Dipty Jain1, Prachi Atmapoojya2, Roshan Colah3 and Pooja Lodha4
1 Professor and Head, Dept. of Pediatrics, Government Medical College & Hospital, Nagpur.
2 Senior Resident, Dept. of Pediatrics, Government Medical College & Hospital, Nagpur.
3 Former Scientist G & Director In-Charge, National Institute of Immunohaematology, Mumbai.
4 Consultant, Fetal Medicine and Fetal Therapy, Ruby Hall Clinic &Director, Kangaroo Cradle; The Fetal Care Clinic, Pune.
Correspondence to: Dipty Jain. Professor and Head, Dept. of Pediatrics,
Government Medical College & Hospital, Nagpur. E-mail:
dipty47@rediffmail.com
Published: July 1, 2019
Received: May 4, 2019
Accepted: May 30, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019040 DOI
10.4084/MJHID.2019.040
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Sickle cell disease (SCD) is the
most common inherited hemoglobinopathy and is associated with increased risk of
complications and early mortality. Nowadays, with improved health care
facilities, antibiotic prophylaxis, vaccination, and availability of drugs like
hydroxyurea, the life expectancy of SCD patients has improved. More women are
reaching reproductive age group and are expressing their desire to reproduce.
Though SCD adversely affects pregnancy, leading to increased incidence of
maternal and perinatal complications like pre-eclampsia, preterm labor, IUGR,
abortions etc., adequate care throughout pregnancy ensures a better outcome.
Also, recent advancements in the fields of prenatal diagnosis and
pre-implantation genetic diagnosis, help couples suffering from SCD to have a
healthy baby. This paper focuses on the effects of SCD on pregnancy outcomes
and effective management of complications during pregnancy, also comparing
maternal and perinatal outcomes in studies conducted in different countries.
The second part of the paper summarizes pregnancy management in SCD for better
maternal and fetal outcomes.
|
Introduction
Sickle
cell disease (SCD) is the most common inherited hemoglobinopathy with
approximately 300,000 neonates born globally every year, predominantly
in countries like Nigeria, India and Democratic Republic of Congo.[1]
The
term sickle cell disease includes different genotypes of homozygous HbS
sickle cell anemia (SS) and the double heterozygote states of sickle
hemoglobin C disease (SC), sickle beta plus thalassemia (Sβ+Thal), sickle beta zero thalassemia (Sβ0thal), sickle cell anemia with alpha thalassemia (SS αthal), and sickle cell anemia with high fetal hemoglobin (SS+F).[2]
Strictly,
sickle cell anemia (SCA) is caused by a homozygous mutation (hemoglobin
S) and presents as chronic anemia accompanied by painful episodes. The
main defect triggering these events is impaired microcirculation due to
sickling of erythrocytes.
Until the 1970s, the management of
patients with SCA was weak, and pregnancy was associated with high
maternal and fetal mortality. Nowadays with newborn screening
techniques and preventive measures such as vaccination and antibiotic
prophylaxis since birth, overall disease outcomes and patient survival
have improved and there is a significant reduction in maternal and
neonatal mortality rates as well.[3] However, despite
all advances, pregnancy in SCD is still associated with higher clinical
and obstetric complications compared to the general population.
The
physiological adaptations that occur in the circulatory, hematologic,
renal and pulmonary systems during pregnancy can overburden organs that
already have chronic injuries secondary to SCD, increasing the rate of
obstetric complications like eclampsia and pre-eclampsia, worsening of
vasocclusive crises and acute chest syndromes. Though pregnancy in SCD
carries a higher risk of maternal and fetal complications, it can be
managed by ensuring adequate perinatal care.
This paper provides
an overview of the literature on maternal, perinatal morbidities and
their management in pregnancy with SCD, with a special focus on the
public health implications of prenatal screening in low and
middle-income countries (LMIC) of the South. Pre-existing anemia and
malnutrition in pregnant women, highly prevalent in LMIC are important
factors that might affect pregnancy outcomes and fetal growth.[2]
Material and Methods
This
is a rapid review of various articles published on pregnancy and sickle
cell disease in recent years. Articles were identified through a PubMed
search, including studies of women with SCD with known maternal and/or
perinatal outcomes, as well as any known characteristics of
reproductive history. The paper begins by outlining the
significance of the major issues affecting the pregnancy outcomes in
women with SCD in the first half below. Next part focuses on
effective management of SCD pregnancies.
Fertility in women with SCD. SCD patients have delayed physical as well as sexual development.[4]
These are consequences of various factors like poor nutrition,
repetitive infections, blood transfusions, painful crisis, and frequent
hospital admissions.[5] The onset of menarche is delayed in women with SCD4,[6] and is strongly associated with the HbSS phenotype compared to the HbSC phenotype.[7]
Women with SCD have unique risk factors that may affect their ability
to conceive, including chronic inflammation, oxidative stress,
transfusion-related hemochromatosis, and ovarian sickling, causing
ischemia and reperfusion injury to the ovaries.
