Mariagiovanna Cefalo1,*, Ermanno Puxeddu2, Loredana Sarmati3, Giovangiacinto Paterno1, Carla Fontana4, Daniela Nasso1,Gloria Pane2, Eleonora De Bellis1, Raffaele Palmieri1, Elisa Buzzati1, Federico Meconi1, Roberta Laureana1, Paola Casciani1, Anna Giulia Zizzari1, Paola Rogliani2, Paolo de Fabritiis1, Luca Maurillo1, Francesco Buccisano1, Maria Cantonetti1, William Arcese1, Adriano Venditti1 and Maria Ilaria Del Principe1.
1 Hematology, Department of Biomedicine and Prevention, University of Rome "Tor Vergata", Rome, Italy.
2
Division of Respiratory Medicine, Department of Experimental Medicine
and Surgery, University of Rome "Tor Vergata", Rome, Italy.
3 Clinical Infectious Diseases, Department of Systems Medicine, University of Rome "Tor Vergata", Rome, Italy.
4 Clinical Microbiology Laboratories, Department of Experimental Medicine, Tor Vergata University of Rome, Rome, Italy.
Correspondence to: Dr. Mariagiovanna Cefalo, Hematology,
Department of Biomedicine and Prevention, University of Rome "Tor
Vergata", Viale Oxford, 81, 00133 Rome, Italy. Tel. +39 0620903236/
Fax. +39 0620903221. E-mail:
mariagiovanna.cefalo@hotmail.it
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Although bronchoalveolar lavage (BAL) measurements of galactomannan
antigen (GM) seems to be more sensitive than serum testing to detect
invasive fungal infection (IFI), a consensus on the most appropriate
diagnostic threshold of the BAL GM test is still unclear. Moreover,
there is uncertainty as to whether BAL is a safe procedure in patients
with hematological malignancies (HM) and thrombocytopenia.
Objectives:
Based on this background, 102 adult patients with HM and associated
thrombocytopenia were retrospectively analyzed with the dual aim of 1)
determining whether BAL is a safe and feasible procedure; and, 2)
identifying the most appropriate threshold for GM positivity in the
diagnosis of IFI.
Patients/Methods:
each BAL was considered as one case/patient. One hundred twelve BALs
were carried out in 102 HM patients: at the time of the BAL, the median
platelet count (PLTs) in all patients was 47x109/L (1-476), and 31 patients (27%) had PLTs< 20x109/L. Results:
complications from the BAL were infrequent (3.5%) and mild. No bleeding
was reported. The BAL GM cut off of >0.8 was associated with the
best diagnostic accuracy (sensitivity 72.97% and specificity 80%).
Antifungal treatment of patients with BAL GM >0.8 resulted in a
clinical-radiological improvement in 35/41patients (85%).
Conclusions:
BAL was a safe procedure also in thrombocytopenic patients, permitting
an IFI diagnosis not otherwise identifiable using EORTC/MSG criteria.
Our data suggest that a BAL GM value of>0.8 represents the most
useful cut-off in terms of sensibility and specificity. Further
prospective studies on a more significant number of patients are needed
to confirm these results.
|
Introduction
Patients
affected by hematological malignancies (HM) have an increased risk of
invasive fungal infections (IFI) due to prolonged neutropenia, severe
immunosuppression, and chemotherapy-induced damage to mucosal barriers.[1,2]
Considering that IFI remains a major cause of morbidity and mortality
in these patients, timely diagnosis and treatment are required.[3] The diagnosis of IFI relies on clinical, radiological and microbiological criteria.[4] Among microbiological tests, detection of galactomannan (GM), a polysaccharide component of the cell wall of Aspergillus spp., has proven to be more sensitive than culture for an IFI diagnosis.[5] GM is measurable in peripheral blood (serum and plasma) and other biological fluids, such as bronchoalveolar lavage (BAL),[3]
and may be released into the blood and other body fluids in the early
stages of fungal infections, even before clinical and radiological
evidence.[4] However, false-positive results of GM
detection may occur because of co-medications and/or host factors. In
addition, the sensitivity of serum GM tests may decrease in case of
antifungal prophylaxis or empirical/preventive therapy.[3,6,7] For a diagnosis of IFI, the GM test sensitivity is higher with BAL samples than blood samples because airway Aspergillus
invasion always precedes fungal migration into blood vessels followed
by vascular dissemination, resulting in higher quantities of GM in the
bronchial fluid.[4] Current European guidelines5
establish the optimal cut off for GM positivity assay using BAL samples
as between 0.5 and 1, yet the best value within this range has not yet
been further defined.
