both were admitted to the ICU, and one of them passed away. The
deceased patient was on Zanamivir inhalation instead of Oseltamivir
because she was ventilated.
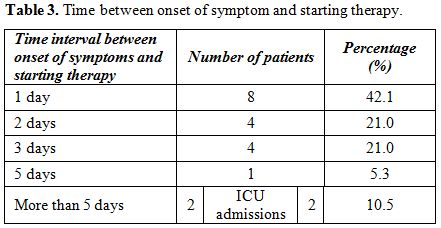 |
Table 3. Time between onset of symptom and starting therapy. |
Fifteen
patients (78.95%) completed a 5-day course of Oseltamivir. Two patients
received six days of therapy. Another two patients received seven days
of Oseltamivir. At the discretion of the respiratory and infectious
consultants, one of them received the last two days as an outpatient at
home.
Six patients (31.58%) needed oxygen therapy, and 2 (10.53%) were admitted to the intensive care unit (ICU).
Except the two patients who were admitted to the ICU, the earliest delivery was two weeks after the confirmed H1N1 infection.
The
first ICU admission was the case of a 41-year-old lady who had
essential hypertension, and a history of ICU admission due to a
penicillin allergy. She had three children and one miscarriage,
P3+1(previous three caesareans). She presented at 38+1 weeks for urgent
cesarean section due to a non-reassuring CTG. The cesarean section went
uneventfully with the delivery of a healthy male fetus weighing 3.7 kg
with 1-minute APGAR score of 8/9. On the first postoperative day, she
started to complain of SOB, cough, and a fever. She was started on
antibiotics and went home on the second postoperative day. She
presented on the 3rd postoperative
day with severe respiratory distress and hypoxia. Her chest radiograph
showed severe infiltration and opacification (chest radiographs 1-4, shown).
She was admitted to the ICU and intubated. H1N1 influenza infection was
suspected, so a tracheal aspirate was taken and was positive for H1N1
(she was the first case in the hospital to be diagnosed with H1N1
infection). A total of 6 days were between the initial presentation and
the initiation of therapy. She was then intubated, and over a
deteriorating course of 15 days with multi-organ failure, she passed
away.
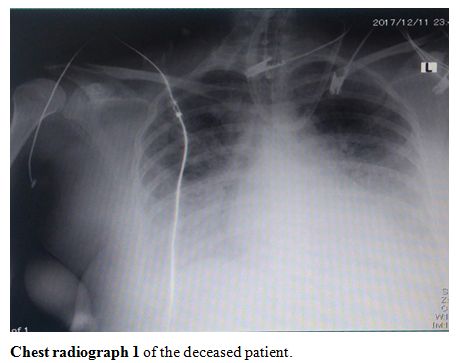 |
Chest radiograph 1 of the deceased patient. |
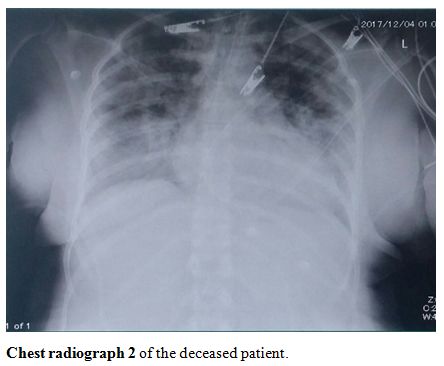 |
Chest radiograph 2 of the deceased patient. |
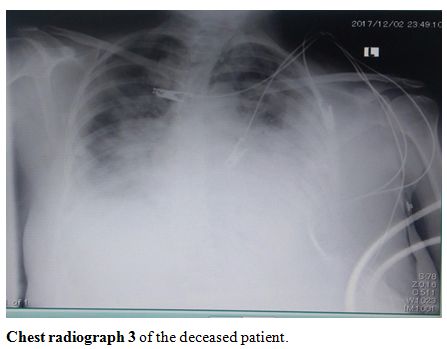 |
Chest radiograph 3 of the deceased patient |
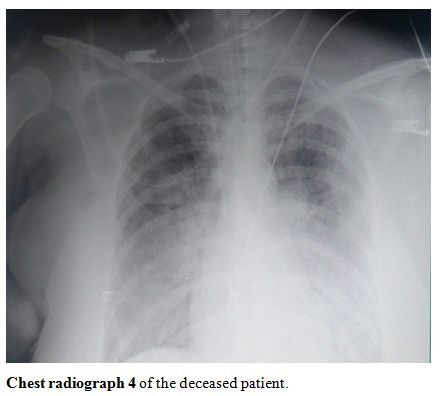 |
Chest radiograph 4 of the deceased patient. |
The
second patient admitted to the ICU was a 32-year-old lady, P3 (all were
NVD). She has a history of seasonal allergy and angioedema. She was
