Daniele Ignazio La Milia1*, Sara Vincenti1*, Barbara Fiori2, Fabio Pattavina1, Riccardo Torelli1, Andrea Barbara2, Malgorzata Wachocka1 , Umberto Moscato2, Simona Sica2, Viviana Amato2, Walter Ricciardi2 and Patrizia Laurenti2.
1 Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma, Italia.
2 Università Cattolica del Sacro Cuore, Roma, Italia.
*Authors
participated equally in this work
Published: November 1, 2019
Received: June 13, 2019
Accepted: October 6, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019062 DOI
10.4084/MJHID.2019.062
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Building-work
activities could cause dust contamination and fungal spores’
dissemination. A significant relationship was found between
building-work activities and the incidence of invasive aspergillosis,
in profoundly immunocompromised patients.
Renovation-works
activities were carried out by four building sites of the hematology
ward in a Teaching Hospital without the interruption of clinical
activities. These sites were monitored by environmental sampling to
determine the particles and fungi count. Clinical surveillance was made
using galactomannan antigen test as a proxy for invasive aspergillosis
diagnosis. A definitive diagnosis of IA was confirmed by clinical and
radiological features.
The galactomannan antigen test showed no
significant difference between presence (2,75%) and absence (5,03%) of
renovation work activities (p=0,522). During the renovation activities,
an increment of IA cases with respect to the control period was not
recorded. The particle counts showed higher values of small and
big-diameter particles before the renovation works if compared to the
end of the activities. It was probably due to the containment measures
implemented during and immediately after the final phases of the
building site. The Fungi counts showed no significant differences
between the phase before and after the renovation activities.
Our
findings show that is possible to perform renovation work, during
clinical activities, by increasing clinical and environmental
surveillance.
|
Introduction
Construction
and renovation activities are an ever-constant phenomenon in Hospitals,
causing dust contamination and dissemination of fungal spores.
Different studies describe a strict association between dust
contamination, as a consequence of building-work activities, and the
dispersion of a large number of fungal spores in the environment.[1,2] In particular the Aspergillus
spores spread in great amount during construction and renovation work.
Several work-related aspergillosis outbreaks have been described in
literature.[3] Aspergillus
is a large genus of ubiquitous filamentous fungi, that can cause
invasive aspergillosis (IA) through the inhalation of the airborne
conidia.[3,4]
The factors that influence with
overall fungi load in building-work activities are: first of all,
construction work characteristics (active construction work, greater
surface area of construction site and demolition are associated with a
higher fungi concentration); later of course, season (lower fungi
concentration in cloudy periods if compared to sunny periods),
temperature (higher temperature associated with higher concentration)
and relative humidity (higher concentration in case of higher relative
humidity).[5,6,7,8] The most frequent environmental
sources of fungi in building-work activities are: inflow of unfiltered
outside air, backflow of contaminated air, unclean air filters,
fireproofing materials, air conditioning out of order, duct systems and
dust above false ceilings.[9,10] Reports about
airborne fungal contamination related to the type of building-work
activities and climatic conditions are described also in Italy.[11]
A review estimates that the overall mortality rate of building work activities-associated fungal infections was almost 50%.[12]
A significant relationship between fungal contamination of air and
surfaces in hematology wards and the incidence of IA has been
demonstrated in non-epidemic situations.[2] For the highly immunocompromised patients the mortality rates associated with IA range from 40% to 90%.[13,14] Besides, IA occurs in <5% of autologous and 5%–10% of allogeneic hematopoietic stem cell transplant recipients.[15,16]
Due
to the difficulties in performing an early diagnosis, IA is associated
with a high mortality rate. In recent years several methods, in
addition to clinical and radiological data were developed to earlier
diagnose IA, including circulating biomarkers, and among these, serum
galactomannan detection has markedly improved the diagnosis of invasive
aspergillosis.[17]
Besides, fluid galactomannan
quantification in BAL fluid has shown excellent sensitivity and
specificity to assist clinical decision-making in confirming or
excluding a diagnosis of IA when clinical findings are not clear.[18]
The
purpose of the present work is to evaluate the possibility of carrying
out renovation activities without stopping the clinical activities in
the high-risk ward, such as the Hematology ward, through the
environmental monitoring (fungi and particle counts) during different
phases of renovation works and measuring the incidence of IA by Fluid
Galattomannan Quantification in biological samples and confirming that
with the clinical and the radiological features of the patients.
Materials and Methods
Building
renovation works, classifiable as type D construction sites according
to the Canadian IPAC, were carried out in the Hematology ward of a
Teaching Hospital in Italy between December 2016 and June 2017.[19]
During the whole period, the clinical activities were not interrupted.
