Florence Urio1,2, Humphrey George1*, Furahini Tluway2, Thomas B. Nyambo1, Bruno P. Mmbando2,3 and Julie Makani2,4.
1 Department of Biochemistry, Muhimbili University of Health and Allied Sciences, Dar es salaam, Tanzania.
2
Sickle Cell Programme, Department of Haematology and Blood Transfusion,
Muhimbili University of Health and Allied Sciences, Dar es salaam,
Tanzania.
3 National Institute for Medical Research, Tanga, Tanzania.
4 Department of Haematology and Blood Transfusion, Muhimbili University of Health and Allied Sciences, Dar es salaam, Tanzania.
* Diseased
Correspondence to: Florence Urio, Department of Biochemistry and Sickle
Cell Programme, Muhimbili University of Health and Allied Sciences P.
O. Box 65001, Dar-es-Salaam, Tanzania. Phone: +255716894860 Email:
flosu28@gmail.com
Published: September 1, 2019
Received: March 26, 2019
Accepted: August 8, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019054 DOI
10.4084/MJHID.2019.054
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
The distribution of human parvovirus B19 (HPV B19) infection is
ubiquitous and occurs worldwide. The virus has high tropism to red
blood cells progenitor`s cells leading to temporary infection of bone
marrow and transient arrest of erythropoiesis. People with frequent
episodes of hemolytic anemia including sickle cell disease (SCD) and
thalassemia are at increased risk of infection. This study aimed at
assessing prevalence and factors associated with HPV B19 infections
among hospitalized SCD patients.
Methodology:
This was a cross-sectional hospital-based study among SCD patients
hospitalized at Muhimbili National Hospital. HPV B19 was detected using
RT-PCR. Hematological and Chemistry tests were done using Sysmex
XT2000i and Chemistry analyzer respectively.
Results:
A total of 329 SCD patients, median age 15 years (interquartile range
7-22 years) were tested for HPV B19. The prevalence of HPV B19 was 29%.
In multivariate logistic regression model, HPV B19 infection was
associated with pain (OR=4.28, 95%CI: 1.20–15.19; p=0.025), low
neutrophil counts (OR=0.57, 95%CI: 0.35–0.92, p=0.022) and MCH
(OR=0.92, 95%CI: 0.85–0.99; p=0.033). Individuals infected with HPV B19
had slightly higher prevalence of severe anaemia (30.4% vs. 20.3%,
p=0.054) and HIV infection (6.0% vs. 2.1%, p=0.083) in the univariate
analysis. Considering the effect of HPV B19 virus on spleen, aplastic
anemia and platelet counts in SCD patients, our study did not find any
association with these parameters (p=0.244; p= 0.205 and p=0.567
respectively).
Conclusion:
The prevalence of HPV B19 among hospitalized SCD patients at MNH was
high. SCD patients with HPV B19 were more likely to present with pain,
low neutrophils levels, and MCH. HIV infection might be associated with
a high risk of HPV infection in SCD patients; however, this requires
further investigation.
|
Introduction
Parvovirus
B19 is a small, single-stranded DNA virus of family parvoviridae and
genus Erythrovirus which shows tropism to bone marrow and has been
implicated in erythema infectiosum and other hematological disorders.[1,2] The virus has high tropism towards red blood cells (RBCs) progenitors.[1-3]
Sickle cell disease (SCD) patients are at high risk of infection due to
an increase in RBC progenitor division; this is for compensating the
deficiency of circulating RBC which is a common feature in SCD.[4]
Infection
with human parvovirus B19 (HPV B19) is relatively common, mildly
contagious, occurring sporadically or in epidemics. It has been
estimated that the peak incidence of infection occurs in children
between 6 and 14 years old. The most common route of transmission
appears to be through respiratory droplets because HPV B19 DNA has been
found in respiratory secretions at the time of viremia. Transmission is
mostly common occurs by close contacts from person to person. The rate
of transmission is almost 50% in household contact, but it varies from
10% to 60% in school and daycare exposure.[1]
HPV B19 is of interest because it causes transient aplastic anemia in SCD and is mostly associated with hemolytic disease.[5]
HPV B19 is currently considered a disease of public health importance,
particularly among patients with SCD. Prevalence of severe anemia in
non-SCD patients with Parvovirus B19 has been reported previously to be
2.7% in Kenya and 30.2% in Papua New Guinea (PNG).[4,6]
A prevalence of 23.3%, 27.3% and 32.1% in non SCD with aplastic anemia
were also reported in Northern Nigeria, India and Brazil respectively.[5,7,8] However, a prevalence of 37.6% was reported in SCA population in Eastern Saudi Arabia.[1]
Several
complications have been associated with HPV B19 like erythema
infectiosum; arthropathy; transient aplastic crisis; chronic red cell
aplasia; papular, purpuric eruptions on the hands and feet (“gloves and
socks” syndrome); and hydrops fetalis. It is thus important that like
many other diseases, HPV B19 should be clearly understood in its
virology, pathogenesis, clinical manifestation, diagnosis, laboratory
management, and its epidemiology for proper prevention and control.