also admitted to the ICU and intubated ten months before her current
pregnancy due to pulmonary hemorrhage, implying a probable residual
lung disease or damage. She was confirmed to have H1N1 infection at 30
weeks +3 days gestation. She presented with a dry cough, shortness of
breath, palpitations, headache, chills, generalized fatigue, myalgia,
and hemoptysis. Her physical examination revealed tachycardia (130
beats/minute), wheezes and hypoxia. She was diagnosed to have H1N1
infection with a secondary bacterial infection and started on
Oseltamivir, nebulizers, and antibiotics three days after her onset of
symptoms. Her CXR showed diffuse bilateral ground-glass opacification
and pleural effusion. In the ICU she developed anemia and was given a
blood transfusion. She developed severe respiratory distress with
hypoxia at 33 weeks and underwent urgent cesarean section under
epidural anesthetic with an outcome of an alive baby weighing 2
kilograms (Kg) and an APGAR score of 8/9. The baby was admitted to the
NICU due to prematurity. She was kept in the ICU. Her respiratory
condition started to improve and continued improving over the
postoperative course. She recovered well and was discharged with her
baby in good condition.
Except these two patients, all other
patients delivered 2 or more weeks after their infection; babies were
not separated from them and they were encouraged to breastfeed. A total
of 5 patients (26.31%) delivered before the 37 weeks were completed.
Most of our patients (73.68%) delivered beyond term. 36.84% of our
patients delivered vaginally while 12 patients (63.16%) were delivered
by cesarean section, most of them were elective caesareans (Table 4).
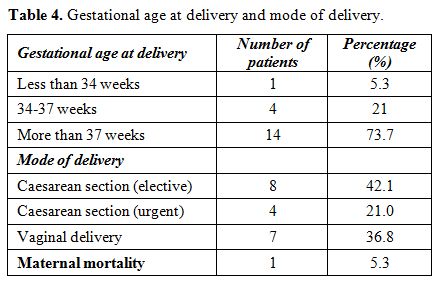 |
Table
4. Gestational age at delivery and mode of delivery. |
Nine
babies out of 20, (45%) weighed more than 3 kg. Four babies (20%), 2 of
which were twins, weighed less than 2 kg. 18 babies (90%) had APGAR
scores more than 8 at 1 and 5 minutes after delivery. Five babies (25%)
were admitted to the NICU (Table 5).
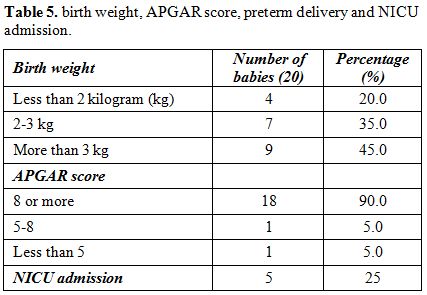 |
Table 5. Birth weight, APGAR score, preterm delivery and NICU admission. |
One
patient was pregnant with twins and delivered three weeks after H1N1
admission (admitted at 28+6 weeks, delivered at 35+4 weeks) by cesarean
section due to intra-uterine growth restriction (IUGR), decreased
liquor and decreased fetal movement and spontaneous decelerations on
CTG. She started therapy three days after the onset of her symptoms.