Two hospital rooms have been renovated at a time, in order to allow
activities in the rooms adjacent to the building site. A total of four
building sites were set up overtime for the renovation works, named
Building site 1 (BS1), Building site 2 (BS2), Building site 3 (BS3) and
Building site 4 (BS4) respectively (Figure 1).
During the renovation activities, the following preventive actions were
applied, in order to avoid dust dispersion and fungal spores
dissemination: each area of activities was isolated with appropriate
barriers from the rest of the ward through the construction of an
anteroom, and only authorized personnel were required to pass through
the anteroom. Ceiling barriers were also applied. The HEPA filter-air
extractors were installed as an infection protection measures. As
recommended by CDC[20] thought the HEPA filter-air
extractors, a negative pressurization of the work area was maintained
at all time. The doors of the patient rooms had to be closed all the
time and the cleaning shifts were intensified.
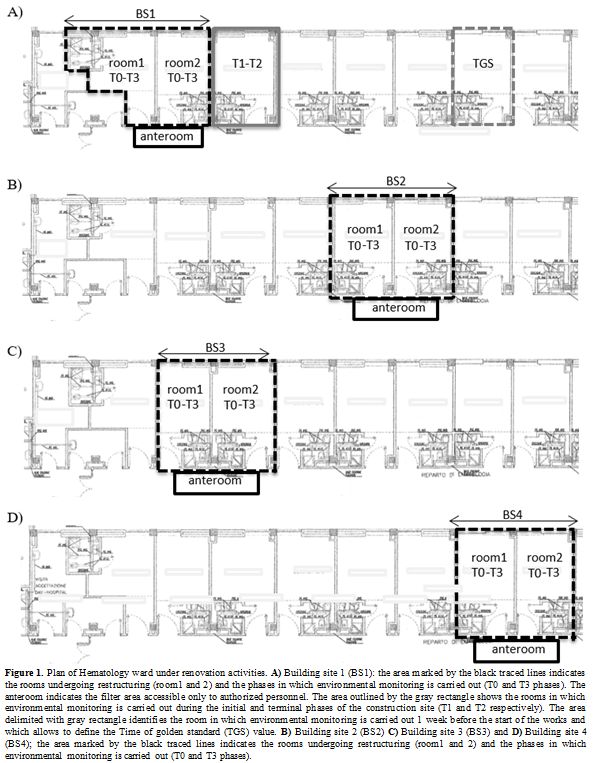 |
Figure 1. Plan of
Hematology ward under renovation activities. A) Building site 1 (BS1):
the area marked by the black traced lines indicates the rooms
undergoing restructuring (room1 and 2) and the phases in which
environmental monitoring is carried out (T0 and T3 phases). The
anteroom indicates the filter area accessible only to authorized
personnel. The area outlined by the gray rectangle shows the rooms in
which environmental monitoring is carried out during the initial and
terminal phases of the construction site (T1 and T2 respectively). The
area delimited with gray rectangle identifies the room in which
environmental monitoring is carried out 1 week before the start of the
works and which allows to define the Time of golden standard (TGS)
value. B) Building site 2 (BS2) C) Building site 3 (BS3) and D)
Building site 4 (BS4); the area marked by the black traced lines
indicates the rooms undergoing restructuring (room1 and 2) and the
phases in which environmental monitoring is carried out (T0 and T3
phases). |
Before
the beginning of the renovation activities, pressure measures (anteroom
vs corridor) were performed with (TESTO-480, TESTO s.p.a.).
At the
end of the renovation activities, and before the employing of the
patient rooms, proper cleaning procedures were applied.
Environmental monitoring.
Environmental sampling was carried out in the patient rooms by a
laboratory technician dedicated to this study. Air sampling was
conducted with the active volumetric Surface Air System sampler (SAS
Super ISO, VWR International Srl). We used plates containing a
selective culture medium for fungi (Sabouraud Dextrose Agar, Liofilchem
S.r.l, (TE) Italy). The volume of air sampled was 1000 m3
(1000 liters). The Sabouraud plates were incubated at 25°C for five
days, and they were checked daily. The number of colonies recovered on
the air sample plates was adjusted for multiple impacts; a positive
hole correction was used to determine the likely number of fungi
passing through the orifices of the grid. This correction was
calculated as reported in the proper instrument user manual according
to J. M. Macher.[21] The concentration of airborne fungi was expressed as the number of colony-forming units per cubic meter of air (CFU/m3).
The isolated colonies were also identified by a lactophenol cotton blue
wet mount preparation and slides were observed under the optical
microscope.
The airborne particle count (APC) in the range size of
0.5-10 μm in diameter was performed using a LIGHTHOUSE HANDHEL 3016 IAQ
portable counter (IQ Air, Incen AG, Goldach, Switzerland) according to
ISO 14644:2015 part 1.[22] This device had a storage capacity up to 3000 records. The data can be normalized to m3.