Another important
reason for infertility is hypogonadotrophic hypogonadism due to
deposition of iron in the hypothalamo-pituitary axis because of
multiple blood transfusions and iron overload. A single-center study in
Egypt demonstrated that adolescents with SCD and excessive iron stores
have significantly lower levels of follicle stimulating hormone (FSH),
luteinizing hormone (LH), and estrogen when compared to those without
excessive iron stores.[8]
Like all other organs,
intravascular occlusion due to sickling of RBCs can occur in ovaries
too, leading to infarction, ovarian dysgenesis, and primary ovarian
insufficiency.[9] Also, NSAIDs which are very widely
used for VOCs in SCD, have been shown to inhibit ovulation in mammalian
species, likely due to inhibition of cyclooxygenase 2 (COX-2), thereby
reducing prostaglandin synthesis. The result is impairment in
ovulation, fertilization, and implantation.[10]
Pregnancy in SCD.
Pregnancy in sickle cell disease can be complicated as both prospective
mother and neonate are at increased risk of adverse outcomes. The
physiological changes of pregnancy like increased metabolic demand,
increased blood viscosity and hyper-coagulability gets aggravated in
SCD patients leading to increased incidence of complications like a
vaso-occlusive crisis, acute chest syndrome, osteonecrosis, hepatic
necrosis, leg ulcers, and thromboembolic events. Vaso-occlusion
also occurs in placenta leading to villous fibrosis, necrosis, and
infarction, thereby causing impaired uteroplacental circulation, which
leads to chronic fetal hypoxia and adverse fetal outcomes.[11,12]
Early
reports on the outcome of pregnancy in women with sickle cell anemia,
depicted an almost universal adverse outcome for mother and child, but
with improvements in medical care, especially the introduction of
preconception care, the outcome has dramatically improved. This
improvement in feto-maternal outcome is poorly reflected in sub-Saharan
Africa where the prevalence and complications of sickle cell disease in
pregnancy is highest in the world, and a maternal mortality rate of
0.38 - 1.29/100,000 births and perinatal mortality rate of 1.21 -
2.50/100,000 births are still being reported.[13]
This has been attributed to modest medical and antenatal care
facilities, and scarce so, or non-existence of preconception care
facilities in most communities in sub-Saharan Africa.
Obstetric and non-obstetric complications.
Pregnancy in SCD is associated with increased risk of obstetric
complications like pre-eclampsia and eclampsia, so, their incidence is
significantly higher in SCD patients as compared to the general
population.[14]
The risk of gestational diabetes is also found to be high, though not statistically significant.[15]
Micro-vascular damage and decreased uteroplacental circulation in these
mothers leads to an exaggerated risk of spontaneous abortions and
stillbirths. Other factors contributing to adverse fetal outcomes
include poor general health of the mother and drug abuse like tobacco,
alcohol and narcotics.[16]
Pregnancy exacerbates
the pre-existing anemia in SCD women, leading to a higher incidence of
severe anemia and increased requirement of blood transfusions. There is
a higher rate of cesarean deliveries in SCD patients,
though this disease is not an indication in itself.[12]
Further,
the incidence of sickle cell disease-related complications like VOCs,
ACS is increased during pregnancy. Defective splenic functions in SCD
due to auto-splenectomy, superimposed with the immune-compromised state
of pregnancy leads to increased risk of infections like pneumonia,
pyelonephritis, UTIs, postpartum infections, etc. Pregnancy, by
involving a hypercoagulable state, predisposes SCD women to
thromboembolic complications like deep vein thrombosis and cerebral
venous thrombosis.
Hence, due to a number of obstetric and
non-obstetric complications, maternal mortality is significantly higher
in SCD women compared to the general population. Though better health
care facilities and increased awareness in developed countries have
reduced maternal mortality, the situation is still the same in
developing countries. Table 1, below, summarizes the factors associated with maternal outcomes in women with SCD.