The babies weighed 1.5, and 1.51 kg and both were admitted to the NICU.
The 4th baby who was admitted to the
NICU was delivered at 34 weeks due to fetal distress during induction
of labor because of oligohydramnios. The mother was H1N1 infected at
28+3 weeks, with 2 days between onset and therapy. The baby weighed 1.7
kg and was admitted for one week. The same baby was admitted again 2
weeks after discharge due to a chest infection. One term baby, who was
delivered at 38 weeks, vaginally, in a private hospital, weighing 2.6
kg with an APGAR score of 5, was admitted to the NICU for 5 days due to
hypoxia and wheezes. The detailed diagnostic workup is not available.
The baby was discharged and now is doing very well. The mother was
infected at 19 +2 weeks, with 3 days between onset and therapy. Discussion
Around half of our patients were above 31 years old, and more than half of them were infected in the second trimester. Prabhu,[12]
however, found that 90.6% of H1N1 infected pregnant patients in the
series were in the age group between 21 and 29 years, 65.6% were
primigravidae, and 87.5% were diagnosed in the third trimester.
According to Siston AM et al.,[23] third-trimester infection was associated with the highest death compared with first and second trimesters.
The
three most common clinical symptoms in our patients were cough, fever,
and chills. These were moderate symptoms. Jamieson DJ et al.,[5] Louie JK, et al.[10] and Hewagama S et al.[16] reported similar results.
Shortness
of breath, hemoptysis, hypoxia, wheezes, tachypnea, and decreased air
entry were found in patients with severe symptoms who required chest
radiographs with positive findings, oxygen therapy and intubation with
intensive management. Liu L et al.,[17] reported that
in a general population, critical cases were associated with severe
hypoxemia, multisystem organ failure, and a requirement for mechanical
ventilation.
94.73% of our patients were hospitalized to complete
their minimum of a 5-day course, and two patients (10.53%) were
admitted to the ICU. One patient with mild symptoms declined
hospitalization and completed her therapy at home with no
complications. There was only one maternal death (5.26%). She
deteriorated very quickly and, because of the low index of suspicion of
the H1N1 infection, 6 days passed before confirming a diagnosis and
starting therapy. The delay in initiating therapy, the urgent cesarean
delivery due to fetal distress, and the postpartum status were the most
probable contributing factors to her death, as she had only a history
of essential hypertension and penicillin allergy as co-existing
clinical conditions. After that case, and because of public and medical
staff fear and awareness, particularly in pregnant women, the threshold
to test patients for the H1N1 was lowered, and therefore most our
patients (42.10%) were diagnosed and started on therapy within one day
of their disease onset. The rest of our patients started therapy within
5 days of onset of symptoms, except the maternal mortality case and the
other patient who was admitted to the ICU (more than 5 days). Meijer WJ
et al.[18] reviewed and judged 294 reports according
to the STROBE guidelines or CONSORT statement. In all, 100 studies,
published between 1961 and 2015, were included and reported that,
compared to the general population, pregnant women are more often
hospitalized and admitted to an intensive care unit due to influenza
virus infection. Our approach of early testing and initiation of
therapy in confirmed cases contributed significantly to the good
outcome, a lower rate of ICU admissions, less need for oxygen (only 4
excluding the ICU patients) and low complications in our series. Early
treatment with Oseltamivir is associated with a reduced risk of severe
disease.[18] Heba V et al.[19]
found that initiation of oseltamivir within 48 hours of symptom onset
was associated with fewer complications in patients hospitalized with
2009 influenza A (H1N1). Viasus D et al.[20] reported
that timely oseltamivir administration has a beneficial effect on
outcomes in hospitalized adults with A (H1N1), even in those who are
admitted beyond 48 h after onset of symptoms. Higuera Iglesias AL et
al.[21] found that earlier initiation of Oseltamivir
therapy, even when initiated more than 48 hours after the onset of
symptoms, significantly reduced occurrence and severity of pneumonia
and shortened hospitalization in pandemic H1N1 2009. Based on these
results, patients affected by future influenza pandemics would benefit
from early therapy.