While there is adequate information from developed countries, there is
currently no information on the prevalence of HPV B19 and its
associated factors in SCD in Tanzania.
The present study aimed
at assessing the prevalence of HPV B19 infection and associated factors
among hospitalized SCD patients and its possible impact on
hematological parameters, biochemical parameters [Alanine
Aminotransferase (ALT), Aspartate transaminase (AST), Bilirubin (direct
and total) and Lactate dehydrogenase (LDH)] and clinical parameters.
Methodology
Study Area.
The study was conducted at Muhimbili National Hospital (MNH). MNH is a
National referral hospital located in Dar es Salaam; it receives
referral cases from all over Tanzania. About 4,000 cases of SCD
patients are seen at MNH annually.[9]
Study Population.
This was a nested study investigating factors associated with severe
anemia among patients admitted at Muhimbili National Hospital.[10]
The study population was hospitalized SCD patients with various
complications between February and April 2016. Patient care and
management for recruited study population is well described in the
published protocol by Tluway et al.[10] Inclusion
criteria were: 1) aged between 0-45 years 2) confirmed SCD-(SS/SβO) by
High-Performance Liquid Chromatography (HPLC), and 3) hospitalized at
MNH during the study period. Patients on hydroxyurea, those who had
received blood transfusion within the past four weeks and re-admission
within the past four weeks were excluded from this study.[10]
The study was granted ethical approval by Muhimbili University of
Health and Allied Science (MUHAS) Institutional Review Board. This work
was part of a study for the Master of Science in Biochemistry.
Laboratory procedures.
Venous blood samples were collected from the antecubital or femoral
vein in EDTA tubes. Hemoglobin level (g/dl), red blood cell (RBC)
counts (x10^6/µl), mean cell volume (MCV) (fl), mean cell hemoglobin
(MCH) (pg), reticulocyte count (%), white blood cell (WBC) count
(x10^3/µl) and platelet counts(x10^3/µl) were determined by automated
hematology analyzer (Sysmex XT 2000i Kobe, Japan). Whole blood was
collected in serum tubes and was used for microbiology (HIV Test, which
was performed according to the National HIV Rapid Test Algorithm and
Parasitology), Alanine Aminotransferase (ALT), Aspartate transaminase
(AST), Bilirubin (direct and total) and Lactate dehydrogenase (LDH)
were done using a chemistry analyzer (Roche Cobas integral 400plus) and
Malaria test was performed using Malaria rapid diagnostic kit.
Buffy
coat was collected by spinning the EDTA blood at 3000rpm for 10
minutes, then were separated to obtain plasma and buffy coat and was
stored in graduated cryopreserve tubes. The DNA extraction was
performed with QIAamp DNA Min kits, (Germany), HPV B19 DNA viral
detection was undertaken using in-house optimized TaqMan real-time PCR
machine and primers designed for the study.
Data Analysis.
Data were entered into a MySQL database and analyzed using STATA
version 11 (Stata Corp, College Station, TX). Description of the study
participants was summarized by proportions for categorical variables,
while continuous variables were presented as means with standard
deviations. The mean differences of continuous variables between groups
were compared using the independent t-test. Proportions were compared
using the Chi-square test or the Fisher exact test. All estimates were
presented within 95% confidence intervals, and p-value less than 0.05
was deemed significant.