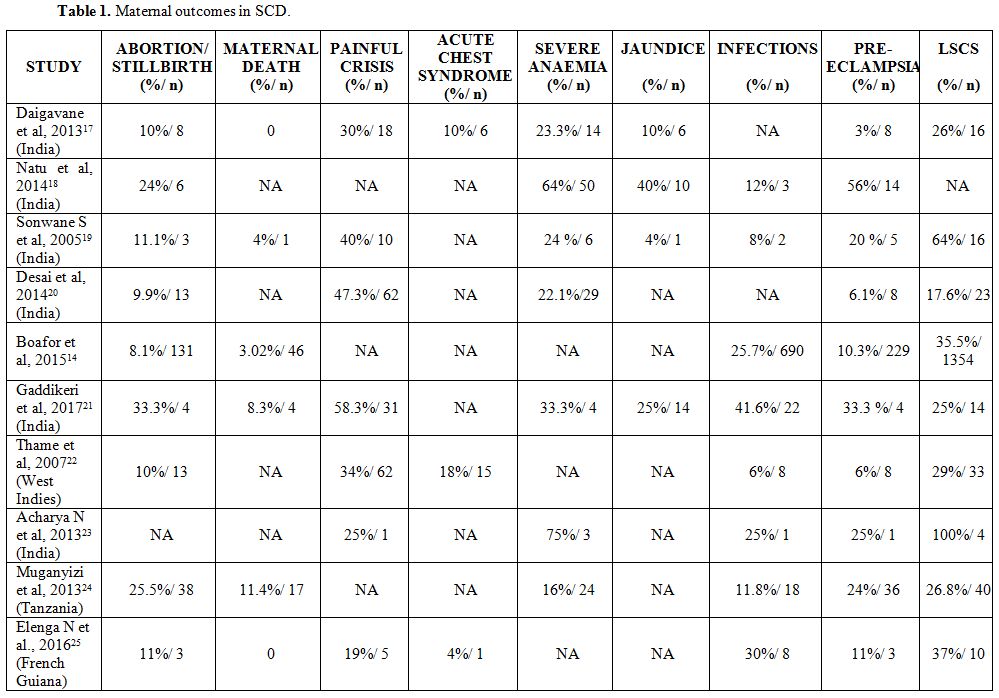 |
Table
1. Maternal outcomes in SCD. |
Perinatal morbidities.
Hypoxia and anemia seen in patients with sickle cell disease are
important factors that affect fetal growth. Anemia in the mother causes
impaired placental perfusion and thereby reduces the nutritional
substrate transport and oxygen delivery to the fetus. All this is
associated with an increased incidence of IUGR in SCD pregnancy. In
low-income countries, other factors like maternal malnutrition,
multiple pregnancies, and reduced health care facilities also play a
crucial role in adverse perinatal outcomes.[2]
Incidence
of preterm deliveries is high in SCD pregnancy, the exact mechanism is
still unclear, but increased production of prostaglandin has been
implicated.[2] Other reasons for it are anemia,
urinary tract infections, abruption placenta, placenta previa and
toxemia of pregnancy, which are more commonly seen in pregnant women
SCD.[2]
Table 2 below summarizes the factors associated with adverse perinatal outcomes in women with SCD, as reflected in the review.
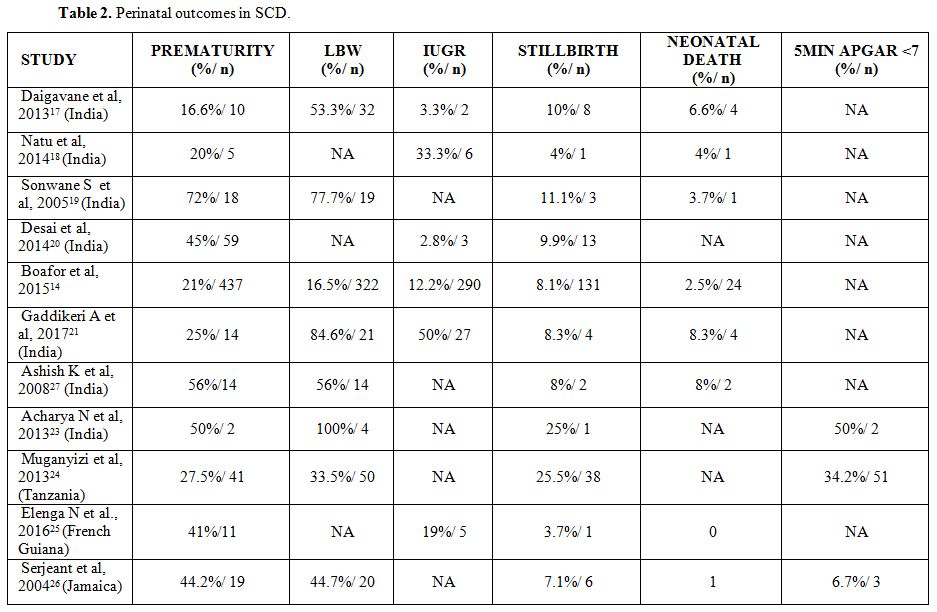 |
Table 2. Perinatal outcomes in SCD. |
There
is also an increased incidence of other neonatal complications like
HIE, RDS, and jaundice in neonates born to SCD mothers[18,27]
leading to increased neonatal admissions. Five- minute APGAR score was
compared in an Indian study, it was found that 50% of neonates born to
SCD mothers had 5 min APGAR score < 7.[28]
Management of Adverse Events in Pregnancy with SCD
Six major adverse events need planning with effective management for better maternal and neonatal outcomes, as described below:
1. Painful crisis –
pregnant women presenting with vaso-occlusive crises should be
hospitalized, adequate bed rest and fluid intake should be ensured. For
pain relief, paracetamol and other NSAIDs should be given. If pain is
not relieved narcotic analgesics may be used. However, meperidine
should be avoided because of associated toxicity and risk of
convulsions.[29]
2. Acute chest syndrome (ACS) – Pregnant
women with SCD presenting with complaints of severe cough and chest
pain should be evaluated for ACS. Pulmonary infiltrates on chest x-ray,
leukocytosis, blood, and sputum cultures is done to ascertain
infectious complications. Treatment includes appropriate antibiotics,
oxygen support, hydration, analgesics and if required blood
transfusion.[29]
3. Pulmonary Embolism
– women presenting with chest pain and respiratory distress with normal
chest x-ray should be suspected to have pulmonary embolism. Treatment
should be started with LMWH awaiting the confirmation of the diagnosis.