The sampler was positioned at the height of about 1 m above the floor,
at the potential height of a patient’s “breathing area zone” in bed.
The
monitoring activities in the building site (BS1, BS2, BS3, and BS4)
were performed in several phases, as reported below and shown in figure 1:
Building site 1 (Figure 1 A):
1.
Time of Gold Standard phase (TGS): 1 week before the start of the
renovation activities, particles and fungi counts were performed. The
environmental monitoring in the TGS phase was performed in the farthest
room respect to room object of renovation activities. TGS phase was
useful to determine the “baseline value” of particles and fungi.
2.
T1: At the beginning of the renovation activities. The environmental
monitoring (particles and fungi counts) was carried out "at rest" with
the furniture but without patients inside in the room next to the
building site.
3. T2: At the end of the period
of construction, before the sanification of renovated rooms and after
the removal of the anteroom. The environmental monitoring was performed
in the same patient’s room of T1 phase.
4. T3:
after the sanification of the renovated rooms. Environmental monitoring
was performed "at rest" in the renovated rooms with furniture but
without the patient.
Building site 2:
According
to the results of the environmental monitoring of the BS1 site, the
environmental monitoring of TGS and T2 phases were not performed for
BS2. Phases monitored in the BS2 were reported below (Figure 1 B).
1. T0: before the beginning of renovation works within the room object of renovation activities.
2.
T3: after the sanification of the renovated rooms. Environmental
monitoring was performed "at rest" in the renovated rooms after
cleaning and before patient occupancy.
Building site 3 and Building site 4 were monitored for the same phases reported for BS2 (Figure 1 C and D)
IA at-risk group patients classification:
The clinical data of the patients hospitalized in the Hematology ward
during the renovation work from December 2016 to June 2017 (case
period) and one year later from December 2017 to June 2018 (control
period) were reviewed to identify any cases of IA.
The patients
were categorized as: i) no evidence of risk (group 1), ii) increased
risk (group 2), iii) high risk (group 3) and iiii) very high risk
(group 4), according to their degree of immunocompromise as reported in
the box1 of Talento et al. (i.e. degree of neutropenia, graft versus
host disease, number of allogeneic and autologous HSCT, etc.[23] If more than one risk groups were identified within a specific cohort, the higher risk group was selected (group 4).
Differences
in the percentage of at-risk group categorization during the case and
the control period were evaluated through the chi-squared test or
Fisher exact test, as appropriate. A probability of p<0,05 was
considered statistically significant. All statistical tests were
two-sided. Statistical analysis was performed using Stata IC 14 for Mac
(Intercooled Stata 14 for MacIntosh, Stata Corporation Lakeway, USA,
2015).
Therapeutic regimens:
Antifungal prophylaxis was performed mainly with posaconazole
(300mg/day), and in few cases with fluconazole (400mg/day). The
antifungal treatment was carried out with amphotericin B
(3mg/kg/day) or caspofungin (50mg/day) and voriconazole
(400mg/day). In case of febrile neutropenia, empiric antimicrobial
treatment was performed with piperacillin/tazobactam generic formula
(13.5 mg/day).
Galactomannan detection:
Positivity to galactomannan in bronchoalveolar lavage (BAL) and serum
galactomannan antigen test were used as a proxy for invasive
aspergillosis (IA) diagnosis. Serum specimens from patients admitted to
Hematology ward were collected between December 2016 and May 2017 (case
period) and between December 2017 and May 2018 (control period). These
clinical samples were routinely sent to a microbiological laboratory
for galactomannan (GM) detection. The GM test was performed according
to the manufacturer’s instructions for the Platelia Aspergillus kit
(Bio-Rad Laboratories, CA, USA). The optical density (OD) value for
each hole of the plate was read, and the GM detection value in the
serum or BALF samples was derived as follows: specimen OD value divided
by standard OD value. A serum GM value of 0.5 or higher was considered
positive.[24] If GM detection was negative in the
serum of patient with high suspicious of IA, also the bronchoalveolar
lavage (BAL) was collected, and the Galactomannan antigen test was
performed. The GM positive tests were correlated with clinical and
radiological features for the definitive diagnosis of IA.
Statistical Methods:
The percentage of positive samples was calculated considering the only
samples positive to GM for the single patient: if the serum of a
specific patient was negative to GM, while BAL was positive to GM, we
considered only BAL sample and not the serum.
Differences in
percentage during case and control period were evaluated through the
chi-squared test or Fisher exact test, as appropriate. A probability of
P<0,05 was considered statistically significant. All statistical
tests were two-sided. Statistical analysis was performed using Stata IC
14 for Mac (Intercooled Stata 14 for MacIntosh, Stata Corporation
Lakeway, USA, 2015).