Results
Three
hundred and twenty-nine (329) hospitalized SCD patients fulfilled the
inclusion criteria and were enrolled in the study; their median age was
14 years (IQR 7–22 years). HPV B19 viral DNA was detected in 94 (28.6
%) patients of whom males were 51 (54.2%). The median age for
hospitalized SCD patients with HPV B19 was 15 years (IQR; 7-22), and
there was no statistically significant difference in HPV status within
age groups.
The tables 1A and 1B
show that pain (p=0.053), severe anemia (p=0.054), neutrophil counts
(p=0.069) and HIV infection (p=0.083) are features that are marginally
associated with HPV B19 infection. However, results from
multivariate logistic regression model of variables with P<0.2 show
that HPV B19 infection was associated with pain (OR=4.28, 95%CI:
1.20–15.19; p=0.025), neutrophil counts (OR=0.57, 95%CI: 0.35–0.92,
p=0.022) and mean cell hemoglobin (OR=0.92, 95%CI: 0.85–0.99; p=0.033).
Hemoglobin concentration, severe anemia (Hb<5g/dL) and HIV
infections were not statistically associated with HPV B19 infections in
the multivariate model, and these were excluded from the parsimonious
model. Even though the HIV infection was non-significant in the
multivariate model, the direction of effect was still positive
(OR=1.75, 95%CI: 0.335–9.13, 0.507).
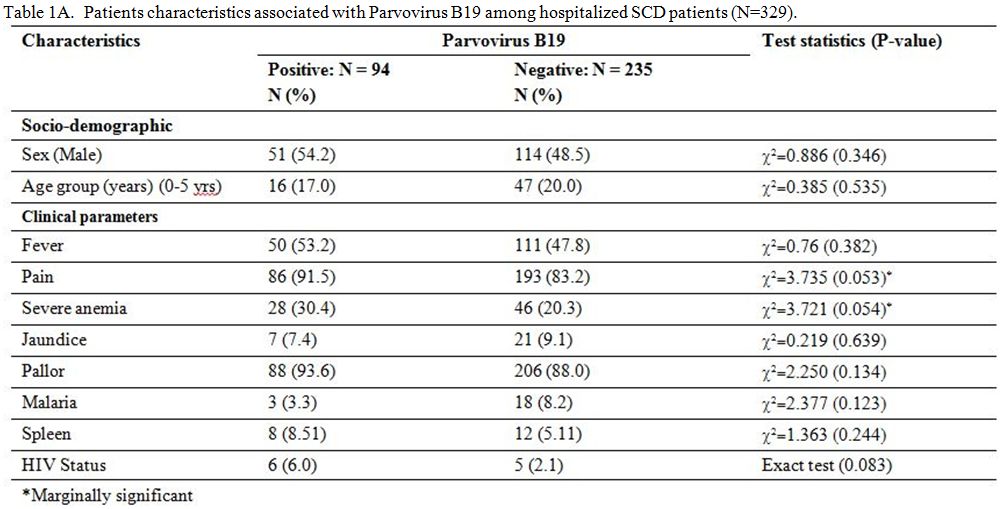 |
Table 1A. Patients characteristics associated with Parvovirus B19 among hospitalized SCD patients (N=329). |
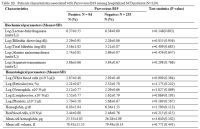 |
Table 1B. Patients characteristics associated with Parvovirus B19 among hospitalized SCD patients (N=329). |
Discussion
In
this study, the prevalence of HPV B19 among hospitalized SCD patients
was 29%. We also found an association between HPV B19 with pain, severe
anemia, HIV infection, mean cell hemoglobin, and neutrophil counts
among hospitalized SCD patients.