It is to be noted that elevated D-dimer will not confirm the diagnosis
as it can rise in other conditions like ACS and acute painful episodes.[29]
4. Strokes – Infarctive
and hemorrhagic strokes should be suspected in any female presenting
with acute neurological impairment. Treatment of choice is emergency
exchange transfusion. Thrombolysis is not helpful to treat stroke in
SCD.[29]
5. Hematological Complication –
Anemia is the most common complication of pregnancy. Blood loss, bone
marrow suppression by parvovirus infection and nutritional deficiencies
are the causes.[2] Prophylactic red blood cell
transfusion is done in some centers as it is believed that the risk of
complications like stroke, ACS, sequestration is decreased. However,
RCOG guidelines (Royal College of Obstetrician and Gynecologists) do
not recommend the same. Transfusions are only indicated when
Hb<7gm/dl because such low hemoglobin leads to decreased fetal
oxygenation and abnormal fetal outcomes.[29] HELLP
syndrome can develop in up to 10% of women with pre-eclampsia. It can
be managed conservatively or by urgent delivery depending on
gestational age.
6. Infections – The
major sites of infection are the urinary tract and the respiratory
system. Less often, puerperal endometritis, hepatitis, transient
bacteremia, osteomyelitis, and HIV have been encountered. During
infection, fever and acidosis lead to increased sickling and worsening
anemia. Appropriate antibiotics should be started at the earliest to
avoid further complications. Acute cholecystitis can also occur during
pregnancy and presents with fever, chills, and right upper quadrant
pain. Such attacks may simulate sickle hepatopathy, hepatitis or
hepatic sequestration. Liver function tests and ultrasound assessment
will help in diagnosis. Antibiotics and symptomatic management,
followed by elective cholecystectomy, is advised in the postpartum
period.[2]
Prenatal Diagnosis
Prenatal
diagnosis remains an important option for couples at- risk of having a
child with SCD. With increasing awareness in the community, more
couples are opting for prenatal diagnosis.[30]
First-trimester prenatal diagnosis by chorionic villus sampling at 10
to 12 weeks of gestation and DNA analysis is the method of choice.[31]
Often couples at risk are identified late in the second trimester, and
they can still be offered amniocentesis at 14 -15 weeks gestation and
DNA analysis or fetal blood sampling by cordocentesis at 18 to 19 weeks
gestation and HPLC analysis of the fetal blood to look for the
percentage of adult and sickle hemoglobin present.[31]
Celocentesis
for aspiration of celomic fluid at 7-9 weeks gestation allows earlier
prenatal diagnosis for monogenic disorders like beta-thalassemia and
sickle cell anemia. However, there is a problem of maternal cell
contamination. It has been recently shown that this can be
overcome through Embryo-fetal erythroid precursors selection using anti
CD 71 Microbeads or by direct micromanipulator pick up of the cells
selected based on their morphology.[32]
Preimplantation
genetic diagnosis (PGD) is not a replacement for prenatal diagnosis but
another option particularly for couples who would like to have a
healthy baby but who do not wish to terminate an affected pregnancy as
is most often done after prenatal diagnosis as it involves the
selective transfer of unaffected embryos following in-vitro
fertilization (IVF). This approach is also valid for couples with an
unsuccessful reproductive history who are opting for undergoing
assisted reproduction. However, there can be technical problems of
allele drop out and contamination leading to misdiagnosis.[33]
Pregnancy Management in SCD
Pre-conceptual care.
