Baran Cengiz Arcagok1 and Birol Karabulut2.
1 Acibadem Mehmet Ali Aydinlar University, Pediatrics, Division of Neonatology, Istanbul, Turkey.
2
Izmir Katip Celebi University Ataturk Training and Research Hospital,
Pediatrics, Division of Neonatology, Karabaglar, Izmir, Turkey.
Correspondence to: Birol Karabulut. Izmir Katip Celebi University
Ataturk Training and Research Hospital, Pediatrics, Division of
Neonatology, Karabaglar, Izmir, Turkey, E-mail:
dr.birolkarabulut@icloud.com
Published: September 1, 2019
Received: July 2, 2019
Accepted: August 8, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019055 DOI
10.4084/MJHID.2019.055
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Neonatal sepsis (NS) is a common systemic disease that causes morbidity
and mortality in newborns. But there is no ideal biomarker that can be
used in the early diagnosis of NS. In recent studies, platelet to
lymphocyte ratio (PLR) has been reported to play a critical role in the
inflammatory process. In this study, we aimed to contribute to the
research about whether or not PLR can be used as an early predictor of
the diagnosis of NS.
Methods:
This retrospective cohort study was conducted among the newborns born
in İzmir Buca Maternity and Pediatric Hospital between March
2015-February 2016. During these twelve months, 611 neonates with
Early-Onset Sepsis (EOS) were admitted to our neonatal intensive care
unit. One hundred and forty-nine neonates with suspected EOS, 67
neonates with proven EOS and 92 healthy neonates were enrolled in the
study.
Results: Platelet
to lymphocyte ratio (PLR) values of the three groups were
calculated 56.5 ± 17.8 vs. 62.4± 14.9 vs. 15.3 ± 2.1, respectively. PLR
values of suspected or proven EOS group were significantly higher than
the control group. PLR has AUC 0.89 to 0.93, the cutoff value of 39.5
to 57.7, the sensitivity of 88.9% to 91.3% and specificity of 94.7% to
97.6%, the positive predictive value of 94.3% to 97.4%, and negative
predictive value of 88.6% to 91.8% in suspected and proven sepsis
diagnosis.
Conclusions: Our results suggest that PLR can be used as a parameter in the prediction of neonatal sepsis.
|
Introduction
Neonatal sepsis (NS) is one of the major causes of morbidity and mortality in neonatal age.[1]
NS are classified, according to the absence or the presence of the
positive blood culture, in Clinical Sepsis and Proven Sepsis.
Concerning the time of symptoms onset, they are defined as Early-Onset
Sepsis (EOS) and Late-Onset Sepsis (LOS). When the blood culture is
negative, but the neonate presents clinical and inflammation signs, and
biomarker increase, the sepsis is defined as Clinical Sepsis.
Conversely,
in Proven Sepsis, the neonate presents clinical, and laboratory signs
of infection/inflammation, and the blood cultures are positive.[2]
The time of onset defines the type of sepsis. The ones developing in
the first three days of life are called EOS, whereas those developing
from 4 to 28 days of life are called LOS.[3] It is
believed that EOS is mainly due to the maternal-fetal transmission of
microorganisms during pregnancy or perinatally. Microorganism
transmission to the blood circulation of neonates causes immune system
reaction leading to systemic inflammatory response syndrome (SIRS),
which may progress into sepsis, multiple organ failure, and death.[4] Early diagnosis and therapy may inhibit the progression of SIRS and prevent sepsis-related morbidity and mortality.[5]
Determination of maternal risk factors and clinical and laboratory
features are used for diagnosis of EOS. Important risk factors for EOS
include the maternal medical history of urinary infection, vaginitis,
early membrane rupture, and chorioamnionitis.[6]
Clinical signs are nonspecific and subtle in neonatal EOS. The
unspecific clinical symptoms in neonates and the lack of sufficiently
accurate biomarkers can lead to delay in diagnosis and initiation of
the therapy, unnecessary hospital admissions, and antibiotic resistance
secondary to antibiotic misuse.[7] Blood culture is
the gold standard laboratory test in the diagnosis of NS; however this
method has significant limitations, which include false negativity
secondary to maternal antibiotic use or low microorganism
concentration, need 48 to 72 hours to get the results, false positivity
secondary to contamination. Actually, the blood culture sensitivity in
the diagnosis of sepsis is reported to be around 19%.[8]
Given that, a “magic” biomarker to early diagnose EOS is to find. Many
biomarkers have been tested for the accuracy in EOS diagnosis,
including acute phase reactants, interleukins, and immunoglobins.[9-11]
C-reactive protein (CRP) is the most frequently studied inflammatory
marker, which is also used in the follow-up of therapy. CRP is a
sensitive but not a specific marker to diagnose sepsis, because of the
increase in multiple non-infectious inflammatory events, other than
sepsis, and the delay in the increase (10 to 12 hours).[12]
Another inflammatory marker, procalcitonin (PCT), increases in the
first 3 to 4 hours from the beginning of symptoms and decreases to
normal level in 24 hours.[13] Since peripheral blood
smear test, another inflammatory marker, necessitates both appropriate
laboratory conditions and personal experience, it’s reliability in
sepsis diagnosis in low.[14] All of these limitations
regarding inflammatory markers cause the absence of a reliable
biomarker that can be used in the early diagnosis of NS. Recent studies
reported that platelet and lymphocytes have a critical role in the
inflammatory process. PLR is an indicator of the balance between
inflammation and thrombosis. Thus, the inflammatory status results in
accelerated megakaryocyte proliferation and associated thrombocytosis.