The prevalence reported in this
study is higher than that reported in Kenya (2.7%) from 264
hospitalized non-SCD children with severe anemia and 23.3% and 27.3%
from non-SCD with aplastic anemia in Northern Nigeria and India
respectively.[4,5,7] However, other
studies reported a higher prevalence of HPV B19, 30.2% in non SCD with
severe anemia in PNG, and 32.1% in Brazil.[6,11]
Eastern Saudi Arabia reported a higher prevalence of HPV B19 37.6% in
SCD patients compared to this study. The latter difference may be
attributed to the different SCD haplotypes found in these two
countries. Another reason may be due to the age group of study
participants; our study had a wider range of age group from 0 to 45
years of age whereas other studies were conducted only to children less
than five years. As expected, studies done on SCD population had mostly
higher prevalence compared to those done in non-SCD population because
SCD patients have frequent episodes of hemolytic anemia. The
prevalence of HPV B19 reported in Kenya is much lower compared to other
studies due to the small number of study participants.
We report
no evidence of an association between the HPV B19 infection and age,
although the trend indicated an increase in the risk of infection with
increasing age; this is contrary to finding reported by Iwalokun et al.[12] Transmission of HPV B19 was reported to occur frequently in a school-age child.[13]
HPV B19 infection is known to induce acute splenic sequestration crisis (ASSC).[14]
The risk of developing ASSC is mostly for infants and toddlers with
SCD, who produce sickled red blood cells and have not yet developed
splenic infarctions and organ complexities. In our study, we observed
no association between HPV B19 and spleen size. Our study population
had a majority of children between the ages of 11-20 years. Only 5% of
children below five years had HPV B19 infection. The small sample size
of children below five years could explain the lack of association
between HPV B19 and spleen size. Patients with chronic hemolytic anemia
such as SCD tend to have transient aplastic crises due to acute
infection of HPV B19. They may have life-threatening anemia due to the
shortened red cell survival.[15] Aplastic anemia has
been shown to mainly affect the pediatric age group with the median age
of onset of 8 years, and it is very rarely seen after the age of 15
years in SCD patients.[16] In this study, we did not
find an association between HPV B19 with aplastic anemia. 7% of the HPV
B19 positive SCD patients had aplastic anemia. The smaller sample size
of the pediatric age group patients could explain the lack of
association between these two parameters. We recommend further
studies with larger sample size of pediatric age group SCD patients to
evaluate this association.
This study found that HPV B19 was
associated with low levels of neutrophil counts and severe anemia.
However, in the multivariate model, the association was more evident
with low levels of neutrophil counts and mean cell hemoglobin but not
severe anemia. Our findings are similar to previous studies performed
by Whitley et al., who conducted an epidemiological study of HPV B19 in
children with SCD; he reported an association with low levels of
neutrophils.[17] A study by Sakai et al. also
suggested that HPV B19 virus may not have a direct effect on the low
levels of neutrophils but which instead is due to secondary removal and
consumption of leukocytes.[18] We did not observe an
association between HPV B19 with platelets counts in SCD patients. This
may be because leukocytopenia and thrombocytopenia sometimes do occur
in addition to erythrocytopenia to patients with HPV B19.[19]
Our study did not find a statistically significant difference in red
blood cell count between HPV B19 positive and negative population.
Wildig et al. conducted a study on SCD patients with HPV B19 and
reported an association with hemoglobin.[6] We
expected to find an association with hemoglobin due to the pathogenesis
of the disease but did not find an association in this study.
Our
findings showed that hospitalized SCD individuals with HIV infection
are more likely to be infected with HPV B19. Although the association
was not significant because of low statistical power, further
investigation with a larger sample size is required to evaluate the
association. It has been postulated that mechanism involved in the
persistence of HPV B19 in HIV patients may be due to lack of production
of anti-parvovirus antibody, possibly secondary to a basic defect in
antigen presentation by the macrophages or by dysfunctional T cells.[20]
Previous studies have shown persistent HPV B19 infection to be an
important factor in the development of chronic anemia in HIV-infected
individuals.[21] There is a hypothesis that immune suppressed people with HPV B19 infection may be infectious for long periods;[21] however, there is limited information on this hypothesis, and hence, more research is required.
In
our study, SCD patients with HPV B19 had more painful crises (4 times
more) compared to those without HPV B19. At variance with the cases
reported by Smith-Whitleyet et al.,[22] and by others,[23] the pain crises, in our circumstances, were not related to aplastic episodes and bone marrow necrosis.