Results
Between
December 2016 and June 2017, four building sites (BS1, BS2, BS3, and
BS4) were carried out for a total of 8 patients’ rooms involved in the
renovation activities of the Hematology ward. Each building site
involved two rooms (room1 and room2) at a time (Figure 1). We registered differential pressures of about 5 Pa between the anteroom vs the corridor for all four BS.
In figure 2 the results of the particle counts were reported. Figure 2
shows the levels of APC at different phases, both in the presence
(phase TGS, T1 and T2) and in the absence of the renovation activities
(phase, T0 and T3). The APC values (0.5 μm, 1.0 μm, 5.0 μm and 10 μm)
of the T0 phase (Figure 2 A-C-E-G) are higher respect of the values observed in the T3 phase (Figure 2 B-D-F-H). The APC values of the T1 phase is lower than the T2 phase and similar to the TGS values.
Specifically, the APC values of the analyzed particles (FIgure 2 A-C-E-G)
in the T2 phase of BS1, BS2 (room2), BS3 (room1) and BS4 (room1
and room2) are higher than the TGS value, with the exception of the
room1 of the BS3 (Figure 2 C-E-G). Regarding the APC values of the T3 phase (Figure 2 B-D-F-H), the BS1 (room1 and room2) and BS2 (room2) for APC of 0.5 µm and 1.0 µm were higher than TGS values.
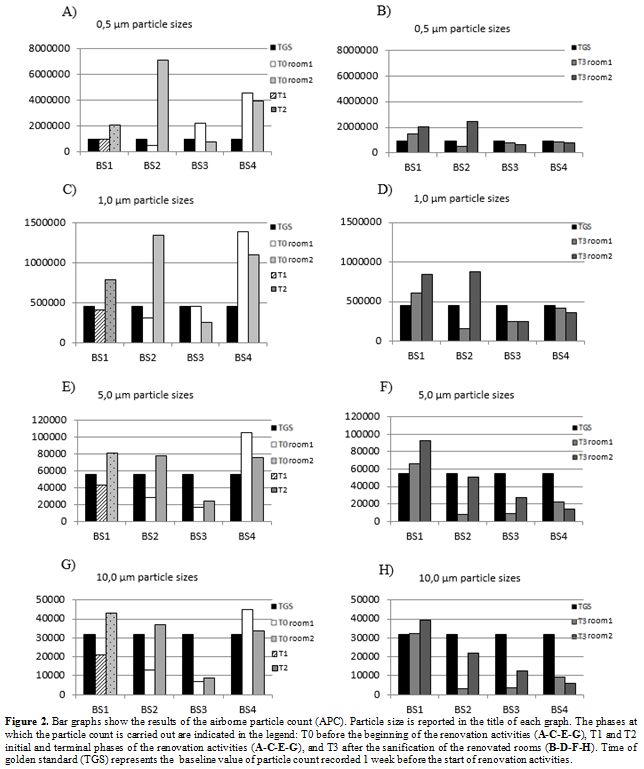 |
Figure 2. Bar graphs show
the results of the airborne particle count (APC). Particle size is
reported in the title of each graph. The phases at which the particle
count is carried out are indicated in the legend: T0 before the
beginning of the renovation activities (A-C-E-G), T1 and T2 initial and
terminal phases of the renovation activities (A-C-E-G), and T3 after
the sanification of the renovated rooms (B-D-F-H). Time of golden
standard (TGS) represents the baseline value of particle count
recorded 1 week before the start of renovation activities. |
Airborne fungi counts were reported in figure 3.
The airborne fungal trend in the phases before the beginning of
renovation activities (T0) and in the phases after renovation (T3),
shows a higher airborne dispersion of fungi in T3 phase. Nevertheless,
a very high concentration of fungi was observed in T0 phases of BS4.
The asterisk reported in figure 3, indicated the presence of Aspergillus spp. A very low concentration of Aspergillus spp. was detected: 1 UFC/m3 for BS1 (T1-room1 and T3-room2), 1 UFC/m3 for BS2 (T0-room1), 1 UFC/m3 for BS3 (T3-room1) and 2 UFC/m3
for BS4 (T0-room1). Considering the importance of the categorization in
at-risk groups for IA during the hospital construction/renovation
works,[24] we classified the patients in at-risk
groups during the control period and the renovation activity period
(case period) as reported in table 1.
The percentage of patients categorized at risk group 4 was higher
during the case period (51,92%), respect to the control period
(33,09%). This difference was statistically significant (p=0.004).