In a systematic review and meta-analysis of observational studies, Dominik Mertz et al.,[22]
found that in influenza infection, pregnancy is associated with a
higher risk of hospital admission than non-pregnant individuals with
similar risk of mortality. This increased susceptibility was described
for various pathogens including H1N1 influenza virus.[5]
Changes in the immune, cardiac and respiratory systems are the likely
reasons that pregnant women are at increased risk for severe illness
with influenza.[14,15] These facts were taken into account during the management approach of our patients.
The only patient with significant co-existing medical diseases was the previously mentioned 2nd
ICU patient. No other cases had significant medical problems. This fact
could have contributed to the good outcome. Jamieson DJ et al.[5]
mentioned the reporting of six deaths in pregnant women to the CDC. All
were in women who had developed pneumonia and subsequent acute
respiratory distress syndrome requiring mechanical ventilation.[5]
Among the 788 pregnant patients in the USA with 2009 Influenza A (H1N1)
infection, 30 died (3.8%). Patients who started their therapy more than
4 days after disease onset were more likely to be admitted to an ICU.
Authors concluded that early treatment was associated with fewer ICU
admissions and fewer deaths.[23] Fatima S Dawood et al.[24]
found that the estimate of respiratory and cardiovascular mortality
associated with the 2009 pandemic was 15 times higher than the reported
laboratory-confirmed deaths. There are several risk factors and medical
conditions that increase the severity, complications, admission to the
ICU and death among H1N1 infection.[10,25,26,27]
Although
31.58% of our patients were infected in the second trimester and 5.26%
in the first trimester, only 5.26% delivered before 34 weeks. Only
21.05% delivered between 34 and 37 weeks and most patients (73.68%)
delivered after 37 weeks. The increased overall prematurity rate of
26.31% could be caused by H1N1 infection. Two patients who were
infected around 28 weeks (one at 28 weeks plus 3 days and the other at
28 weeks plus 6 days) developed decreased liquor and IUGR and underwent
cesarean section due to fetal distress at 34 weeks and 35 weeks plus 4
days. This rate is much higher than reported in the general population.[28]
There were no cases of intra-uterine fetal death (IUFD) or stillbirths
in our cases. NICU admission in our series was 25%. Pierce M et al.,[29]
found that perinatal mortality is increased due to an increased rate of
stillbirth, increased prematurity, increased rate of NICU admission due
to secondary pneumonia. Overall around two-thirds of our patients were
delivered by cesarean section mostly elective obstetric indications.
The mode of delivery was dictated by obstetric reasons except one
patient who underwent a caesarean section because of severe respiratory
compromise.
Forty-five percent of babies weighed more than 3 kg.
Four babies (20%), 2 of them were twins, weighed less than 2 kg. The
newborn babies were well off as indicated by an APGAR score of more
than 8 at 1 and 5 minutes after delivery in 90% of babies. Fell DB et
al.[30] in a systematic meta-analysis of comparative
studies found that in the subgroup of the highest-quality studies two
reported significantly increased preterm birth following severe 2009
pandemic H1N1 (pH1N1) influenza illness, whereas those assessing
mild-to-moderate pH1N1 or seasonal influenza found no association. They
found no association with small for gestational age (SGA). They
concluded that comparative studies of preterm birth, SGA birth and
fetal death following maternal influenza disease are limited in number
and quality. An association between severe pH1N1 disease and preterm
birth and fetal death was reported by several studies; however, these
limited data do not permit firm conclusions on the magnitude of any
association.[30] There was one case of mortality in our report. William L Callaghan et al.,[31]
found that 12 pregnancy-related deaths could be due to possible or
confirmed H1N1 infection in the 2009-2010 pandemic. A CDC study of 347
pregnant women found that prompt use of antiviral drugs during the 2009
H1N1 influenza pandemic improved survival among severely ill pregnant
women.[32] Neuraminidase inhibitors (NI) are likely
to reduce mortality in hospitalized patients and are effective at
reducing secondary symptomatic influenza transmission.[33]
Because pregnancy is a high rather risk situation, all our patients
were given NI, even with the potential significant side effects. The US
Centres for Disease Control and Prevention recommend chemoprophylaxis
with either Oseltamivir or Zanamivir against H1N1 influenza
For people at risk of complications, including pregnant women.[34]
The use of NIs is reassuring to pregnant and lactating women as they
aren’t associated with adverse outcomes or congenital malformations
even with early pregnancy exposures.[35,36] Although cardiac side effects (1.8%) and transient neonatal hypoglycemia in the newborns were reported,[37,38] we did not encounter such side effects in our newborns.
