Ηypothyroidism is a common endocrinopathy complicating patients with TDT, mainly Thalassemia Major. Τhe toxicity of iron excess accumulated in thyroid tissues due to the high iron annual input is considered the key pathogenic factor. The prevalence of hypothyroidism in TDT patients varies widely from 5-29%.[1,2] Higher incidences (>35%) were reported in cohorts comprised exclusively of adult TDT patients.[3-5] This wide variation is due to extensive diversity of the cohorts and thyroid dysfunction related factors like genotype, age, clinical severity, frequency of transfusions, and compliance, and efficiency of chelation therapy.[1] The occurrence of HT was recently evaluated in patients on long-term treatment with oral chelators. A study of 83 TDT patients aged 23 ± 12.6 yrs- following Deferasirox (DFX) monotherapy for 3-10 years, failed to detect any HT cases.[6] In contrast prolonged mono-therapies with either Deferiprone (DFP) or Desferrioxamine (DFO) in adolescents showed a ~9.0% HT prevalence.[7]
Splenectomy was also postulated to influence the development of HT in TDT patients. Belhoul et al. reported a 6.3% incidence of HT in 392 TDT patients (30 with splenectomy) aged 15.4 ± 7.6 years. During the study, patients with ferritin >2,500 μg/l had a 3.53 times higher chance of developing hypothyroidism compared to patients with <1000 μg/l and hypothyroidism rate was higher in splenectomized (8/30, 26.6%) than non-splenectomized patients (16/362,4.4%). Ferritin levels were significantly higher in patients with HT (mean 4,101 ± 2,668 μg/l) versus those without (2,597 ± 1976 μg/l), while the ratio of splenectomized with ferritin >2,500 μg/l was 6/8 (75%) versus 9/16 (56%) of non–splenectomized.[8] Severe iron overload (ferritin >2,500μg/l) has been previously linked to higher incidences of HT in TDT.[3]
Tavazi et al. conducted a comparative analysis of NTDT patients concerning splenectomy. Based on the membrane-bound free iron, the total non-heme iron, and the red cell glutathione concentration, they reported excessive oxidative red cells membrane damage in splenectomized patients and postulated that intact spleen represents a pool of excess iron including non-transferrin bound iron.[9]
To investigate the role of splenectomy in the pathogenesis of HT in TDT we selected a group of 52 TDT/HT patients followed and treated in our Unit during 2014. The patients, aged 41-50 years when studied (2014), were part (45.2%) of a large cohort of 115 TDT patients of similar ages (born between 1965-1974) with homogeneous clinical, hematological and biochemical follows and treatment regimens of transfusions and chelation, in accordance to continuously revised national management guidelines. The original cohort comprised of 212 patients- 97 (45.7%) of whom died from complications related to severe iron load. The majority (>75%) died of heart failure due to high iron content.[10] Thus the 115 survivals are patients on relatively efficient chelation, preserving a long-term moderate or mild grade of iron load.
During the first decade, patients’ transfusions included pre-transfusion Hb levels of 6-7 g/dl gradually adjusted to a regimen of pre-transfusion Hb of 9-10 g/dl and transfusion with 10-15 ml Packed Red Cells (PRC)/Kg/BW every 2-3 weeks since the second decade. At this time, chelation started with DFO in low doses and poor compliance, improving progressively. In 1995 a good percentage of patients achieved balanced iron input to output with a mean ferritin reduction to 3,286 ± 1,574 μg/l compared to 4,456±992 μg/l in 1985.[11] The efficiency of chelation enhanced further on intensive monotherapy using DFO and oral chelators DFP and DFX or combined with DFO. By 2014 more than 65% of patients had ferritin levels < 1000 μg/l.
