 |
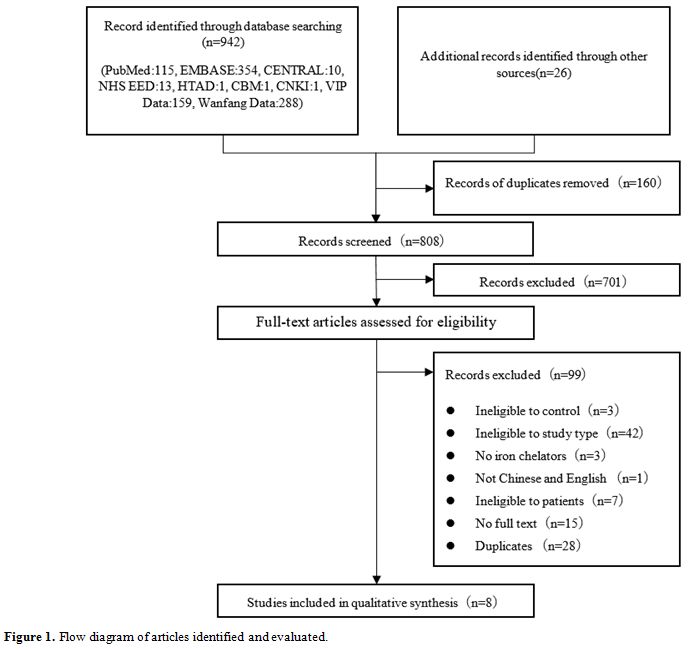
Figure 1. Flow diagram of articles identified and evaluated. |
Properties of the included papers. Table 1 provides the characters of the included papers, and Table 2 displays the results of the included studies. Remarkably, one paper49 adopted two perspectives for the analysis, including the perspective of the public payer for health services in Poland (Polish National Health Fund, NHF) and the perspective of the patient NHF. However, the results for both perspectives were identical. The three health states in Ho et al.[47] were β-thalassemia with complications, β-thalassemia without complications, and death. The complications included cardiac disease, diabetes mellitus, hypogonadism, hypoparathyroidism, and hypothyroidism.
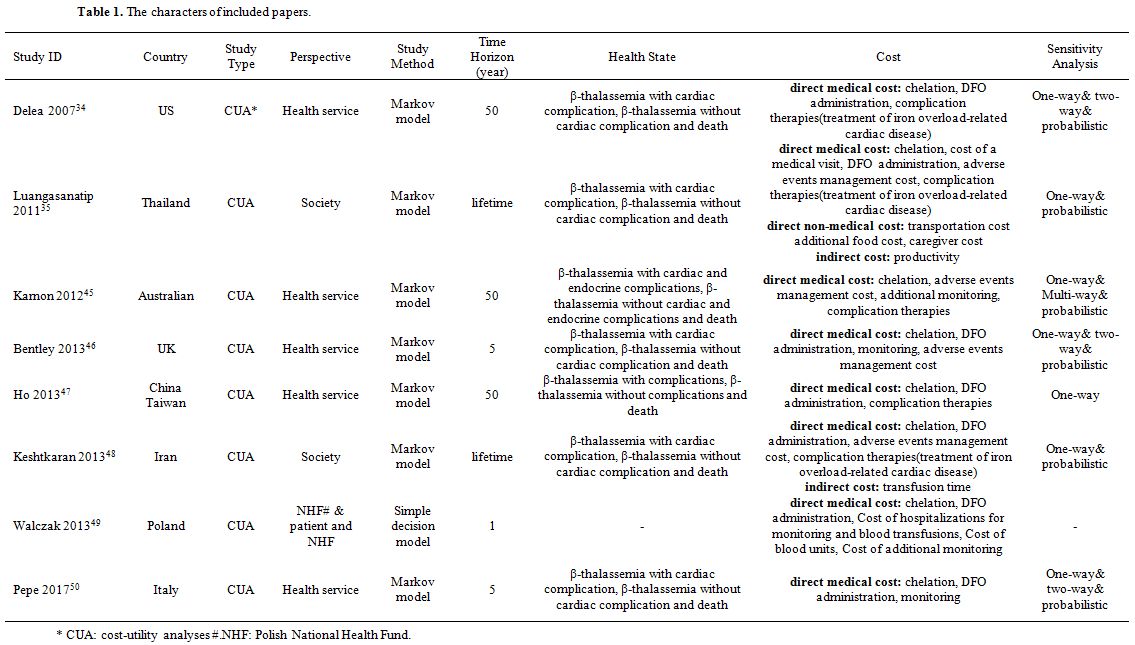 |
Table 1. The characters of included papers. |
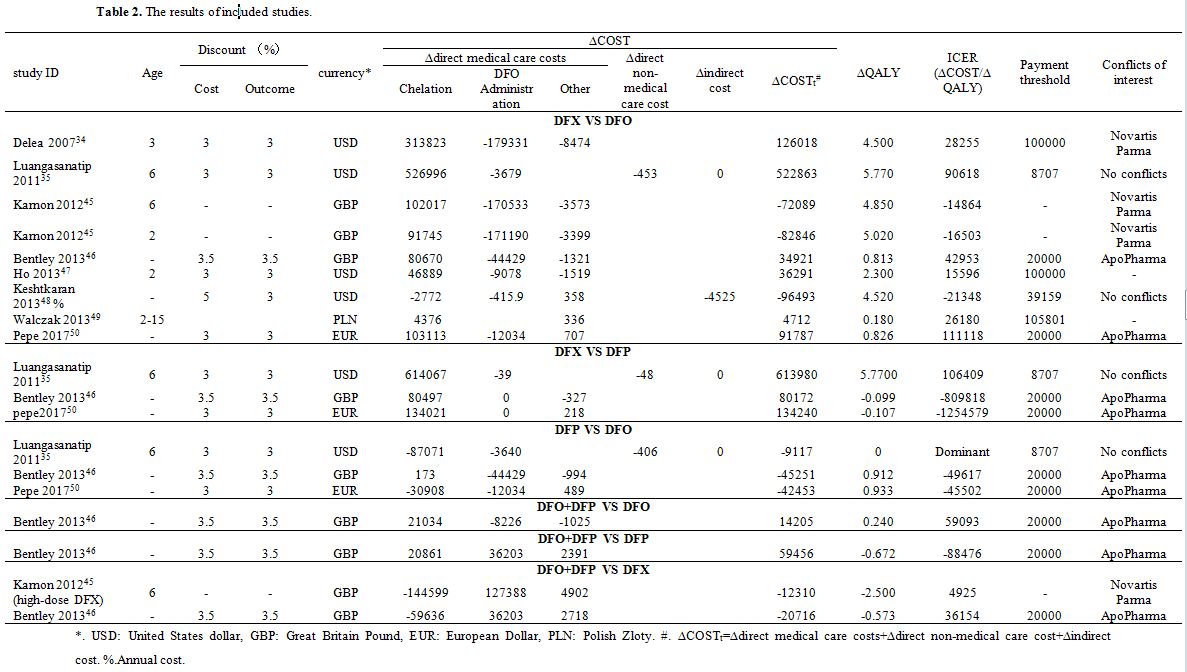 |
Table 2. The results of included studies. |
The cost of three main components was considered in the included papers: direct medical cost, direct non-medical cost, and indirect cost. The different papers have collected different cost data. The specific costs are shown in Table 1 and Table 2. As can be seen in Table 2, the costs affecting the economy of the four chelation regimens were predominantly constituted by the chelation cost, DFO administration cost, and indirect cost.
Methodological quality assessment. The results of the methodological quality assessment using the Drummond checklist are presented in Table 3. Out of the eight papers, six papers (75.00%)[34,35,46-48,50] were considered as A, one paper (12.50%)[45] as B, and one paper (12.50%)[49] as C, without D. The papers scored well on questions 1, 2, 3, 5, 6, 8, 9, and worse on questions 4, 7, 10.