All women with SCD in reproductive age should be provided with relevant
information on how SCD affects pregnancy and what measures should be
taken for better maternal and fetal outcomes. It is during this period
that she should be made aware of the importance of partner screening
and the options for prenatal screening.
A complete medical and
social history of the mother should be obtained, including her
vaccination status, current medications, any other co-morbid condition,
and any drug abuse. Vaccination against all encapsulated organism,
including Neisseria meningitides, Streptococcus pneumonia, and
Haemophilus influenza should be updated. In addition, Hepatitis B and
Influenza vaccine should be given. Folic acid (5 mg) should be given
once daily both preconception and throughout pregnancy. Iron is
recommended if there is evidence of iron deficiency, but most of the
women have iron overload. Most of the women are on hydroxyurea. It is
recommended that drugs like hydroxyurea, ACE (angiotensin-converting
enzyme) inhibitors, and iron chelators should be discontinued at least
3 months before conception due to the risk of teratogenic side effects.[34]
Screening for complications like pulmonary hypertension by 2D
echocardiography, retinal screening for proliferative retinopathy, iron
overload, renal and liver function studies to rule out sickle
nephropathy and hepatic involvement should be done yearly.[2]
Ante-natal care.
The first prenatal visit should be a comprehensive assessment. Routine
blood investigations like complete blood count, HIV, HBs Ag, HCV should
be done along with urine examination. Mother should be explained
the importance of a regular antenatal visit; it is recommended to visit
obstetrician every other week during the first two trimesters.[35]
Blood pressure and urinalysis should be performed at each consultation,
and midstream urine for culture performed monthly as these women are
prone to pre-eclampsia and increased risk of urinary tract infections.[2]
Mothers
should be explained to avoid precipitating factors of sickle cell
crises such as exposure to extreme temperatures, dehydration, and
overexertion. Also, repeated vomiting can cause dehydration and
precipitate crisis. Hence, she should seek medical advice at the
earliest.
Women who are at increased risk of pre-eclampsia are
advised to take low-dose aspirin 75 mg from 12 weeks of gestation
unless they have aspirin sensitivity.[36]
Early
studies recommended prophylactic transfusion during pregnancy as there
was a decrease in maternal morbidity and perinatal mortality among
transfused women, but these are not recommended due to risks of
alloimmunization, iron overload, transfusion reactions and infections.[37]
Also, recent studies demonstrated that prophylactic transfusion
decreased the incidence of maternal, painful crises but did not
influence fetal or maternal outcome.[38,39]
Transfusion is indicated in case of severe anemia, and exchange
transfusion is recommended in case of stroke and acute chest syndrome.
Intrapartum care.
Delivery of SCD mother should be conducted in a center equipped with
all health care facilities to manage high risk pregnancies. Pregnant
women with SCD who have a normally growing fetus should be offered
elective birth through induction of labor at 38 to 40 weeks of
gestation, as SCD by itself is not a contraindication to attempt
vaginal delivery or vaginal birth after cesarean section. If there is
any indication or impending complications, cesarean delivery should be
considered. Women should be kept warm and given adequate fluid during
labor. Pain can be managed with adequate use of analgesics. Epidural
anesthesia is particularly useful in this regard, and efforts should be
made to shorten the duration of labor as well. Continuous intrapartum
electronic fetal heart rate monitoring is recommended owing to the
increased risk of fetal distress. Blood should be cross-matched
and kept ready at the time of delivery.
Post-partum care.
In the postpartum period, it is crucial to assess the degree of anemia
aggravated by blood loss during labor and delivery, and replacement
instituted when indicated. Hydration and oxygenation should be
maintained, and early mobilization encouraged. Crises should be managed
as for non-pregnant women. NSAIDs are routinely administered in the
postpartum period and can be used during breastfeeding. Breastfeeding
should be encouraged, as in women without SCD. Thromboprophylaxis in
the form of low-molecular-weight heparin is recommended for seven days
following a vaginal delivery or a period of 6 weeks following cesarean
section. Antithrombotic stockings are also recommended. Screening
of newborn for sickle hemoglobin is recommended. Mother should be
advised regarding contraception, progestogen-containing contraceptives
such as the progesterone only pill, injectable contraceptives, and the
levonorgestrel intrauterine system are safe and effective in SCD.