The iron load was followed on sequential ferritin assessments (3-4 times annually) since 1977, while thyroid function was monitored by annual assessments of TSH, FT4, and/or T4, after the age of 10 years. The diagnosis of hypothyroidism was considered after two consecutive measurements (1-2 months apart). For primary HT diagnosis, elevated TSH (>4.6 mIU/L) with low FT4 < 0.7 ng/dl (<9,5 pmol/l) and/ or low T4 (<6.1 μg/dl, <80 nmol/l) for overt and elevated TSH with normal FT4 and T4 values for sub-clinical were considered. Central (secondary) HT, was characterized by low FT4 orT4 with normal TSH values.
Patients were classified into two groups, I and II, including 25 splenectomized and 27 nonsplenectomized patients, respectively. Each group was further subdivided into three subgroups: primary, central, and total (primary and central combined) hypothyroidism. Evaluation of the possible impact of splenectomy on hypothyroidism relied on two key factors: age and iron load grade (ferritin levels) at HT diagnosis and last assessment. (Table 1).
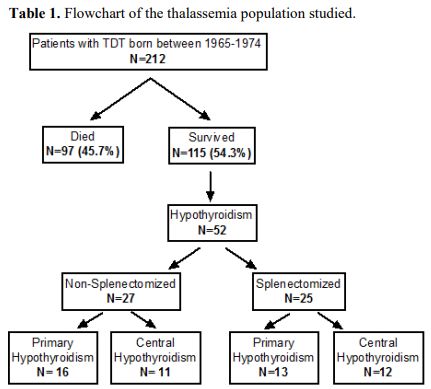 |
Table 1. Flowchart of the thalassemia population studied. |
For statistical analysis, Graph Pad Prism version 8.0.0 for Mac, Graph Pad Software, San Diego, California USA was used, www.graphpad.com. We run descriptive statistics to calculate the mean value and SD for the two variables (age of diagnosis and iron overload) for each different group and subgroup. We compared ferritin levels at diagnosis of HT and last assay in 2014, for all subgroups. Since our values were unpaired and non-parametric, the Man-Whitney tests were used. The p-value was considered statistically significant at< 0.05. The data obtained from the analysis are illustrated in figures 1 and 2.
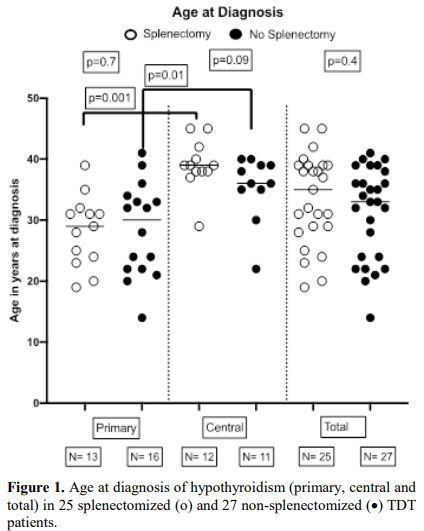 |
Figure 1. Age at diagnosis of hypothyroidism (primary, central and total) in 25 splenectomized (o) and 27 non-splenectomized (•) TDT patients. |
The age at diagnosis of hypothyroidism in splenectomized and non-splenectomized patients was similar with no statistical differences (p values >0.1) for all subgroups. Statistically significant differences in the age at diagnosis were recorded between primary and central hypothyroidism in both splenectomized (p=0.001) and nonsplenectomized (p=0.01) patients. (Figure 1)
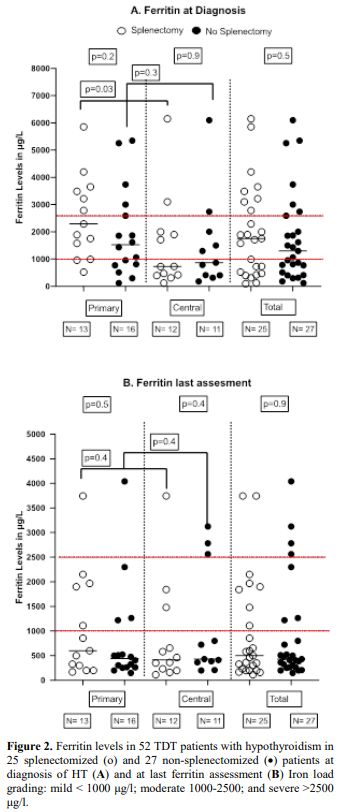
Figure 2 illustrates the distribution of serum ferritin levels and the grade of iron load in the subgroups of hypothyroidism at diagnosis of HT and last ferritin assay in 2014, comparing the results between splenectomized and non-splenectomized patients, separately for primary, central and total hypothyroidism. Statistical analysis showed no differences between splenectomized and non splenectomized patients with primary (p=0.13); central (p=0.92); and total (p=0.38) HT at diagnosis. In all subgroups, ferritin levels at last measurement were lower to those at diagnosis due to the progressive improvement of chelation treatment. However, no statistical differences between the same subgroups were recorded.