Moreover, increased platelet counts and decreased lymphocyte counts
have been shown to be related to both aggregation and inflammation, and
thus, represent risk indicators.[15-18] In the
present study, the PLR which are parts of a complete blood count, were
compared with the traditional parameters CRP and PCT for the ability to
predict EOS in neonates with or without positive blood cultures.
Materials and Methods
Patients.
An observational, retrospective cohort study was conducted to evaluate
newborns born in Buca Gynecology, Obstetrics and Pediatrics Hospital,
Izmir, Turkey between March 2015 and February 2018. We calculated that
a sample size of 64 in the study group and 64 in the control group
would allow us to detect differences between the 2 groups (α = 0.05,
power = 80%).[19] Our patient group included neonates
with the gestational age of 37 to 42 weeks according to Ballard Score
or ultrasonography performed before week 20, appropriate for
gestational age (AGA) and diagnosed with EOS. Exclusion criteria
included less than 37 weeks or more than 42 weeks, small for
gestational age (SGA), intrauterine growth restriction (IUGR),
perinatal asphyxia, congenital abnormality, congenital heart disease,
chromosomal abnormality, preeclampsia, and lack of data. Newborns with
a maternal history of urinary tract infection, vaginitis, early
membrane rupture, and clinical or histological chorioamnionitis in last
trimester were followed up for 72 h for clinical signs related to
sepsis, and sepsis screening was performed for newborns with clinical
findings 12 h postnatally. Sepsis screening was performed for newborns
without clinical signs related to sepsis at 12–24 h of the newborn with
a maternal history of urinary tract infection, vaginitis, early
membrane rupture, and clinical or histological chorioamnionitis in last
trimester. Sepsis screening included a complete blood count (CBC), CRP,
PCT, and peripheral blood smear. Lumbar puncture was performed for
newborns with fever or seizure or neurological findings or positive
blood culture. European Medicines Agency (EMA), Report on the Expert
Meeting on Neonatal and Pediatric Sepsis criteria were used for the
diagnosis of sepsis.[20] Clinical signs were: (1)
Respiratory instability: apnea, tachypnea, increased oxygen
requirements, or requirement for ventilation support; (2)
Cardiovascular instability: bradycardia, tachycardia, rhythm
instability, reduced urinary output (less than 1 mL/kg/h), hypotension,
mottled skin or impaired peripheral perfusion; (3) Modified body
temperature: hypothermia, hyperthermia, or temperature instability; (4)
Gastrointestinal instability: feeding intolerance, poor sucking, or
abdominal distention; (5) Skin and subcutaneous lesions: petechial rash
or sclerema; (6) Non-specific: irritability, lethargy, or hypotonia.
Sepsis screening was performed at 12–24 h of life for these laboratory
signs: (1) White blood cell (WBC) count < 4,000 × 109 cells/L or > 20,000 × 109 cells/L; (2) Immature to total neutrophil ratio (I/T) greater than 0.2; (3) Platelet count < 100,000 × 109
cells/L; (4) CRP >15 mg/L or PCT ≥ 2 ng/mL; (5) Glucose intolerance
confirmed at least two times, hyperglycemia (blood glucose > 180
mg/dL), or hypoglycemia (glycemia < 45 mg/dL); (6) Metabolic
acidosis with base excess (BE) < −10 mEq/L or serum lactate > 2
mMol/L. Neonates with two or more clinical signs and two or more
laboratory signs were diagnosed as suspected EOS (Group 1) and admitted
for the treatment. Blood culture positive for these newborns was
considered as proven EOS (Group 2). The control group (Group 3)
consisted of healthy newborns with 37–42 gestational weeks, AGA and
suspicious EOS negatively detected. Maternal risk factors, demographic
and perinatal data, and laboratory signs of the newborns were recorded
in newborn files.
Statistical Analysis.
Statistical analyses were performed using the statistical package SPSS
for Windows version 22.0 (SPSS Inc., Chicago, IL, USA). The paired
sample t-test and independent-sample t-test were used for continuous
variables. Continuous variables were presented as the mean ± SD, and
categorical variables were given as frequencies and percentages. A
p-value of less than 0.05 was considered statistically significant. The
performance of laboratory features in the diagnosis of EOS was
calculated by using the ROC curve.
Results
During
the study period, 6,539 newborns were born in our hospital. Part of
those neonates (n = 1,747) had a maternal history of urinary tract
infection, vaginitis, early membrane rupture, and clinical or
histological chorioamnionitis in last trimester. In addition, 384 of
1,747 neonates with maternal risk factors and 227 of 4,792 neonates
without maternal risk factors were admitted to our unit with a
diagnosis of EOS. Of those admitted patients, 210 of 384 newborns with
maternal risk factors and 185 of 227 newborns without maternal risk
factors were excluded from the study. Thus, 149 of newborns admitted
with suspected EOS (Group 1), 67 proven EOS (Group 2), and 92 healthy
newborns as a control group (Group 3) were included the study (Figure 1).