Estrogen-containing contraceptives should be used as second-line
agents. Barrier methods are as safe and effective in women with SCD as
in the general population.[36]
Hydroxyurea and Pregnancy
Hydroxyurea
has emerged as a wonder drug for SCD patients, thereby reducing
morbidities like VOCs, acute chest syndrome, and also decreasing the
requirement of blood transfusion. Hydroxyurea is classified as an
S-phase anti-neoplastic agent (pregnancy category D). It has been shown
to be potentially teratogenic due to its ability to initiate damage to
genetic material (i.e. DNA).[40] Toxicities of hydroxyurea have been reported, including cytopenias, rash[41] and the potential for teratogenicity was demonstrated in pregnant mammalian models using high doses of hydroxyurea[42,43,44]
although such reports are lacking in humans. It is recommended that
hydroxyurea should be discontinued at least three months before
conception to avoid the risk of teratogenicity.[36]
There
has always been an ethical dilemma about whether all couples at risk of
having a child with sickle cell disease require prenatal diagnosis as
many affected babies may have a milder clinical presentation.[45] It has been suggested that evaluating genetic modifiers may help.[46]
However, the fact remains that it is impossible to predict the severity
of the disease. The decision to terminate or continue an affected
pregnancy should be taken by the couple and not be the counselor.
Conclusions
Since
the literature on perinatal outcomes in SCD is limited, and the
potential impact of additional improvements in modern obstetric care
and treatment for SCD is significant, there is a substantial need for
additional studies of pregnancy-associated complications and outcomes
for women with SCD. This review aims to assess the studies which have
assessed the prevalence of maternal complications during the
intrapartum and postpartum periods for women with SCD. The review also
highlights the effective management of pregnancy and its complications
in women with SCD to ensure successful maternal and neonatal outcomes.
Acknowledgements
Dr. Sangeeta Chattoo, University of York.
References
- Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams
TN. Global burden of sickle cell anaemia in children under five,
2010-2050: modelling based on demographics, excess mortality, and
interventions. Plos Med. 2013;10(7):e1001484 https://doi.org/10.1371/journal.pmed.1001484 PMid:23874164 PMCid:PMC3712914
- Koshy M. Sickle cell disease and pregnancy. Blood Rev. 1995;9(3):157-64. https://doi.org/10.1016/0268-960X(95)90021-7
- Rogers
DT, Molokie R. Sickle cell disease in pregnancy. Obstetrics and
Gynecology Clinics of North America. 2010; 37(2):223-237. [pubmed:
20685550] https://doi.org/10.1016/j.ogc.2010.02.015 PMid:20685550
- Luban
NL, Leikin SL, August GA. Growth and development in sickle cell anemia.
Preliminary report. Am J Pediatr Hematol Oncol. 1982;4:61-65.
- Jesus
AC, Konstantyner T, Lôbo IK, Braga JA. Socioeconomic and nutritional
characteristics of children and adolescents with sickle cell anemia: a
systematic review. Revistapaulista de Pediatria. 2018 Dec;36(4):491-9. https://doi.org/10.1590/1984-0462/;2018;36;4;00010 PMid:30540112 PMCid:PMC6322809
- Zemel
BS, Kawchak DA, Ohene-Frempong K, et al. Effects of delayed pubertal
development, nutritional status, and disease severity on longitudinal
patterns of growth failure in children with sickle cell disease.
Pediatr Res. 2007;61:607-613 https://doi.org/10.1203/pdr.0b013e318045bdca PMid:17413865
- Carvalho
FA, Souza AI, Ferreira ALCG, et al. Profile of reproductive issues
associated with different sickle cell disease genotypes. Rev Bras
Ginecol Obstet. 2017;39(8):397-402 https://doi.org/10.1055/s-0037-1604179 PMid:28683515
- Hagag
AA, El-Farargy MS, Elrefaey S, et al. Study of gonadal hormones in
Egyptian female children with sickle cell anemia in correlation with
iron overload: single center study. Hematol Oncol Stem Cell Ther 2016;
9(9):1-7.
- Chase
AR, Howard J, Oteng-Ntim E. Ovarian sickling as a proposed mechanism
for premature ovarian failure necessitating ovum donation. Menopause
Int. 2009;15:70-71. https://doi.org/10.1258/mi.2009.009015 PMid:19465672
- Gaytan
M, Morales C, Bellido C, et al. Non-steroidal anti-inflammatory drugs
(nsaids) and ovulation: lessons from morphology. Histol Histopathol.
2006;21:541-556.
- Villers
MS, Jamison MG, De Castro LM, James AH. Morbidity associated with
sickle cell disease in pregnancy. American Ijournal of Obstetrics and
Gynecology. 2008 Aug 1;199(2):125-e1. https://doi.org/10.1016/j.ajog.2008.04.016 PMid:18533123
- Hassell K. Pregnancy and sickle cell disease. Hematology/Oncology Clinics. 2005 Oct 1;19(5):903-16. https://doi.org/10.1016/j.hoc.2005.07.003 PMid:16214651
- Rahimy
MC, Gangbo A, Adjou R, Dequenon C, Goussanou S, et al.(2000) Effect of
active prenatal management on pregnancy outcome in sickle cell disease
in an African setting. Blood 96:1685-1689
- Boafor
TK, Olayemi E, galadancin,Hayfron-Benjamin C, Dei-Adomakoh Y, Segbefia
C, Kassim AA, Aliyu MH, Galadanci H, Tuuli MG, Rodeghier M, debaun MR.