This
study has some limitation, including the exclusion of SCD patients on
HU and those that were readmitted within the past four weeks. Including
this group could have removed any bias and estimated the actual
prevalence of HPV B19 in SCD patients. MNH is a national referral
hospital where most patients are referred from peripheral facilities.
Therefore, our study site may be a limitation, as most of these
patients would have received interventions before reaching MNH.
Conclusion
This
study has highlighted the prevalence of HPV B19 among hospitalized SCD
patients at MNH. The prevalence was 29%. Further research is required
to evaluate the clinical and laboratory factors on a larger study
population. Furthermore, the prospective follow-up to evaluate outcome
would be of great value as this would assist in understanding the
complications that arise in SCD individuals with HPV B19 infection. In
turn, this would improve the care and management of SCD individuals.
Acknowledgments
The
authors would like to thank the SCD patients hospitalized at Muhimbili
National Hospital without whom this study would not be possible.
Special thanks go to staff in Sickle cell program, Hematology and Blood
Transfusion Department and Biochemistry Department of MUHAS. Finally,
we would like to thank the MUHAS staff who supported the principal
investigator in all possible ways during his study and research period.
Contributions
HG
participated in designing the study, data collection, data analysis and
wrote the first draft of the manuscript; FU assisted in designing the
study, participated in data analysis and wrote the manuscript; FT
designed the research, collected data and reviewed the manuscript; BPM
analyzed the data, interpreted results and participated in writing the
manuscript; TBN reviewed the manuscript. JM designed the research and
reviewed the manuscript. All authors have read and approved the final
manuscript.
Funding
This
study was a nested study within the Muhimbili Sickle Cohort (MSC), and
it was financially supported by the Wellcome Trust, UK, as part of
Julie Makani fellowship award (no. 093727).
References
- Obeid OE. Brief Original Article Molecular and
serological assessment of parvovirus B19 infections among sickle cell
anemia patients. J Infect Dev Ctries 2011;5(7):535-339. https://doi.org/10.3855/jidc.1807 PMid:21795822
- Servey JT, Reamy BV, Hodge J. Clinical presentations of parvovirus B19 infection. Am Fam Physician 2007;75(3).
- Bukar
AA, Abjah UAM, Kagu MB, et al. Seroprevalence of parvovirus B19 and its
clinical effect among anaemic SCA patients in Northeastern Nigeria
Department of Haematology and Blood Transfusion University of Maiduguri
Teaching Department of Medical Microbiology University of Maiduguri
Teachin 2013;4(2):195-200. https://doi.org/10.5251/ajsir.2013.4.2.195.200
- Wildig
JW, Cossart Y, Peshu N, et al. Parvovirus B19 infection and severe
anaemia in Kenyan children: a retrospective case control study. BMC
Infect Dis. BioMed Central 2010;10(1):88. https://doi.org/10.1186/1471-2334-10-88 PMid:20361872 PMCid:PMC2861060
- Bukar
AA, Kagu MB, Abjah UAM, Ladu AL. Prevalence of Parvovirus B 19
Infection in Children with Aplastic Anemia. Am J Sci Ind Res
2013;4(2):195-200. https://doi.org/10.5251/ajsir.2013.4.2.195.200
- Wildig
J, Michon P, Siba P, Mellombo M, et al. Parvovirus B19 infection
contributes to severe anemia in young children in Papua New Guinea. J
Infect Dis 2006;194(2):146-53. https://doi.org/10.1086/505082 PMid:16779719
- Vineeta
Gupta IS and GN. Prevalence of Parvovirus B 19 Infection in Children
with Aplastic Anemia. Indian Pediatr 2013;50:489-91. https://doi.org/10.1007/s13312-013-0149-2 PMid:23255678
- Sant
ALM, Rita A, De C, et al. Study of chronic hemolytic anaemia patients
in rio de janeiro : Prevalence of anti-human parvovirus b19 IgG
antibodies and the development of transient aplastic crises
2002;44(4):187-90. https://doi.org/10.1590/S0036-46652002000400002 PMid:12219109
- Makani
J., Cox SE., Soka D., et al. Mortality in sickle cell anemia in africa:
A prospective cohort study in Tanzania. PLoS ONE 2011;6. e14699. https://doi.org/10.1371/journal.pone.0014699 PMid:21358818 PMCid:PMC3040170
- Tluway
F, Urio F, Mmbando B, et al. Possible Risk Factors for Severe Anemia in
Hospitalized Sickle Cell Patients at Muhimbili National Hospital,
Tanzania: Protocol for a Cross-Sectional Study. JMIR Res Protoc.