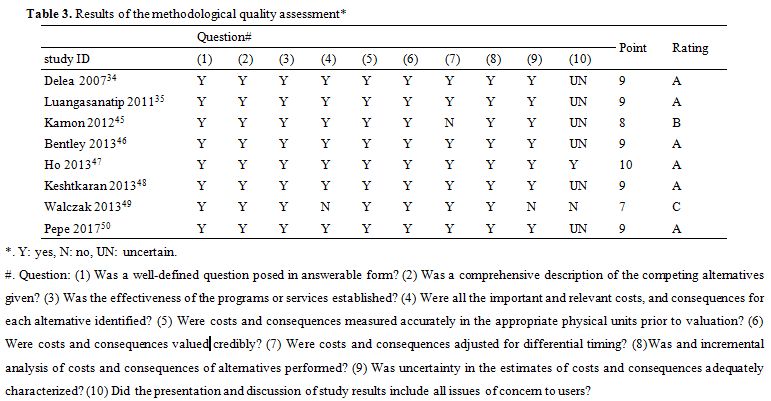 |
Table 3. Results of the methodological quality assessment* |
The reason for that the answer of Walczak et al.[49] to question 4 was “NO” was as follows. Because β-thalassemia major patients with DFO treatment need lengthy infusions, the lengthy infusions cost much time, and which leads to much indirect cost, such as the productive loss, wages loss, etc. As a result, if a patient perspective for the analysis is adopted, the indirect cost will be essential and should be identified. However, Walczak et al.[49] adopted a patient perspective and did not identify the indirect cost. The answer of Karnon et al.[45] to question 7 was “NO” because the costs and consequences of this paper had no discount with a study time horizon of 50 years. The answer of Walczak et al.[49] to question 10 was “NO” because the paper of Walczak et al. had no discussion. The answer of Delea et al.,[34] Luangasanatip et al.,[35] Karnon et al.,[45] Bentley et al.,[46] Keshtkaran et al.,[48] Pepe et al.[50] to question 10 were “UNCERTAIN” because the discussion of these papers lacked some issues of concern to users. The papers of Delea et al.,[34] Luangasanatip et al.,[35] and Keshtkaran et al.[48] did not discuss the generalizability of their research results to other patients or settings. The paper of Pepe et al.[50] did not compare its research results with those of others who have investigated the same question. The papers of Karnon et al.[45] and Bentley et al.[46] did not discuss the impact of foreign utility data on their research results.