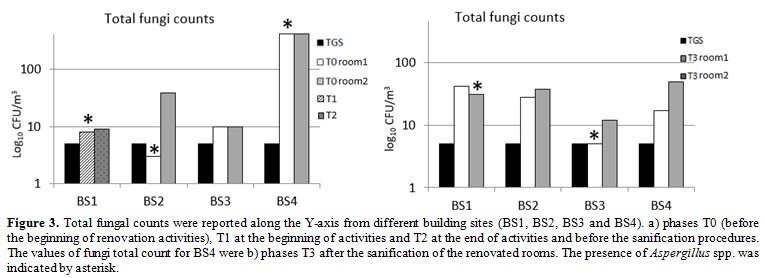 |
Figure 3. Total fungal counts were
reported along the Y-axis from different building sites (BS1, BS2, BS3
and BS4). a) phases T0 (before the beginning of renovation activities),
T1 at the beginning of activities and T2 at the end of activities and
before the sanification procedures. The values of fungi total count for
BS4 were b) phases T3 after the sanification of the renovated rooms.
The presence of Aspergillus spp. was indicated by asterisk. |
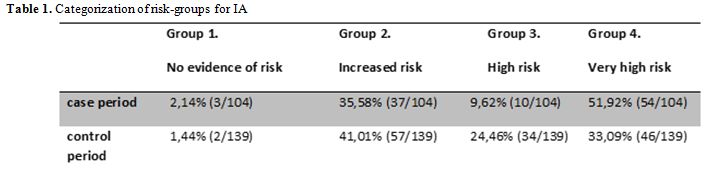 |
Table 1. Categorization of risk-groups for IA |
During
the period of the renovation activities, patients were monitored to
track putative hospital-acquired aspergillosis. Currently, serum
Galactomannan (GM) detection is considered a microbiological diagnostic
criterion for fungal infection in neutropenic patients, according to
the guidelines of the European Organization for Research and Treatment
of Cancer/Invasive Fungal Infections Cooperative Group and the National
Institute of Allergy and Infectious Diseases Mycoses Study Group
(EORTC/MSG).[23] Recently, bronchoalveolar lavage
fluid GM detection was also strongly recommended in the 2016 Infectious
Diseases Society of America guidelines as a test providing high-quality
evidence in neutropenic patients.[25,26] .During the
case period, namely between December 2016 and May 2017, and during the
control period (December 2017 and May 2018) a total respectively of 104
and 139 clinical samples were collected and analyzed to detect GM.
Ninety
of the 104 samples were collected from the blood of patients and 14
from BAL. A total of 4 samples were positive to GM assay (3.85%): 2
samples from blood and 2 samples from BAL. During the control period of
139 samples: 17 were collected from BAL and 122 from blood. Of these, a
total of 8 samples were positive to GM assay (5.76%), 6 samples from
BAL and 2 samples from the blood.
As reported above, 4 and 8
patients were positive to the GM tests during the case and the control
period, respectively. Six of 12 patients positive to GM test, were
treated with piperacillin/tazobactam generic formula. For 10
patients (3 patients in the case period and 7 patients in the control
period), IA diagnosis was confirmed by radiological and clinical data.
The 2 patients that showed positivity to GM assay, but a not supported
IA diagnosis by radiological and clinical data, were simultaneously
subjected to the antimicrobial treatment. Excluding the false positive
to GM test, the rate of patients that shown positivity to GM during the
control period in hematology ward was slightly higher (5,03%) than the
rate of patients positive to galactomannan during the building work
activities (2,75%). This difference was not statistically significant
(P=0,5227).
The standard practice of antifungal chemoprophylaxis
is supported by studies conducted in specific high-risk patient
populations, especially those receiving treatments for hematological
malignancies.[27,28] According to European guidelines,[29]
during the case period, a total of 25 patients were subjected to
antifungal prophylaxis and/or treatment. Three of these showed
positivity to the GM test. For the remaining [22]
patients, who underwent antifungal prophylaxis and/or treatment, the
diagnosis of IA was never confirmed by the clinical and radiological
features. Surprisingly, we found out the same results during the
control period.
Discussion
Demolition,
construction and/or renovation works in Hospitals, may pose a severe
risk to the patients, in particular, those immunocompromised patients.[30,31]
Our study was conducted in the Hematology ward of a large teaching
hospital in Rome during the renovation activities. During the
renovation works, the care activities were maintained. To restructure
the ward, it was necessary to carry out four building sites (BS1,
BS2, BS3, and BS4) sequentially (Figure 1).
Our
findings show that the APC values in the four building sites were
higher in the T0 phase if compared to the values recorded in the T3 and
TGS phases. This is especially true for particles with smaller
diameters (0.5-1.0 µm) because smaller particles persist longer in the
air. This trend can be justified by the fact that during the
construction activities the HEPA filter-air extractors were kept on
night and day, and at the end of the renovation activities, very
accurate and exclusively wet cleanings were performed. Furthermore,
before final sanitization, sterile water was sprayed into the air in
order to precipitate more quickly the bigger particles that are those
that potentially contain microorganisms.