2018;7(2):e46 https://doi.org/10.2196/resprot.7349 PMid:29490896 PMCid:PMC5856920
- Sant'anna
ALM, Garcia R de CNC, Marzoche M, et al. Study of chronic hemolytic
anaemia patients in Rio de Janeiro: Prevalence of anti-human parvovirus
B19 IgG antibodies and the developement aplastic crises. Rev Inst Med
Trop Sao Paulo. Instituto de Medicina Tropical de São Paulo
2002; 44(4):187-90. https://doi.org/10.1590/S0036-46652002000400002 PMid:12219109
- Iwalokun
BA, Iwalokun SO, Hodonu SO, et al. A study on the association between
parvovirus B19 infection, serum tumour necrosis factor and C-reactive
protein levels among Nigerian patients with sickle cell anaemia.
Singapore Med J 2012;53 0037-5675
- Kelly
HA, Siebert D, Hammond R, et.al. The age-specific prevalence of human
parvovirus immunity in Victoria , Australia compared with other parts
of the world. Epidemiol Infect. 2000; 124(3): 449-57. https://doi.org/10.1017/S0950268899003817 PMid:10982069 PMCid:PMC2810931
- Saad
AA, Beshlawi I, Al-Rawas AH, et al. Human Parvovirus B19 in Children
with Sickle Cell Disease; Poking the Spleen. Oman Med J. 2017; 32(5):
425-428. https://doi.org/10.5001/omj.2017.79 PMid:29026475 PMCid:PMC5632704
- Serjeant
BE, Hambleton IR, Kerr S, et al. Hematological response to parvovirus
B19 infection in homozygous sickle-cell disease. The Lancet 2001; 358:
1779-1780 https://doi.org/10.1016/S0140-6736(01)06807-6
- Serjeant
GR, Serjeant BE, Thomas PW, et al. Human parvovirus infections in
homozygous sickle cell disease. Lancet 1993, 341:1237-1240. https://doi.org/10.1016/0140-6736(93)91145-C
- Smith-Whitley
K, Zhao H, Hodinka RL, et al. Epidemiology of human parvovirus B19 in
children with sickle cell disease. Blood 2004;103(2):422-7 https://doi.org/10.1182/blood-2003-01-0069 PMid:14525777
- Sakai
N, Sawada K, Koizumi K, et al. Human parvovirus-induced transient
anemia and leukopenia after delivery. Rinsho Ketsueki 1992;
33:1077-83
- Yoto
Y, T. Kudoh, N. Suzuki, S, et al. Thrombocytopenia induced by human
parvovirus B19 infection. Eur. J. Haematol 1993; 50:255-257 https://doi.org/10.1111/j.1600-0609.1993.tb00158.x
- Prasad Rao Koduri. Parvovirus B19-Related Anemia in HIV-Infected Patients. Aids Patient Care and STDs 2000;14:1 https://doi.org/10.1089/108729100318082 PMid:12240888
- Frickhofen
N, Abkowitz JL, Safford M, et al. Persistent BI9 parvovirus infection
in patients infected with human immunodeficiency virus type 1 (HIV-I):
a treatable cause of anemia in AIDS. Ann Intern Med 1990; 113:926-32. https://doi.org/10.7326/0003-4819-113-12-926
- Smith-Whitley
K, Zhao H, Hodinka RL, Kwiatkowski J, Cecil R, Cecil T, Cnaan A,
Ohene-Frempong K. Epidemiology of human parvovirus B19 in children with
sicklecell disease. Blood. 2004 Jan 15;103(2):422-7.
10.1182/blood-2003-01-0069
- Conrad
ME, Studdard H, Anderson LJ. Aplastic crisis in sickle cell disorders:
bone marrow necrosis and human parvovirus infection. Am J Med Sci.
1988; 295(3):212-5. https://doi.org/10.1097/00000441-198803000-00009 PMid:2833101.