BAL is considered an accurate and safe procedure;[8,9]
however, complications such as significant bleeding, pneumothorax, and
respiratory distress, although infrequent, have been reported.[1,10-15]
For this reason, the routine use of BAL in patients with HM, who often
present with severe neutropenia and thrombocytopenia, is still a matter
of debate.
The aims of the current study were: 1) to demonstrate
the feasibility and safety of BAL in HM patients; and, 2) to identify a
more specific GM BAL value between 0.5 and 1 that significantly
correlates with a diagnosis of IFI.
Patients and Methods
Study Population.
This retrospective study was conducted between January 2013 and
December 2017 in the Department of Hematology of Fondazione Policlinico
Tor Vergata of Rome, Italy. All consecutive BAL procedures, performed
in adult patients affected by HM, were reported. Each BAL procedure was
considered as one case/patient. BAL was performed within 48-72 hours
following a High-Resolution Computerized Tomography (HRCT) scan of the
chest. HRCT was routinely performed as initial evaluation before the
start of chemotherapy, in the case of a fever persisting more than 72
hours from antibiotic therapy initiation or in the presence of
respiratory signs/chest pains not otherwise explained. Patients records
were analyzed for the following variables: age, gender, hematological
disease, smoking and complications after BAL. BAL complications were
defined as the occurrence of any of the following adverse events during
the procedure and up to 48 hours post-procedure: dyspnea, new or
increased oxygen requirement, fever, hemoptysis, stridor, and
pneumothorax or hemothorax.[16]
White blood
cells (WBC), absolute neutrophil count (ANC), lymphocytes count,
platelets values (PLTs), transfusions, antifungal prophylaxis,
diagnostic microbiology (GM assay in serum and in BAL), imaging,
antifungal therapy and date of death were also recorded. Radiological
experts reviewed all radiological images. Patients were classified as
having possible, probable, proven, or no
IFI, based on the revised European Organization for Research and
Treatment of Cancer/Invasive Fungal Infections Cooperative Group and
the National Institute of Allergy and Infectious Diseases Mycoses Study
Group (EORTC/MSG) criteria.[17,18]
The efficacy of antifungal treatments, based on clinical evaluation and imaging studies,[5]
was evaluated on day 30. Mortality was ascribed to IFI (AMR) if the
patient died within 30 days after microbiological, clinical or imaging
evidence of AMR and if all other alternative causes were excluded.
All personal information was treated secretly, and all clinical data were analyzed anonymously.
Bronchoscopy. Pre-Procedure Preparation:
In patients with suspected IFI, electrocardiogram (ECG), coagulation
studies, platelet count, and hemoglobin concentration were performed
and evaluated prior to BAL. Informed consent was obtained from each
patient before BAL execution. BAL was performed according to the
British Thoracic Society guidelines.[19] Patients with a PLTs count<20x109/L
received pooled PLTs transfusion immediately before the procedure. The
BAL target site was chosen based on chest HRCT images acquired before
the procedure.
BAL Procedure:
BAL was performed using a flexible fiber-optic bronchoscope (Olympus BF
1T 180; Olympus, Hamburg, Germany), according to the American Thoracic
Society guidelines.[20] A small amount of lidocaine
(<200 mg) was used for topical anesthesia. The bronchoscope was
placed in a wedge position within the selected bronchopulmonary
segment, and a volume of 100 ml in 20 ml aliquots of pre-warmed normal
saline solution (at room temperature) was instilled through the
bronchoscope and gently aspirated with negative suction pressure of
less than 100 mm Hg. A minimal sample volume of 5 ml of pooled BAL
fluid was used for BAL microbiological analysis and the GMassay.
GM detection:
BAL fluid and serum specimens were sent to the microbiology laboratory
of Fondazione Policlinico Tor Vergata of Rome for GM assessment, which
was performed using a double-sandwich ELISA test known as Platelia Aspergillus
kit (Bio-Rad Laboratories, CA, USA). The test was run according to the
manufacturer’s instructions. The absorbance (optical density units) of
specimens and controls was determined with a spectrophotometer set at
450 and 620/630 nm wavelength. The whole process was automated using
Evolis Twin Plus (Bio-Rad Laboratories, Mississauga, ON).
The
presence or absence of GM antigen in each sample was determined using
an index value. The index value is the optical density value (ODI) of
the specimen divided by the mean optical density of the wells
containing Cut-off Control Serum: ODI = ODI experimental sample/Mean
Cut-off Control ODI.