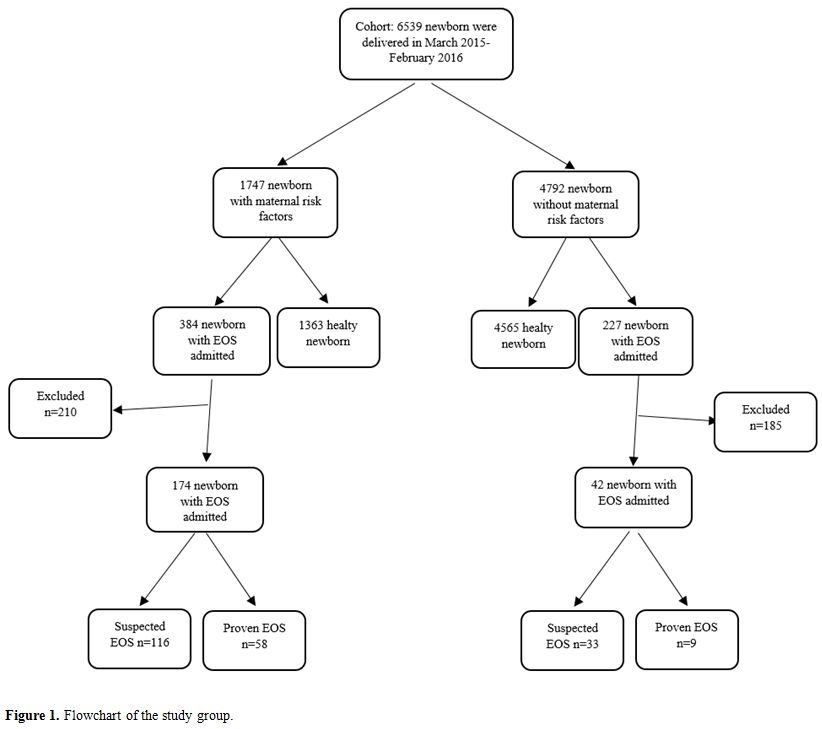 |
Figure
1. Flowchart of the study group. |
Demographic characteristics of groups are summarized in Table 1.
There was no difference between groups regarding demographical and
perinatal data. Comparison of hematological parameters of groups is
summarized in Table 2. PLR,
CRP, PCT, I/T ratio, and WBC counts were higher in group 1 and 2
compared to group 3. The mean platelet count of group 1, group 2, and
group 3 were 245.7 ±66.1, 227.1 ±54.3 and 283.4 ±77.6 (Group 1–3: p =
0.98, Grup 2-3: p = 0.11), respectively. The mean lymphocyte count of
group 1, group 2, and group 3 were 7.4 ± 2.1, 6.5 ±1.3 and 13.1 ±2.9
(Group 1–3: p < 0.001, Group 2–3: p < 0.001), respectively. The
mean PLR of group 1, group 2, and group 3 were 56.5 ±17.8, 62.4 ±14.9
and 15.3 ±2.1 (Group 1–3: p < 0.001, Group 2–3: p < 0.001),
respectively. The mean CRP values of group 1, group 2, and group 3 were
27.5 ± 6.3 mg/L, 56.9 ± 21.7 mg/L, and 4.6 ± 1.1 mg/L (Group 1–3: p
< 0.001, Group 2–3: p < 0.001), respectively. The mean PCT values
of group 1, group 2, and group 3 were 2.2 ± 0.09 ng/mL, 3.4 ±1.2 ng/mL,
and 0.03 ± 0.01 ng/mL (Group 1–3: p < 0.001, Group 2–3: p <
0.001), respectively, and the mean I/T ratios of group 1, group 2, and
group 3 were 0.25 ± 0.1, 0.33 ± 0.08, and 0.1 ± 0.05 (Group 1–3: p <
0.001, Group 2–3: p < 0.001), respectively. In suspected EOS (Group
1), PLR had an AUC of 0.812 for prediction of EOS. At a cut-off level
of 39.5, RPR had a sensitivity of 88.9%, a specificity of 94.7%, a
positive predictive value (PPV) of 94.3%, and a negative predictive
value (NPV) of 88.6%. In proven EOS (Group 2), PLR had an AUC of 0.847
for prediction of EOS. At a cut-off level of 57.7, PLR had a
sensitivity of 91.3%, a specificity of 97.6%, a PPV of 97.4%, and an
NPV of 91.8%. The performance of CRP, PCT, and I/T ratio in EOS
diagnosis are summarized in Table 3.
The sensitivity of blood culture test was 24.8%, and the most
frequently isolated microorganisms were E.coli (34.2%), coagulase
negative Staphylococcus (28.9%), Staphylococcus aureus (23.6%), and
Klebsiella spp (13.1%). Several CSF cultures (n = 47) obtained from 216
newborns with EOS showed no isolation.