These containment
activities meant that the APC values after the renovation activities
were better than those registered immediately before the renovation
works began.
High levels of all APC values in the T0 phase were
detected for the BS4. It could be because all the furniture had been
removed, but probably the sanitizing procedures were not effectively
carried out, as demonstrated by the presence of dust at the time of
particle counts. Instead, the values of the T3 phase of the BS4 were
comparable with the values of the other building sites.
Fungi
counts showed no distinct differences between the T0 phase and the T3
phase concerning the TGS, and in any case, the recorded values were
slightly higher than the value of the TGS phase. Except for T0 phase of
BS4, which showed a very high level of fungi contamination in
comparison with the other building sites. These data were in accordance
with the APC values registered in the same phase, and it could be
explained as reported above. Even if there are no numerical threshold
guidelines available for Aspergillus spp. Counts a threshold of <5 cfu/m3 inward areas without high-efficiency particulate air (HEPA) filtered rooms have been suggested.[32]
During
the renovation activities, colonies of Aspergillus spp., were isolated
both in T0 phase than in T3 phase; a slightly decreasing trend is
observed in the number of colonies observed in the phase T3 (2 CFU/m3) with respect to the total number of colonies observed in the T0 phase (a total of 4 CFU/m3) respecting the threshold of <5 cfu/m3.[32]
The
at-risk groups' categorization showed a higher percentage of the very
high risk patients (group 4) for IA during the case period (51,92%)
respect the control period (33,9%). The detection of galactomannans in
serum and/or BAL of patients admitted during the case period (2,75%)
and the control period (5,03%) did not show any statistically
significant correlation between the presence of the renovation
activities compared to the absence of these. The GM test is the most
rapid detection method for IA diagnosis; nevertheless, false positive
could be observed in patients simultaneously treated with
piperacillin/tazobactam generic formula.[33] Our
study shows that for 2 patients the positivity to GM test was not
confirmed by clinical and radiological data suggesting an alleged
interference between antimicrobial agent and the GM detection
assay.
Over the past decade, a decreased incidence of IA
has been seen in the Hematological patients, due to improved preventive
measures of isolation and antifungal prophylaxis.[34]
Because
of antifungal mold, active prophylaxis and/or treatment decreases the
sensitivity of serum GM assay, clinical and radiological data of
patients that were negative to GM test, were analyzed in order to find
out the alleged false negative. All the patients that showed negative
results to GM test had a clinical and radiological feature that
confirms a negative diagnosis of IA.
Renovation works represent a major environmental risk factor, necessitating protective measures that have to be implemented.[35] In the literature, renovation activity was linked to increased airborne Aspergillus contamination.[3]
Our results showed that if the appropriate protective measures for the
containment of dust dispersions, were adopted, the renovation
activities may co-exist with those of assistance practices, also in a
ward that accommodate patients at very high risk for invasive
aspergillosis (group 4). The compliance of all the containment measures
such as i) the construction of filter areas accessible only to
authorized personnel, ii) the presence of an extractor with HEPA
filters working day and night, iii) the maintenance of adequate
differential pressures and iiii) the implementation of the procedures
of sanitization with further activities such as the nebulization of
sterile water on air in order to precipitate the bigger particles that
otherwise would take longer to settle.
Although the particle counts are carried out in clean rooms, according to the ISO 14644:2015 part 1,[21]
in order to it assign a specific internationally recognized ISO
classification, its application in environments other than in the
cleanrooms made possible to monitor the dust dispersion trend during
the different phases of the construction and/or renovation activities.
Thus, we think that this count can be useful also for environmental
monitoring.
Conclusions
Environmental
monitoring (particle fungi count) of pre (T0) and post (T3) renovation
activities can be a useful tool to verify if the site activities have
worsened the quality of the environment, and possibly to drive
clinicians towards closer surveillance of patients admitted to the ward
undergoing restructuring. This study suggests the importance of a
multi-professional approach, involving clinicians, hygienists, nurses,
and technicians, that regularly meet to share evidence on environmental
monitoring programs, and results on hospital-acquired infection
incidence. The categorization of the at-risk group,[24]
according to the degree of patients before the beginning of renovation
activities, could be a useful method to potentially reduce the risk of
IA during the renovation works. Environmental data can be correlated
with clinical data in order to preventively evaluate an IA and then
proceed quickly in treating the patient before the onset of clinical
deterioration.
Authors' Contribution
DILM
and SV contributed equally to this work and wrote the original draft;
WR and PL reviewed, edited, and supervised, UM, SS and VA contributed
to the revision of the final manuscript, AB, FP, RT, BF, and MW
contributed reagents/materials/analysis tools, DILM performed
statistical data analysis. All authors contributed to the
revision of the final manuscript.