A high prevalence of HT was noted in patients with low grade iron load (ferritin<1,000 μg/l) when compared to those with severe grade (>2,500 μg/l) at the time of diagnosis and much more at the last ferritin assessment, regardless of splenectomy. This prevalence of HT in the presence of low grade iron load on the last ferritin (68% with and 74% without splenectomy) is strong evidence that splenectomy is not involved in the mechanisms triggering hypothyroidism in TDT. Advanced aged TDT patients, undergoing efficient and prolonged chelation therapies, may develop hypothyroidism despite low grade iron load and efficient treatment, contradicting the general assumption that hypothyroidism is more frequent in patients with severe grade iron load (up to 3.5 times more).[8] It seems that the prevalence of HT in TDT patients is positively related to age[5] and negatively to the efficiency of chelation.
Casale et al. retrospectively studied the changes of iron balance in 22 splenectomized and 22 nonsplenectomized matched control TDT patients six years prior and ten years after splenectomy. On the evaluation of consecutive ferritin assays of splenectomized patients, they report significantly higher ferritin levels prior to splenectomy compared to non-splenectomized patients and significantly lowered ferritin in the first year post-splenectomy. A slow lowering trend in the following five years was recorded, approaching those of the non-splenectomized matched patients in the sixth year.[12] These results coincide with ours and explain the similarity of ferritin at diagnosis of HT in advanced aged TDT patients, splenectomized and non-splenectomized. In our cohort the mean age at splenectomy (15.7 + 9.3 yrs) was significantly lower than at diagnosis of HT (33.7 + 7.6 yrs), (p<0.0001); furthermore in 22 of 25 splenectomized patients the diagnosis of HT occurred 9-30 years after splenectomy, a period when splenectomized and non-splenectomized patients are expected to have similar ferritin levels as documented by Casale et al.
The normalization of ferritin after intensive chelation reversed thyroid function to normal in 10 of 18 patients with TDT and HT treated with thyroxine.[13] On the contrary, in our series, no reversion occurred, and 22 of 52 patients on intensive chelation and low ferritin (<1000 μg/l) developed HT.
Statistically higher ages were recorded at diagnosis of central HT in both splenectomized and nonsplenectomized patients; the peak rate for primary HT was between 25-35 years and for central 35-45 years (Figure 1). Furthermore, the prevalence of HT patients with low ferritin (<1000 μg/l) at diagnosis was much higher in central than in primary HΤ; namely 58.3% versus 28.5% for splenectomized and 54.5% versus 37.5% for nonsplenectomized (Figure 2).
Analyzing the age at diagnosis of HT of splenectomized and nonsplenectomized, advanced age TDT patients showed no differences between the three subclasses of HT (primary, central, and total), indicating that the occurrence of primary or central HT was independent of splenectomy. Statistically significant higher ages at diagnosis were found in central versus primary hypothyroidism in both groups (Figure 1). Interestingly several patients (42%) developed HT within a period when their ferritin was persistently low (<1000μg/l). These findings call for confirmation in a homogeneous group of TDT advanced aged patients, like the thalassemic populations of countries with successful treatment programs.