Pregnancy outcomes in women with sickle-cell disease in low and high
income countries: a systematic review and meta-analysis, BJOG, 2016
Apr;123(5):691-8 https://doi.org/10.1111/1471-0528.13786 PMid:26667608
- Barfield
WD, Barradas DT, Manning SE, Kotelchuck M, Shapiro-Mendoza CK. Sickle
cell disease and pregnancy outcomes: women of African descent. Am J
Prev Med. 2010;38(4):S542-9. https://doi.org/10.1016/j.amepre.2009.12.020 PMid:20331956
- Powars
D R, Sanhu M, Niland-Weiss J, Johnson C, Bruce S, Maming P R. Pregnancy
in sickle cell disease. Obstetgynecol 1986; 67: 217-228 https://doi.org/10.1097/00006250-198602000-00012
- Daigavane
MM, Jena RK, Kar TJ. Perinatal outcome in sickle cell anemia: a
prospective study from India. Hemoglobin. 2013 Dec 1;37(6):507-15. https://doi.org/10.3109/03630269.2013.828301 PMid:23952263
- Natu
N, Khandelwal S, Kumar R, Dave A. Maternal and perinatal outcome of
women with sickle cell disease of a tribal population in Central India.
Hemoglobin. 2014 Apr 1;38(2):91-4. https://doi.org/10.3109/03630269.2013.869501 PMid:24417305
- Sonwane
S., Zodpey S. Pregnancy outcome in women with sickle cell
disease/trait. J Obstet Gynecol India Vol. 55, No. 5: September/October
2005 Pg 415-418.
- Desai
G, Anand A, Shah P, Shah S, Dave K, Bhatt H, Desai S, Modi D. Sickle
cell disease and pregnancy outcomes: a study of the community-based
hospital in a tribal block of Gujarat, India. Journal of Health,
Population and Nutrition. 2017 Dec;36(1):3. https://doi.org/10.1186/s41043-017-0079-z PMid:28109314 PMCid:PMC5251338
- Gaddikeri
A, Pajai SP, Rathod AD, Pregnancy and its outcomes in sickle cell
hemoglobinopathies: A study of central India. J South Asian Feder Obst
Gynae 2017; 9(4):399-403 https://doi.org/10.5005/jp-journals-10006-1537
- Minerva
Thame DM, dma HT, Graham Serjeant MD. The mechanisms of low birth
weight in infants of mothers with homozygous sickle cell disease.
Pediatrics. 2007;120:e686. https://doi.org/10.1542/peds.2006-2768 PMid:17766509
- Acharya
N, Kriplani A, Hariharan C. Study of perinatal outcome in pregnancy
with sickle cell disease. Int J Biol Med Res. 2013; 4(2): 3185- 3188
- Muganyizi
PS, Kidanto H. Sickle cell disease in pregnancy: trend and pregnancy
outcomes at a tertiary hospital in Tanzania. Plos one. 2013 Feb
13;8(2):e56541. https://doi.org/10.1371/journal.pone.0056541 PMid:23418582 PMCid:PMC3572068
- Elenga
N, Adeline A, Balcaen J, Vaz T, Calvez M, Terraz A, Accrombessi L,
Carles G. Pregnancy in sickle cell disease is a very high-risk
situation: an observational study. Obstetrics and Gynecology
International. 2016;2016. https://doi.org/10.1155/2016/9069054 PMid:27403164 PMCid:PMC4926018
- Serjeant
GR, Loy LL, Crowther M, Hambleton IR, Thame M. Outcome of pregnancy in
homozygous sickle cell disease. Obstetrics & Gynecology. 2004 Jun
1;103(6):1278-85. https://doi.org/10.1097/01.AOG.0000127433.23611.54 PMid:15172865
- Ashish
K, Raseswari P, Pruthviraj S. Perinatal outcome in pregnancy with
sickle cell anemia. The Journal of Obstetrics and Gynecology of India.
2008;58:500-3.