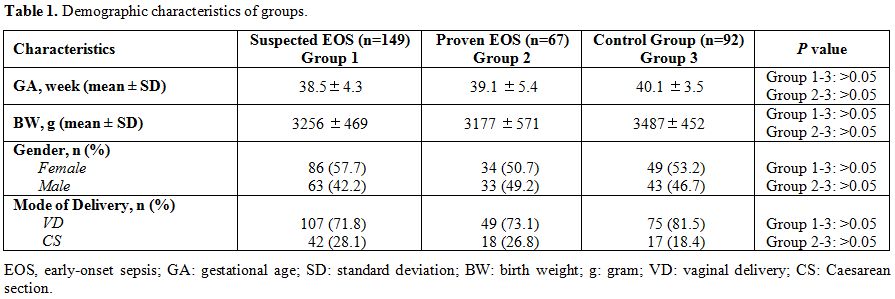 |
Table 1. Demographic characteristics of groups. |
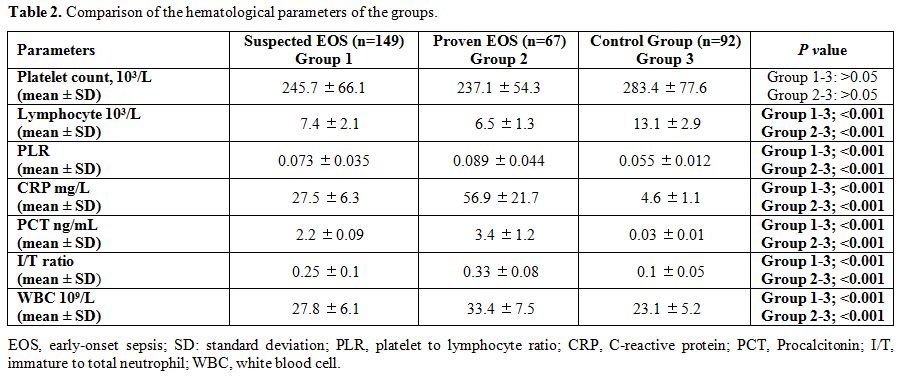 |
Table 2. Comparison of the hematological parameters of the groups. |
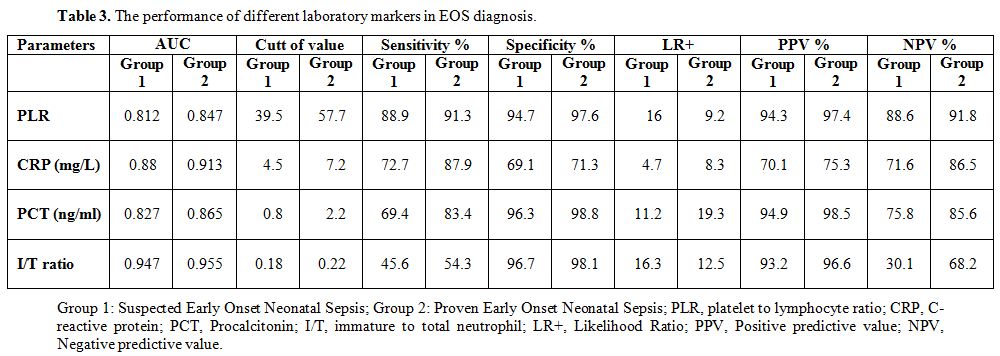 |
Table 3. The performance of different laboratory markers in EOS diagnosis. |
Discussion
In
neonatal sepsis, early diagnosis and therapy are crucial to prevent
morbidity and mortality. However, there is no excellent biomarker to
use in predicting the diagnosis of NS. Many studies have been
evaluating the sensitivity and specificity of the NS diagnostic markers
(e.g., CRP, PCT, immature to total neutrophil ratio, CBC parameters)
and results vary extensively among studies.
Celik et al., while
studying the relationship between CRP and NS, evaluated the accuracy
and cut-off levels of CRP and interleukin-6 (IL)-6 in the diagnosis of
NS and they reported the cut-off values of CRP and IL-6 to be 4.8 mg/L
and 24.65 pg/ml respectively. They determined the sensitivity,
specificity, positive predictive value (PPV), and negative predictive
value (NPV) for CRP to be 67%, 97%, 99%, and 39%, respectively, and for
IL-6 they were 72%, 84%, 95%, and 42%, respectively.[21]
Cetinkaya et al. evaluated the serum amyloid A protein concentrations
together with those of the CRP and PCT in the process of diagnosis and
follow-up of NS in premature infants. They reported the sensitivities
for CRP, PCT, and serum amyloid A to be of 72.3%, 74.8%, and 76.4%,
respectively.[22] In another study, Abdollahi et al.
determined that the simultaneous measurement of PCT, IL-6, and
high-sensitive-CRP (hs-CRP) which is more sensitive in the diagnosis of
NS. They found that the combination of PCT and IL-6 had a sensitivity
of 88%; PCT and hs-CRP had a sensitivity of 82%.[23]
In Ng et al.’s studies, the range of CRP sensitivity and specificity
has been reported to be 35%–94% and 60%–96%, respectively.[24]
Hofer et al. investigated the relationship between CRP and early-onset
neonatal sepsis (EONS). They reported that CRP values might be low due
to the delay in CRP synthesis early in the development of the
infection. CRP was reported to have low sensitivity during the initial
hours of sepsis in previously published studies. Moreover,
non-infectious factors may influence CRP kinetics; for example,
delivery complications have been associated with non-specific
elevations of CRP in the early perinatal period.[25]
Aydemir et al. studied CRP levels in clinical and proven sepsis. They
reported the CRP cut-off to be 7.0 mg/L for proven sepsis. At this
cut-off, the sensitivity, specificity, PPV, and NPV were 76.5%, 98.2%,
94.9%, and 90.5%, respectively. For the diagnosis of clinical sepsis,
with CRP cut-off of 2.6 mg/L, the sensitivity, specificity, PPV, and
NPV were 73.6%, 83.0%, 67.2%, and 86.9% respectively.[26]
In our study, we found the cut-off values of CRP in suspected EOS and
proven EOS 4.5, 7.2 mg/L, respectively. At this cut-off, the
sensitivity, specificity, PPV, and NPV in suspected and proven EOS were
72.7%, 87.9%, 69.1%, 71.3%, 70.1%, 75.3%, and 71.6%, 86.5% respectively.