References
- Alaino A and Bretagne S. Challenges in microbiological diagnosis of invasive Aspergillus infections. F1000Research 2017;6:157. https://doi.org/10.12688/f1000research.10216.1
- Alberti
C, Bouakline A, Ribaud P, Lacroix C, Rousselot P, Leblanc T, Deruin F.
Relationship between environmental fungal contamination and the
incidence of invasive aspergillosis in hematology patients. J Hosp
Infect 2001;48:198-206. https://doi.org/10.1053/jhin.2001.0998
- Goodley
JM, Clayton YM, Hay RJ. Environmental sampling for aspergilla during
building construction on a hospital site. J Hosp Infect 1994; 26:27-35.
https://doi.org/10.1016/0195-6701(94)90076-0
- Fournel
I, Sautour M, Lafon I, Sixt N, L’Ollivier C, Dalle F, Chavanet P,
Couillaud G, Caillot D, Astruc K, Bonnin A, and Aho-Gle´le´ LS, Dijon.
Airborne Aspergillus contamination during hospital construction works:
Efficacy of protective measures. Am J Infect Control 2010;38:189-194. https://doi.org/10.1016/j.ajic.2009.07.011
- Leenders
AC, van Belkum A, Behrendt M, Luijendijk A, Verbrugh HA. Density and
molecular epidemiology of Aspergillus in air and relationship to
outbreaks of Aspergillus infection. J Clin Microbiol 1999;37:1752-7.
- Brenier-Pinchart
MP, Lebeau B, Quesada JL, et al. Influence of internal and outdoor
factors on filamentous fungal flora in hematology wards. Am J
Infect Control 2009;37:631-7. https://doi.org/10.1016/j.ajic.2009.03.013
- Panackal
AA, Li H, Kontoyiannis DP et al. Geoclimatic influences on invasive
aspergillosis after hematopoietic stem cell transplantation. Clin
Infect Dis 2010; 50: 1588–1597.
- Pilmis
B, Thepot-Seegers V, C. Angebault C, et al. Could we predict airborne
Aspergillus contamination during construction work? American Journal of
Infection Control 45 (2017) 39-41 https://doi.org/10.1016/j.ajic.2016.08.003
- Haiduven D. Nosocomial aspergillosis and building construction. Med Mycol 2009; 47(suppl 1): S210–6 https://doi.org/10.1080/13693780802247694
- Moscato U, Borghini A, Teleman AA. HVAC Management in Health Facilities. SpringerBriefs in Public Health, Springer 2017. https://doi.org/10.1007/978-3-319-49160-8_9
- Pini
G, Faggi E, Donato R, Sacco C, Fanci R. Invasive pulmonary
aspergillosis in neutropenic patients and the influence of hospital
renovation. Mycoses 2008; 51:117–22 https://doi.org/10.1111/j.1439-0507.2007.01453.x
- Kanamori
H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks
and infection prevention in healthcare settings during construction and
renovation. Clin Infect Dis. 2015 Aug 1; 61(3): 4 -44 https://doi.org/10.1093/cid/civ297
- Upton
A, Kirby KA, Carpenter P, Boeckh M, Marr K. Invasive aspergillosis
following hematopoietic cell transplantation: outcomes and prognostic
factors associated with mortality. Clin Infect Dis 2007; 44: 531–540. https://doi.org/10.1086/510592
- Neofytos
D, Treadway S, Ostrander D et al. Epidemiology, outcomes, and mortality
predictors of invasive mold infections among transplant recipients: a
10-year, single-center experience. Transpl Infect Dis 2013; 15: 233–242
https://doi.org/10.1111/tid.12060
- Marr
K, Carter R, Crippa F, Wald A, Corey L. Epidemiology and outcome of
mold infections in hematopoietic stem cell transplant recipients. Clin
Infect Dis 2002;34:909–917. https://doi.org/10.1086/339202
- Grow
W, Moreb J, Roque D, et al. Late onset of invasive Aspergillus
infection in bone marrow transplant patients at a university hospital.
Bone Marrow Transplant 2002;29:1519. https://doi.org/10.1038/sj.bmt.1703332
- Zhou
W, Li H, Zhang Y, Huang M, He Q, Li P, Zhang F, Shi Y, Su X, Diagnostic
Value of Galactomannan Antigen Test in Serum and Bronchoalveolar Lavage
Fluid Samples from Patients with Nonneutropenic Invasive Pulmonary
Aspergillosis. Journal of Clinical Microbiology, 2017 ;2153-2161 https://doi.org/10.1128/JCM.00345-17
- Heng
SC, Morrissey O, Chen SC, et al . Utility of bronchoalveolar lavage
fluid galactomannan alone or in combination with PCR for the diagnosis
of invasive aspergillosis in adult hematology patients: a systematic
review and meta-analysis. Crit Rev Microbiol. 2015 Feb;41(1):124-34. https://doi.org/10.3109/1040841X.2013.804033
- IPAC-Canada. Construction-related infection resources. IPAC – Canada (http://www.ipac canada.org/links_construction.php[last accessed April 2019]
- Health
Canada. 2001. Construction-related Nosocomial Infections in Patients in
Health Care Facilities, Decreasing the Risk of Aspergillus, Legionella
and Other Infections, Division of Nosocomial and Occupational
Infections, Bureau of Infectious Diseases, Centre for Infectious
Disease Prevention and Control, Population and Public Health Branch,
Health Canada PL 0603E1, Ottawa, Ontario, Canada K1A0L2
- J.M.