- Acharya
N, Kriplani A, Hariharan C. Study of perinatal outcome in pregnancy
with sickle cell disease. Int J Biol Med Res. 2013; 4(2): 3185- 3188
- Boga
C, Ozdogu H. Pregnancy and sickle cell disease: a review of the current
literature. Critical reviews in oncology/hematology. 2016 Feb
1;98:364-74. https://doi.org/10.1016/j.critrevonc.2015.11.018 PMid:26672916
- Vermaic.Hemoglobinopathies
in India - An overview. Proc. Indo-French Symposium on Recent Trends in
Clinical, Diagnostic and Reserch Aspects of hemoglobinopathies. Kochi
Nov 21-24, 2004; p 2-4
- Colah
RB, Gorakshakar AC, Nadkarni AH. Invasive & non-invasive approaches
for prenatal diagnosis of haemoglobinopathies: experiences from
India.Indian J Med Res. 2011;134:552-60
- Giambona
A Embryo-fetal erythroid cell selection from celomic fluid allows
earlier prenatal diagnosis of hemoglobinopathies. Prenat Diagn
2016;36(4):375-81 https://doi.org/10.1002/pd.4793 PMid:26891446
- Traeger-Synodinos
J, Vrettou C, Kanavakis E. Prenatal, noninvasive and preimplantation
genetic diagnosis of inherited disorders: hemoglobinopathies. Expert
Rev Mol Diagn. 2011 ;11:299-312 https://doi.org/10.1586/erm.11.7 PMid:21463239
- Andemariam
B, Browning SL. Current management of sickle cell disease in pregnancy.
Clinics in Laboratory Medicine. 2013 Jun 1;33(2):293-310.
https://doi.org/10.1016/j.cll.2013.03.023 PMid:23702119
- Hassel K. Pregnancy and sickle cell disease. Hematol Oncol Clin North Am 2005;1:803-16. https://doi.org/10.1016/j.hoc.2005.07.003 PMid:16214651
- No GT. Management of sickle cell disease in pregnancy. London: Royal college of Obstetricians and Gynaecologists. 2011 Jul.
- Cunningham
FG, Pritchard JA, Mason R. Pregnancy and sickle cell
hemoglobinopathies: results with and without prophylactic transfusions.
Obstet Gynecol1983;62:419-24
- Koshy
M, Burd L, Wallace D, Moawad A, Baron J. Prophylactic red-cell
transfusions in pregnant patients with sickle cell disease. A
randomized cooperative study. N Engl J Med 1988;319:1447-5 https://doi.org/10.1056/NEJM198812013192204 PMid:3054555
- Howard
RJ, Tuck SM, Pearson TC. Pregnancy in sickle cell disease in the UK:
results of a multicentre survey of the effect of prophylactic blood
transfusion on maternal and fetal outcome. Br J Obstet Gynaecol
1995;102;947-51 https://doi.org/10.1111/j.1471-0528.1995.tb10900.x
- National
Toxicology Program. NTP-CERHR monograph on the potential human
reproductive and developmental effects of hydroxyurea. NTP CERHR MON.
2008;vii-viii, v, ix-III1.
- Cannas
G, Poutrel S, Thomas X. Hydroxycarbamine: from an Old Drug Used in
Malignant Hemopathies to a Current Standard in Sickle Cell Disease.
Mediterr J Hematol Infect Dis. 2017; 9(1):e2017015. doi:
10.4084/MJHID.2017.015. eCollection 2017. Review. https://doi.org/10.4084/mjhid.2017.015 PMid:28293403 PMCid:PMC5333733
- Brawley
OW, Cornelius LJ, Edwards LR, et al. National Institutes of Health
Consensus Development Conference statement: hydroxyurea treatment for
sickle cell disease. Ann Intern Med. 2008;148:932-938. https://doi.org/10.7326/0003-4819-148-12-200806170-00220 PMid:18458271
- Asano
Y, Okaniwa A. In utero morphological effects of hydroxyurea on the
fetal development in Sprague-Dawley rats. Jikkendobutsu. Exp Animals.
1987;36:143-149. https://doi.org/10.1538/expanim1978.36.2_143
- Khera KS. A teratogenicity study on hydroxyurea and diphenylhydantoin in cats. Teratology. 1979;20:447-452. https://doi.org/10.1002/tera.1420200314 PMid:542896
- Colah
R, Surve R, Nadkarni A, Gorakshakar A, Phanasgaonkar S, Satoskar P,
Mohanty D. Prenatal diagnosis of sickle syndromes in India: dilemmas in
counselling. Prenatdiagn. 2005;25:345-9. https://doi.org/10.1002/pd.1131 PMid:15906420
- Kumar
R, Panigrahi I, Dalal A, Agarwal S. Sickle cell anemia-molecular
diagnosis and prenatal counseling: SGPGI experience. Indian J
Pediatr.2012 ;79:68-74. https://doi.org/10.1007/s12098-011-0510-1 PMid:21713598
[TOP]