PCT
is physiologically produced by thyroid C-cells as a precursor of
calcitonin, an acute-phase protein secreted by several tissues in
response to various endogenous and exogenous stimuli such as cytokines
and lipopolysaccharide, acting as a chemo-attractant factor on blood
monocytes.[27] In healthy neonates, plasma PCT values
increase gradually after birth, reach peak values after 24 h of age
(mean 1.5-2.5 ng/ml, range 0.1-20 ng/ml) and then decrease to normal
values below 0.5 ng/ml by 48-72 h of age. A number of studies in
children and neonates after 72 h of age, demonstrated that PCT values
less than 0.5 ng/ml seem to be normal; increases to 0.5-2 ng/ml seem to
be related to non-infectious inflammation, viral or focal bacterial
infections; increases above a PCT value of 2-2.5 ng/ml, seem to be
related to bacterial or fungal systemic infections.[28-30]
Some
studies on the relationship between PCT and NS report that falsely high
PCT levels have been detected in neonates due to non-infectious
critical diseases. Moreover, normal PCT levels have been reported in
severely infected newborns.[31-33] Although several
studies demonstrated the correlation between a low PCT level
(< 2ng/ml) and Candida infection and high NPV of PCT for Candida
isolation, its role in the management of antifungal treatment is far
from established mainly because of the limitations in study design of
supporting literature. A recently published research agenda on invasive
fungal infections reported the “Utilization of PCT to guide treatment
initiation and duration” as one of the ten priority for future trials
in the field.[34] In another study, Altunhan et al.
compared the PCT levels for the diagnosis of EOS, in neonates with
infectious and non-infectious processes. They did not identify the
difference between the groups’ PCT levels at birth. However, the PCT
levels were significantly higher in newborns with suspected sepsis at
24 h of age, and at a cut-off value of 5.3 ng/mL.They determined that
the specificity, sensitivity, PPV, and NPV were all increased compared
to the cut-off value of 0.59 ng/mL at birth.[35] In
our study, we found the cut-off values of PCT in suspected EOS and
proven EOS 0.8, 2.2 mg/L, respectively. At this cut-off, the
sensitivity, specificity, PPV, and NPV in suspected and proven EOS were
69.4%, 83.4%, 96.3%, 98.8%, 94.9%, 98.5%, and 75.8%, 85.6% respectively.
A
number of the studies have explored the role of various parameters of
complete blood count on the diagnosis of neonatal sepsis, e.g., white
blood cell count (WBC), absolute neutrophil count (ANC), immature/total
leucocyte ratio (I:T ratio), MPV, RDW, PDW, neutrophil and lymphocyte
count. Hornik et al. reported that low WBC count, low ANC, and high I:
T ratio were associated with a higher risk of infection and that these
markers have high specificity and NPV but low sensitivity.[36]
Murphy et al. reported that the combination of two consecutive normal
I: T ratio results and a sterile blood culture has 100% NPV.[37]
Shaaban et al. were investigating MPV value as a diagnostic tool in
early-onset neonatal sepsis (EOS). They reported that MPV was found to
be higher in the sepsis group and sensitivity and specificity on MPV
were 97.1% and 100%, respectively.[38] Patrick et al.
evaluated 156 newborns and demonstrated that MPV measurements were
considerably higher in patients with bacteremia than in newborns
without infection. The authors reported the MPV sensitivity and
specificity for the diagnosis of sepsis to be 42% and 95%,
respectively.[39] Zhang et al. studied the utility of
red cell distribution width (RDW), platelet distribution width (PDW),
neutrophil-lymphocyte count ratio (NLCR), PCT, and CRP in the diagnosis
of neonatal sepsis NS. They found that PCT has the highest sensitivity
(91.7%), and PDW has the highest specificity (84.7%).[40]
In this study, RDW, PDW, NLCR have a sensitivity of 73.3%, 38.3%, and
81.1%; a specificity of 49.2%, 84.7%, and 62.7%; a PPV of 59.1%, 71.5%,
and 68.5%, and a NPV of 64.8%, 57.9%, and 76.8%, respectively.
The
physiological immune response of circulating leukocytes to numerous
stressful events is characterized by a raised neutrophil count and
decreased lymphocyte count. A microbial infection causes an increase of
the total leukocyte and neutrophil counts and results in an
inflammatory reaction. For this reason, these counts might be used as
diagnostic markers of microbial infection.[41-42]
Platelet to lymphocyte ratio (PLR) is a new and easily calculated
value, and it is proven to have a high predictive value at diagnosis of
inflammatory diseases in adults.[15-18] Our study’s
goal was identifying the utility of PLR in the prediction and
suspicious diagnosis of early-onset neonatal sepsis. There is only one
study on PLR in neonatal sepsis so far, to the best of our knowledge.