None
of our patients had received influenza vaccination prior to or during
the current pregnancy. Antenatal influenza vaccination can enhance
fetal growth and can reduce preterm birthrate.[39,40] Maternal influenza immunization is a strategy with substantial benefits for both mothers and infants.[41] Mark G. Thompson et al.,[42]
found that pregnant women vaccinated against flu had a 40% lower risk
for hospitalization if they became ill with the infection compared with
unvaccinated pregnant women. We, therefore, would expect a reduction in
the preterm birth rate, IUGR and NICU admissions should our patients be
vaccinated. This is particularly important in our series since we had a
very low threshold for testing suspected cases and early initiation of
antiviral therapy.
Our one case of twin pregnancy was complicated
by oligohydramnios, IUGR and urgent cesarean section due to fetal
distress. Soydinc et al.,[43] reported that H1N1
influenza infection caused significant fetal and maternal complications
and they had a maternal death of a twin pregnancy infected at 32 weeks
gestation. Hein Bogers et al.,[44] reported 2 cases with severe perinatal complications, one with fetal demise at 24 weeks gestation.
Our
case series are limited in number. We recommend studying all cases of
H1N1 infection at a national level to reach more solid conclusions.
Conclusions
H1N1
influenza A infection in pregnancy is associated with adverse maternal
and perinatal outcomes. Medical and public awareness, low threshold for
testing pregnant patients, very early initiation of antiviral therapy
and multidisciplinary approach in our series decreased the overall
adverse effects of this infection.
References
- Centers for Disease Control and Prevention (CDC)
Hospitalized patients with novel influenza A (H1N1) virus
infection-California, April-May, 2009; MMWR Morb Mortal Wkly Rep; 2009.
pp. 536-41. PMid:19478723
- Collignon P. Swine flu-lessons learnt in Australia; Med J Aust; 2010. pp. 364–5. PMid:20367578
- Kelly HA. A pandemic response to a disease of predominantly seasonal intensity; Med J Aust; 2010. pp. 81–3 PMid:20078407
- Australian influenza surveillance summary report no. 26, 2009, reporting period: 31 October 2009–6 November 2009
- Jamieson
DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS,
Lindstrom S, Louie JK, Christ CM, Bohm SR, Fonseca VP, Ritger KA,
Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML,
Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ. Novel
Influenza A (H1N1) Pregnancy Working Group. H1N1 2009 influenza virus
infection during pregnancy in the USA. Lancet. 2009 Aug 8;
374(9688):451–8. https://doi.org/10.1016/S0140-6736(09)61304-0
- New
South Wales public health network Progression and impact of the first
winter wave of the 2009 pandemic H1N1 influenza in New South Wales,
Australia. Euro Surveill. 2009; 14(42):pii = 19365
- Lindsay
L, Jackson LA, Savitz DA. Community influenza activity and risk of
acute influenza like illness episode among healthy unvaccinated
pregnant and postpartum women. A J Epidemiol 2006;163:838-848 https://doi.org/10.1093/aje/kwj095 PMid:16554352
- Lim
Boon H, Mahmood Tahir AJ. Influenza A H1N1 2009 (swine flu) and
pregnancy. Obstet Gynecol India 2011; 61(4):386-393.
https://doi.org/10.1007/s13224-011-0055-2 PMid:22851818 PMCid:PMC3295877
- Kumar
A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al.