Macher. Positive Hole Correction of MultipleJet-Impactors for
Collecting Viable microorganism. Am. Ind. Hyg. Assoc. J. 1989;50 (11)
561-56. https://doi.org/10.1080/15298668991375164
- ISO
14644:2015 part 1 Cleanrooms and associated controlled environments –
Part 1: Classification of air cleanliness by particle concentration
- Talento
AF, Fitzgerald M, Redington B, O’Sullivan N, Fenelon L, Rogers TR.
Prevention of healthcare-associated invasive aspergillosis during
hospital construction/renovation works. Journal of Hospital Infection
2019;103(1), 1-12. https://doi:10.1016/j.jhin.2018.12.020
- De
Pauw B, Walsh TJ, Donnelly JP, et al.. Revised definitions of invasive
fungal disease from the European Organization for Research and
Treatment of Cancer/ Invasive Fungal Infections Cooperative Group and
the National Institute of Allergy and Infectious Diseases Mycoses Study
Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008; 46:1813–1821.
https://doi.org/10.1086/588660
- Patterson
TF, Thompson GR, III, Denning DW, et al. Executive summary: practice
guidelines for the diagnosis and management of aspergillosis: 2016
update by the Infectious Diseases Society of America. Clin Infect
Dis.2016; 63:433– 442. https://doi.org/10.1093/cid/ciw444
- D’Haese
J, Theunissen K, Vermeulen E, et. al. Detection of galactomannan in
bronchoalveolar lavage fluid samples of patients at risk for invasive
pulmonary aspergillosis: analytical and clinical validity. J Clin
Microbiol. 2012; 50:1258 –1263. https://doi.org/10.1128/JCM.06423-11
- Rogers
TR, Slavin MA, Donnelly JP. Antifungal prophylaxis during treatment for
hematological malignancies: are we there yet? Br J Haematol
2011;153,681-697. https://doi.org/10.1111/j.1365-2141.2011.08650.x
- Ziakas
PD, Kourbeti IS, Mylonakis E. Systemic antifungal prophylaxis after
hematopoietic stem cell transplantation: a metaanalysis. Clin Ther
2014; 36:292-306.e1. https://doi.org/10.1016/j.clinthera.2013.11.010
- Maertens
J, Girmenia C, Brüggemann RJ, et al. European guidelines for
primary antifungal prophylaxis in adult haematology patients: summary
of the updated recommendations from the European Conference on
Infections in Leukaemia. J Antimicrob Chemother 2018;73:3221-3230 https://doi.org/10.1093/jac/dky286
- Iwen
PC, Davis JC, Reed EC, Winfield BA, Hinrichs SH. Airborne fungal spore
monitoring in a protective environment during hospital construction,
and correlation with an outbreak of invasive aspergillosis. Infect
Control Hosp Epidemiol 1994;15:303–306 https://doi.org/10.2307/30146558
- Haiduven D. Nosocomial aspergillosis and building construction. Med Mycol 2009;47:210-6. https://doi.org/10.1080/13693780802247694
- Aspergillosis
Subcommittee of the Health Protection Surveillance Centre Scientific
Advisory Committee. National guidelines for the prevention of
nosocomial aspergillosis. 2018.
- Demiraslan
H, Atalay MA, Eren E, Demir K, Kaynar L, Nedret Koc A, Doganay M.
Assessing the risk of false positive serum galactomannan among patients
receiving piperacillin/tazobactam for febrile neutropenia Medical
Mycology 2017; 55, 535–540. https://doi.org/10.1093/mmy/myw129
- Graf
K, Khani SM, Ott E, Mattner F, Gastmeier P, Sohr D, et al. Five-years
surveillance of invasive aspergillosis in a university hospital. BMC
Infect Dis 2011;11:163. https://doi.org/10.1186/1471-2334-11-163
- Nihtinen
A, Anttila VJ, Richardson M, et al. The utility of intensified
environmental surveillance for pathogenic molds in a stem cell
transplantation ward during construction work to monitor the efficacy
of HEPA filtration. Bone Marrow Transplantation 2007; 40, 457–460. https://doi.org/10.1038/sj.bmt.1705749