Can et al. reported that a neutrophil to lymphocyte ratio (NLR) of 6.76
was the predictive cut-off value of EOS (sensitivity 97.4%; specificity
100%; AUROC curve 0.99; P=0.001), and a PLR of 94.05 was determined as
the predictive cut-off value of EOS (sensitivity 97.4%; specificity
100%; AUROC curve 0.93; P=0.001).[43] In our study,
we identified that the PLR levels of suspected and definite EOS were
significantly higher than that of the control group. PLR value of
neonates with suspected EOS had a cut-off level of 39.5, 88.9%
sensitivity, 94.7% specificity, 94.3% PPV, and 88.6% NPV. PLR value in
neonates with definite EOS had a cut-off level of 57.7, 91.3%
sensitivity, 97.6% specificity, 97.4% PPV, and 91.8% NPV. CRP, in
suspected and definite EOS, had a cut-off level of 4.5-7.2 mg/L,
72.7%-87.9% sensitivity, 79.1%-81.3% specificity, 94.9%-98.5% PPV, and
75.8%-85.6% NPV, respectively. PCT in suspected and definite EOS had a
cut-off level of 0.8-2.2 ng/mL, 69.4%-83.4% sensitivity, 96.3%-98.8%
specificity, 77.6%-82.4% PPV, and 76.9%-87% NPV, respectively. It was
confirmed that PLR has a higher specificity and PPV in comparison with
other biomarkers used in the diagnosis of EOS.
Furthermore,
sensitivity, specificity, PPV, and NPV values of PLR were found to be
higher than CRP and PCT. Based on these findings of our study, we
conclude that PLR is cost-effective, easily calculated, needs a small
amount of blood, is an easy test to perform, and has high sensitivity,
specificity, PPV, and NPV values. We determined that PLR is a reliable
marker to be used in the early prediction of EONS and maybe a good
alternative to others, currently used parameters.
The strengths of
our study include a large sample size and the point that it compared
suspected and definite EOS, proven EOS, and assigning a control group.
Our study also has some limitations: first, it was performed
retrospectively; second, even though we excluded patients with other
inflammatory diseases, accompanying inflammatory comorbidities may have
influenced the reliability of the results. Of course, the specificity
of this test has been evaluated in the context of strict adherence to
the criteria adopted in choosing the subjects studied.
To
summarize, identifying a biomarker with a high predictive value is
significance for early diagnosis, treatment, and prevention of NS.
Based on our results, we consider that PLR can be used as a new
biomarker in the early detection of EOS.
References
- Y. Dong, C.P. Speer, Late-onset neonatal sepsis:
recent developments, Arch. Dis. Child. Fetal Neonatal Ed. 100 (3)
(2015) F257-F263. https://doi.org/10.1136/archdischild-2014-306213 PMid:25425653 PMCid:PMC4413803
- R.A.
Polin, F. Committee on, Newborn, Management of neonates with suspected
or proven early-onset bacterial sepsis, Pediatrics 129 (5) (2012)
1006-1015. https://doi.org/10.1542/peds.2012-0541 PMid:22547779
- Cohen
J, Vincent JL, Adhikari NKJ, Machado FR, Angus DC, Calandra T, et al.
Sepsis: A roadmap for future research. Vol. 15, The Lancet Infectious
Diseases. 2015. p. 581-614. https://doi.org/10.1016/S1473-3099(15)70112-X
- J.S. Gerdes, Diagnosis and management of bacterial infections in the neonate, Pediatr. Clin. N. Am. 51 (4) (2004) 939-959 https://doi.org/10.1016/j.pcl.2004.03.009 PMid:15275982
- Mukhopadhyay
S, Taylor JA, Von Kohorn I, et al. Variation in sepsis evaluation
across a national network of nurseries. Pediatrics 2017;139(3) https://doi.org/10.1542/peds.2016-2845 PMid:28179485
- Mussap M, Noto A, Cibecchini F, Fanos V. The importance of biomarkers in neonatology. Semin Fetal Neonatal Med. 2013;18:56-64. https://doi.org/10.1016/j.siny.2012.10.006 PMid:23164809
- Jyothi
P, Basavaraj MC, Basavaraj PV. Bacteriological profile of neonatal
septicemia and antibiotic susceptibility pattern of the isolates. J Nat
Sci Biol Med 2013;4:306-9. https://doi.org/10.4103/0976-9668.116981 PMid:24082722 PMCid:PMC3783770
- Benitz WE. Adjunct laboratory tests in the diagnosis of early-onset neonatal sepsis. Clin Perinatol 2010;37:421-38. https://doi.org/10.1016/j.clp.2009.12.001 PMid:20569816
- J.R.