Critically ill patients with 2009 influenza A (H1N1) infection in
Canada. JAMA 2009;302:1872-1879. https://doi.org/10.1001/jama.2009.1496 PMid:19822627
- Louie
JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in
pregnant and postpartum women in California . N Engl J Med 2010;
262:27-35. https://doi.org/10.1056/NEJMoa0910444 PMid:20032319
- Mertz,
D., Kim, T. H., Johnstone, J., Lam, P. P., Science, M., Kuster, S. P.,
Fadel, S. A., Tran, D., Fernandez, E., Bhatnagar, N., Loeb, M. (2014).
Populations at risk for severe or complicated Avian Influenza H5N1: a
systematic review and meta-analysis. PloS one, 9(3), e89697. https://doi.org/10.1371/journal.pone.0089697
- Prabhu T.R. H1N1 influenza virus infection in pregnancy: A study of 32 cases. Journal of SAFOG 2014;6(2):93-97. https://doi.org/10.5005/jp-journals-10006-1279
- Brabin B.J. Epidemiology of infection in pregnancy. Rev. Infect. Dis.1985;7:579-603 https://doi.org/10.1093/clinids/7.5.579 PMid:3903938
- Goodnight WH, Soper DE. Pneumonia in pregnancy. Crit Care Med. 2005; 33(10 Suppl):S390-7. https://doi.org/10.1097/01.CCM.0000182483.24836.66 PMid:16215363
- Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Infect Dis. 2006 Nov; 12(11):1638-43. https://doi.org/10.3201/eid1211.060152 PMid:17283611 PMCid:PMC3372330
- Hewagama
S, Walker SP, Stuart RL, Gordon C, Johnson PDR, Friedman ND, O' Reilly
M, Cheng AC, Giles ML. 2009 H1N1 influenza A and pregnancy outcome in
Victoria, Australia. Clin Infect. Diseases 2010; 50:686-690. https://doi.org/10.1086/650460 PMid:20100064
- Liu
L, Zhang RF, Lu HZ, Lu SH, Huang Q, Xiong YY, Xi XH, Zhang ZY.
Sixty-two severe and critical patients with 2009 influenza A (H1N1) in
Shanghai, China. Chin Med J (Engl) 2011; 124(11):1662-1666
- Meijer
WJ, van Noortwijk AG, Bruinse HW, Wensing AM. Influenza virus infection
in pregnancy: a review. Acta Obstet Gynecol Scand 2015; 94(8):797-819. https://doi.org/10.1111/aogs.12680 PMid:26012384
- Hiba
V, Chowers M, Levi-Vinograd I, Rubinovitch B, Leibovici L, Paul M.
Benefit of early treatment with Oseltamivir in hospitalized patients
with documented 2009 influenza A (H1N1): a retrospective cohort study.
J Antimicrob Chemother 2011; 66(5):1150-1155 https://doi.org/10.1093/jac/dkr089 PMid:21393197
- Viasus
D, Pano-Pardo JR, Pachon J, Riera M, Lopez-Medrano F, Paveras A, et al.
Timing of Osetlamivir administration and outcomes in hospitalized adult
patients with pandemic 2009 influenza A (H1N1) virus infection. Chest
2011; 140(4):1025-1032. https://doi.org/10.1378/chest.10-2792 PMid:21415133
- Higuera
Iglesias AL, Kudo K, Manabe T, Corcho Berdugo AE, Corrales Baeza A,
Alfaro Ramos L, et al. Reducing recurrence and severity of pneumonia
due to pandemic H1N1 2009 by early oseltamivir administration: a
retrospective study in Mexico. PLoS One. 2011; 6(7):e21838. doi:
10.1371/journal.pone.0021838. Epub 2011 Jul 8. https://doi.org/10.1371/journal.pone.0021838
- Mertz
D, Geraci J, Winkup J, Gessner B D, Ortiz J R, Loeb M. Pregnancy as a
risk factor for severe outcomes from influenza virus infection: A
systematic review and meta-analysis of observational studies. Vaccine
2017; 35(4):421-428. https://doi.org/10.1016/j.vaccine.2016.12.012 PMid:28024955 PMCid:PMC5359513
- Siston
AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, et al.