Economic evaluation.
a) DFX versus DFO. There were nine cost-utility analyses of DFX versus DFO. In three of nine studies,[34,47,49] the ∆QALY and ∆COST were positive numbers, which meant that using DFX achieved better utility and a higher cost than when using DFO. Due to the lower ICERs of these three studies[34,47,49] than their payment threshold, the cost of using DFX was acceptable, and using DFX was cost-effective. In the three studies from two papers,[45,48] the ∆QALY values were positive, whereas those of ∆COST values were negative, which meant that using DFX led to a better utility and a lower cost compared the use of DFO. As a result, using DFX was cost-effective. In three other studies,[35,46,50] the ∆QALY and ∆COST were positive numbers, and their ∆ICER values were higher than their payment threshold, indicating that the cost of using DFX was unacceptable and suing DFO was more cost-effective.
b) DFX versus DFP. There were three cost-utility analyses of DFX versus DFP. In the three included studies, ∆QALY, and ∆COST values determined by Luangasanatip et al.[35] were both positive numbers, so using DFX resulted in better utility and a higher cost than when using DFP. Unfortunately, the ∆ICER of Luangasanatip et al.[35] was USD 106,409 and higher than its payment threshold. As a result, the cost of using DFX was unacceptable, and using DFP was cost-effective. In two other studies,[46,50] ∆QALY values were negative, whereas those of ∆COST were positive, showing that using DFX led to a worse utility and a higher cost as compared with the cases of using DFP. As a result, the DFX was not cost effective.
c) DFP versus DFO. There were three cost-utility analyses of DFP versus DFO. These three studies revealed that DFP was cost-effective in comparison with the utilization of DFO. In the study of Luangasanatip et al.,[35] ∆QALY value was zero and ∆COST value was USD -9,117, which meant that using DFP led to the same utility and a lower cost compared the use of DFO, and using DFP was cost-effective. In two other studies,[46,50] the ∆QALY values were positive, and ∆COST values were negative, which meant that using DFP led to a better utility and a lower cost compared with using DFO. As a result, the DFP was not cost effective.
d) DFO + DFP versus DFO. Only one study[35] assessed that comparison. In the study of Luangasanatip et al.,[35] ∆QALY and ∆COST were positive numbers, and the ∆ICER was higher than the payment threshold. This outcome meant that the DFO+DFP led to a better utility and a higher cost compared with DFO monotherapy. Because the ∆ICER was higher than its payment threshold, using DFO+DFP was unacceptable in Thailand, and using DFO monotherapy was established as cost-effective.
e) DFO + DFP versus DFP. In the only one study (Bentley et al.)[46] included in that category, ∆QALY value was negative, whereas ∆COST value positive. This result indicated that the DFO+DFP had a worse utility and a higher cost compared with DFP monotherapy. Hence, using DFP monotherapy was more cost-effective.
f) DFO + DFP versus DFX. In the two studies[46,50] in this group, ∆QALY and ∆COST were both negative numbers, which meant the DFO+DFP led to a worse utility and a lower cost in comparison with DFX monotherapy. Therefore, it was impossible to be sure which of two chelation regimens was cost-effective. More related research is required for further analysis.
Sensitivity analyses. Sensitivity analysis was not conducted in only one[49] out of eight papers. The details of the sensitivity analysis of the other seven papers can be found in Table 1. The sensitivity analysis of seven papers[34,35,45-48,50] showed that the influential parameters were drug cost, DFO administration cost, drug compliance, the dose of the drug, the starting age of chelation therapy, the discount rate and the utility values associated with the route of administration or complication.