Delanghe, M.M. Speeckaert, Translational research and biomarkers in
neonatal sepsis, Clin. Chim. Acta 451 (Pt A) (2015) 46-64. https://doi.org/10.1016/j.cca.2015.01.031 PMid:25661089
- Su
H, Chang SS, Han CM, et al. Inflammatory markers in cord blood or
maternal serum for early detection of neonatal sepsis-a systemic review
and meta-analysis. J Perinatol 2014;34:268-74. https://doi.org/10.1038/jp.2013.186 PMid:24457256
- Zhou M, Cheng S, Yu J, et al. Interleukin-8 for diagnosis of neonatal sepsis: a meta-analysis. PLoS One 2015;10:e0127170 https://doi.org/10.1371/journal.pone.0127170 PMid:25996378 PMCid:PMC4440704
- Laborada
G, Rego M, Jain A, Guliano M, Stavola J, Ballabh P, et al. Diagnostic
value of cytokines and C-reactive protein in the first 24 hours of
neonatal sepsis. Am J Perinatol. (2003) 20:491-501. doi:
10.1055/s-2003- 45382 5. https://doi.org/10.1055/s-2003-45382 PMid:14703598
- Chiesa
C, Pacifico L, Osborn JF, Bonci E, Hofer N, Resch B. Early-onset
neonatal sepsis: still room for improvement in procalcitonin diagnostic
accuracy studies. Medicine (Baltimore). 2015;94:e1230. https://doi.org/10.1097/MD.0000000000001230 PMid:26222858 PMCid:PMC4554116
- Hedegaard
SS, Wisborg K, Hvas A-M. Diagnostic utility of biomarkers for neonatal
sepsis-a systematic review. Infect Dis (Lond). 2015;47:117-124. https://doi.org/10.3109/00365548.2014.971053 PMid:25522182
- Wang
Z, Peng S, Wang A, Xie H, Guo L, Jiang N, Niu Y. Platelet-lymphocyte
ratio acts as an independent predictor of prognosis in patients with
renal cell carcinoma. Clin Chim Acta. 2018 May;480:166-172. Epub 2018
Feb 17. https://doi.org/10.1016/j.cca.2018.02.014 PMid:29462592
- Chen
H, Xue H, Liu W, Wu F, Wang Y, Gao H. Meta-analysis of Platelet
Lymphocyte Ratio as A Prognostic Factor for
Non-small Cell Lung Cancer.
Zhongguo Fei Ai Za Zhi. 2019 May 20;22(5):289-298. https://doi.org/10.3779/j.issn.1009-3419.2019.05.05
- Shen
Y, Huang X, Zhang W. Platelet-to-lymphocyte ratio as a prognostic
predictor of mortality for sepsis: interaction effect with disease
severity-a retrospective study. BMJ Open. 2019 Jan 25;9(1):e022896. https://doi.org/10.1136/bmjopen-2018-022896 PMid:30782690 PMCid:PMC6352809
- Djordjevic
D, Rondovic G, Surbatovic M, Stanojevic I, Udovicic I, Andjelic T et
al. Neutrophil-to-Lymphocyte Ratio, Monocyte-to-Lymphocyte Ratio,
Platelet-to-Lymphocyte Ratio, and Mean Platelet Volume-to-Platelet
Count Ratio as Biomarkers in Critically Ill and Injured Patients: Which
Ratio to Choose to Predict Outcome and Nature of Bacteremia? Mediators
Inflamm. 2018 Jul 15;2018:3758068. eCollection 2018. https://doi.org/10.1155/2018/3758068 PMid:30116146 PMCid:PMC6079471
- Faul,
F., Erdfelder, E., Buchner, A., & Lang, A.-G. (2009). Statistical
power analyses using G*Power 3.1: Tests for correlation and regression
analyses. Behavior Research Methods, 41, 1149-1160. https://doi.org/10.3758/BRM.41.4.1149 PMid:19897823
- European Medicines Agency (EMA), Report on the Expert Meeting on Neonatal and Pediatric Sepsis, 2010
- Celik
IH, Demirel FG, Uras N, et al. What are the cut-off levels for IL-6 and
CRP in neonatal sepsis? J Clin Lab Anal 2010;24:407-12. https://doi.org/10.1002/jcla.20420 PMid:21089127
- Cetinkaya
M, Ozkan H, Köksal N, et al. Comparison of serum amyloid A
concentrations with those of C-reactive protein and procalcitonin in
diagnosis and follow-up of neonatal sepsis in premature infants. J
Perinatol 2009;29:225-31. https://doi.org/10.1038/jp.2008.207 PMid:19078972
- Abdollahi
A, Shoar S, Nayyeri F, Shariat M. Diagnostic Value of Simultaneous
Measurement of Procalcitonin, Interleukin-6 and hs-CRP in Prediction of
Early-Onset Neonatal Sepsis. Mediterr J Hematol Infect Dis.
2012;4:e2012028. https://doi.org/10.4084/mjhid.2012.028 PMid:22708043 PMCid:PMC3375671
- Ng PC, Lam HS. Diagnostic markers for neonatal sepsis. Curr Opin Pediatr. 2006;18:125-31 https://doi.org/10.1097/01.mop.0000193293.87022.4c PMid:16601490
- Hofer
N, Zacharias E, Müller W, Resch B. An update on the use of C-reactive
protein in early-onset neonatal sepsis: current insights and new tasks.
Neonatology. 2012;102:25-36 https://doi.org/10.1159/000336629 PMid:22507868
- Aydemir
C, Aydemir H, Kokturk F, Kulah C, Mungan AG. The cut-off levels of
procalcitonin and C-reactive protein and the kinetics of mean platelet
volume in preterm neonates with sepsis. BMC Pediatr. 2018; 18: 253. https://doi.org/10.1186/s12887-018-1236-2 PMid:30068303 PMCid:PMC6090766
- Fendler
WM, Piotrowski AJ. Procalcitonin in the early diagnosis of nosocomial
sepsis in preterm neonates. J Paediatr Child Health. 2008;44(3):114-8. https://doi.org/10.1111/j.1440-1754.2007.01230.x PMid:17927729
- Chiesa
C, Panero A, Rossi N, Stegagno M, De Giusti M, Osborn JF, Pacifico L.