Pandemic 2009 influenza A (H1N1) virus illness among pregnant women in
the United States. JAMA 2010; 303(15):1517-1527. https://doi.org/10.1001/jama.2010.479 PMid:20407061 PMCid:PMC5823273
- Dawood
FS, Luliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, et al. Estimated
global mortality associated with the first 12 months of 2009 pandemic
influenza A H1N1 virus circulation: a modelling study. Lancet Infect
Dis.2012; 12(9):687-695 https://doi.org/10.1016/S1473-3099(12)70121-4
- ANZIC
influenza Investigators., Webb SA, Pettila V, Seppelt I, Bellomo R,
Bailey M, Cooper DJ, et al. Critical care services and 2009 H1N1
influenza in Australia and New Zealand Engl J Med. 2009;
361(20):1925-34.
- The ANZIC Influenza
Investigators. Critical care services and the H1N1 (2009) influenza
epidemic in Australia and New Zealand in 2010: the impact of the second
winter epidemic. Crit care 2011; 15(3):R143. https://doi.org/10.1186/cc10266 PMid:21658233 PMCid:PMC3219015
- Viasus
D, Pa-o-Pardo JR, Pachón J, Campins A, López-Medrano F, Villoslada A,
Fari-as MC, Moreno A, Rodríguez-Ba-o J, Oteo JA, Martínez-Montauti J,
Torre-Cisneros J, Segura F, Gudiol F, Carratalà J. Factors associated
with severe disease in hospitalized adults with pandemic (H1N1) 2009 in
Spain. Clin Microbiol Infect 2011; 17(5):738-746. https://doi.org/10.1111/j.1469-0691.2010.03362.x PMid:20825436
- Changchang
Li Zhijiang Liang, Michael S Bloom, Qiong Wang, Xiaoting Shen, Huanhuan
Zhang, Suhan Wang, Weigin Chen, Yan Lin, Qingguo Zhao, Cunrui Huang.
Temporal trends of preterm birth in Shenzhen, China: a retrospective
study. Reprod Health 2018; 15:47 https://doi.org/10.1186/s12978-018-0477-8 PMid:29534760 PMCid:PMC5851155
- Pierce
M, Kurinczuk JJ, Spark P, Brocklehurst P, Knight M; UKOSS. Perinatal
outcomes after maternal 2009/H1N1 infection: national cohort study. BMJ
2011; 14:342:d3214. https://doi.org/10.1136/bmj.d3214
- Fell
DB, Savitz DA, Kramer MS, Gessner BD, Katz MA, Knight M, Luteijn JM,
Marshall H, Bhat N, Gravett MG, Skidmore B, Ortiz JR. maternal
influenza and birth outcomes: systematic review of comparative studies.
BJOG 2017; 124(1):48-59 https://doi.org/10.1111/1471-0528.14143 PMid:27264387 PMCid:PMC5216449
- William
L Callaghan, Andreea A Creanga, Denise Jamieson. Pregnancy-related
mortality resulting from influenza in the United States during the
2009-2010 Pandemic. Obstet Gynecol 2015; 126(3):486-490. https://doi.org/10.1097/AOG.0000000000000996 PMid:26244541 PMCid:PMC4557717
- Maternal
and infant outcomes among severely ill pregnant and postpartum women
with 2009 pandemic influenza A (H1N1)--United States, April 2009-August
2010. MMWR Morb Mortal Wkly Rep. 2011; 60(35):1193-6(ISSN:
1545-861X)
- Doll Mk,
Winters N, Boikos C, Kraicer-Melamed H, Gore G, Quach C. Safety and
effectiveness of neuraminidase inhibitors for influenza treatment,
prophylaxis, and outlook control: a systematic review of systematic
reviews and/or meta-analysis. J Antimicrob Chemother 2017;
72(11):2990-3007 https://doi.org/10.1093/jac/dkx271 PMid:28961794
- Centres for Disease Control and Prevention. What pregnant women should know aboutH1N1 (formerly called swine flu) virus. http://www.cdc.gov/h1n1flu/guidance/pregnant.htm (accessed May 27, 2009).
- Graner
S et al. Neuraminidase inhibitors during pregnancy and risk of adverse
neonatal outcomes and congenital malformations: Population based
European register study. BMJ 2017 Feb 28; 356:j629. https://doi.org/10.1136/bmj.j629 PMid:28246106 PMCid:PMC5421412
- Tanaka
T, Nakajima K, Murashima A, Garcia-Bournissen F, Koren G, Ito S. Safety
of neuraminidase inhibitors against novel influenza A (H1N1) in
pregnant and breastfeeding women. CMAJ : Canadian Medical Association
Journal. 2009; 181(1-2):55-58. https://doi.org/10.1503/cmaj.090866
- Ehrenstein
V, Kristensen NR, Monz BU, Clinch B, Kenwright A, Sorensen HT.
Oseltamivir in pregnancy and birth outcomes. BMC Infect Dis. 2018;
18(1):519 https://doi.org/10.1186/s12879-018-3423-z PMid:30326840 PMCid:PMC6192366
- Svensson
T, Granath F, Stephansson O, Kieler H. Birth outcomes among women
exposed to neuraminidase inhibitors during pregnancy. Pharmacoepidemiol
Drug Saf. 2011; 20(10):1030-4. https://doi.org/10.1002/pds.2194 PMid:21774030
- Steinhoff
M. C., Omer S. B. A review of fetal and infant protection associated
with antenatal influenza immunization. AJOG 2012; S21-S27 https://doi.org/10.1016/j.ajog.2012.06.071 PMid:22920054
- Steinhoff
MC, Omer SB, Roy E, Arifeen SE, Raqib R, Dodd C, Breiman RF, Zaman K.
"Neonatal outcomes after influenza immunization during pregnancy: a
randomized controlled trial," Canadian Medical Association Journal,
vol. 184, no. 6, pp. 645–653, 2012. https://doi.org/10.1503/cmaj.110754 PMid:22353593 PMCid:PMC3314035
- Zaman
K, Roy E, Arifeen S. E, Rahman M, Raqib R, Wilson E, Omer SB, Shahid
NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza
immunization in mothers and infants. N Engl J Med 2008;
359(15):1555-1564. https://doi.org/10.1056/NEJMoa0708630 PMid:18799552
- Thompson
MG, Kwong JC, Regan AK, Katz MA, Drews SJ, Azziz-Baumgartner E, et al.
Influenza Vaccine Effectiveness in Preventing Influenza-associated
Hospitalizations During Pregnancy: A Multi-country Retrospective Test
Negative Design Study, 2010-2016.Clin Infect Dis. 2018 Oct 11. https://doi.org/10.1093/cid/ciy737.42
- Soydinc
HE, Celen MK, Yildiz B, Sak ME, Evsen MS, Gul T. Pregnancy and H1N1
infection in Southeast Turkey. J Infect Dev Ctries 2012; 6(8):644-649 https://doi.org/10.3855/jidc.1956 PMid:22910572
- Bogers
H, Bos D, Schoenmakers S, Duvekot JJ. Postpandemic Influenza
A/H1N1pdm09 is still Causing Severe Perinatal Complications. Mediterr J
Hematol Infect Dis. 2015;7(1):e2015007. https://doi.org/10.4084/mjhid.2015.007 PMid:25574366 PMCid:PMC4283922
[TOP]