Reliability of procalcitonin concentrations for the diagnosis of sepsis
in critically ill neonates. Clin Infect Dis. 1998;26(3):664-72. https://doi.org/10.1086/514576 PMid:9524841
- Gendrel
D, Assicot M, Raymond J, Moulin F, Francoual C, Badoual J, Bohuon C.
Procalcitonin as a marker for the early diagnosis of neonatal
infection. J Pediatr. 1996;128(4):570-3. https://doi.org/10.1016/S0022-3476(96)70374-8
- van
Rossum AM, Wulkan RW, Oudesluys-Murphy AM. Procalcitonin as an early
marker of infection in neonates and children. Lancet Infect Dis.
2004;4(10):620-30 https://doi.org/10.1016/S1473-3099(04)01146-6
- Monneret
G, Labaune JM, Isaac C, Bienvenu F, Putet G, Bienvenu J. Procalcitonin
and C-reactive protein levels in neonatal infections. Acta Paediatr.
1997;86:209-12. https://doi.org/10.1111/j.1651-2227.1997.tb08870.x PMid:9055895
- Lapillonne
A, Basson E, Monneret G, Bienvenu J, Salle BL. Lack of specificity of
procalcitonin for sepsis diagnosis in premature infants. Lancet.
1998;351: 1211-2. https://doi.org/10.1016/S0140-6736(05)79165-0
- Chiesa
C, Panero A, Rossi N, Stegagno M. Reliability of procalcitonin
concentrations for the diagnosis of sepsis in critically ill neonates.
Clin Infect Dis. 1998;26:664-72. https://doi.org/10.1086/514576 PMid:9524841
- Bassetti
M, Garnacho-Montero J, Calandra T, Kullberg B, Dimopoulos G, Azoulay E,
Chakrabarti A, Kett D, Leon C, Ostrosky-Zeichner L, Sanguinetti M,
Timsit JF, Richardson MD, Shorr A, Cornely OA. Intensive care medicine
research agenda on invasive fungal infection in critically ill
patients. Intensive Care Med. 2017. https://doi.org/10.1007/s00134-017-4731-2 PMid:28255613
- Altunhan
H, Annagür A, Örs R, Mehmetoğlu I. Procalcitonin measurement at 24
hours of age may be helpful in the prompt diagnosis of early-onset
neonatal sepsis. Int J Infect Dis. 2011;15:e854-8. https://doi.org/10.1016/j.ijid.2011.09.007 PMid:22019570
- Hornik
CP, Benjamin DK, Becker KC, et al. Use of the complete blood cell count
in early-onset neonatal sepsis. Pediatr Infect Dis J. 2012;31:799-802. https://doi.org/10.1097/INF.0b013e318256905c PMid:22531231 PMCid:PMC3399972
- Murphy K, Weiner J. Use of leukocyte counts in evaluation of early-onset neonatal sepsis. Pediatr Infect Dis J. 2012;31:16-19. https://doi.org/10.1097/INF.0b013e31822ffc17 PMid:21860335
- Shaaban
HA, Safwat N. Mean Platelet Volume in Preterm: A Predictor of Early
Onset Neonatal Sepsis. J Matern Fetal Neonatal Med. 2018;22:1-6. https://doi.org/10.1080/14767058.2018.1488161 PMid:29886794
- Patrick
CH, Lazarchick J. The effect of bacteremia on automated platelet
measurement in neonates. Am J Clin Pathol. 1990;93:391-4. https://doi.org/10.1093/ajcp/93.3.391 PMid:2309660
- Zang
HB, Chen J, Lan FQ, Ma XJ, Zhang SY. Diagnostic values of red cell
distribution width, platelet distribution width and
neutrophil‑lymphocyte count ratio for sepsis. Experimental and
Therapeutic Medicine. 2016;12:2215-2219. https://doi.org/10.3892/etm.2016.3583 PMid:27698714 PMCid:PMC5038364
- Elawady
S, Botros SK, Sorour AE, et al. Neutrophil CD64 as a Diagnostic Marker
of Sepsis in Neonates. J Investig Med 2014;62:644-9. https://doi.org/10.2310/JIM.0000000000000060 PMid:24463977
- Yoon
NB, Son C, Um SJ. Role of the neutrophil-lymphocyte count ratio in the
differential diagnosis between pulmonary tuberculosis and bacterial
community-acquired pneumonia. Ann Lab Med. 2013;33:105-110 https://doi.org/10.3343/alm.2013.33.2.105 PMid:23482854 PMCid:PMC3589634
- Can
E, Hamilcikan Ş, Can C. The Value of Neutrophil to Lymphocyte Ratio and
Platelet to Lymphocyte Ratio for Detecting Early-onset Neonatal Sepsis.
J Pediatr Hematol Oncol. 2018 May;40(4):e229-e232. https://doi.org/10.1097/MPH.0000000000001059 PMid:29219889