Sera with an ODI < 0.50 were qualified
negative for GM antigen detection, while sera with an ODI ≥ 0.50 were
qualified positive for GM antigen.[21] The manufacturer’s most optimal BAL GM cut-off value for positivity is considered between 0.5 to 1.0.[5]
Statistical analysis.
A comparison of dichotomous variables used the chi-square or Fisher
exact test; the independent test or Mann-Whitney test was used for
continuous variables as appropriate. A p-value less than 0.05 was
considered significant.
Diagnostic performance was expressed as
sensitivity and specificity, diagnostic odds ratio, and error odds
ratio relative to ODI cutoff values using two-way contingency tables. A
95% confidence interval (CI) was calculated for each value. The area
under the receiver operating characteristics (ROC) curve was
constructed to assess how changes in the ODI cutoff for the GM EIA
assay altered the sensitivity and the value of 1-minus specificity. All
analyses were performed using the GraphPad Prism 6.0 (GraphPad
Software, San Diego, CA, USA) software package.
Results
One
hundred twelve BALs were performed in 102 HM patients, of which 73
(82%) were male (median age, 48 years; range 18-78). The most common
hematological diagnosis was acute myeloid leukemia (AML; 45%), followed
by non-Hodgkin lymphoma (NHL; 33%). Fifty-four patients (53%) were
smokers. Of the 35 patients (31%) who underwent BAL before induction
course of chemotherapy, 19 (54%) were affected by AML. At the time of
BAL, 39 (35%) patients had severe neutropenia (ANC<0.5x109/L).[16] Forty-four (39%) patients had a lymphocytes count < 1x109/L (Table 1). The median platelet count of all patients was 47x109/L (range 1-476), 51 (45%) and 30 (27%) patients had a PLTS count <40x109/L and <20x109/L, respectively.
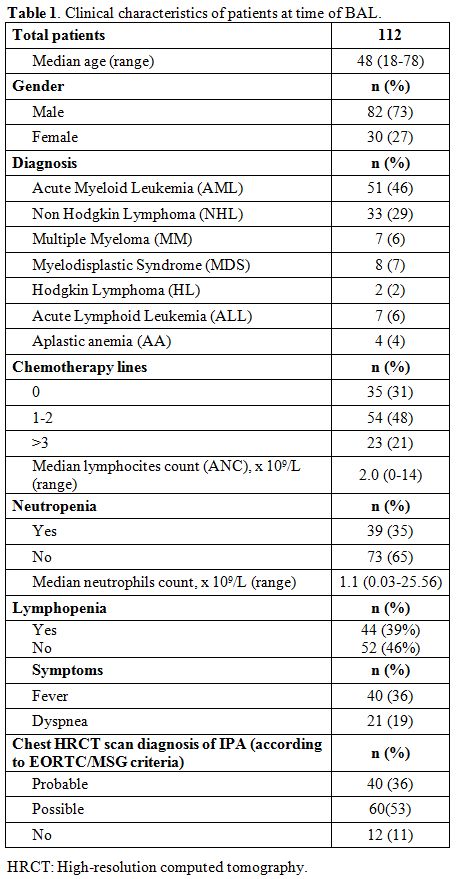 |
Table 1. Clinical characteristics of patients at time of BAL. |
Twenty-nine
patients (26%) underwent BAL while receiving primary or secondary
antifungal prophylaxis (6 posaconazole, 6 voriconazole, 16 fluconazole,
2 liposomal B-amphotericin (L-Amb); 1 caspofungin). Nine patients
underwent multiple BAL procedures in different periods of their
clinical history.
All patients had positive radiological findings.
Forty (36%) patients had an HRCT-fulfilling EORTC/MSG criteria of
probable IFI diagnosis, 60 (53%) a possible IFI diagnosis, and 12 (11%)
had lung infiltrates not classifiable by EORTC/MSG criteria (Table 1).
Of the 112 BAL procedures, GM was found at a value of > 0.5 in 64
patients (57%). Forty-one of these patients had a GM ODI >0.8 and
37/41 had a GM ODI >1. A serum GM assay was performed in 90/112
cases (80%). The median number of serum GM tests was 16.5 (range 3-48).
Among the 90 cases in which both serum and BAL GM were tested, the
number of BAL with a GM >0.5 was significantly higher compared to
the number of serum GM> 0.5 [55/90 (61%) vs 36/90 (40%) p=.004]. The
number of BAL GM positive remained significantly higher than number of
serum GM also when we selected the group of patients with an ODI>0.8
[(37/41(90%) vs. 14/37(37%) p<.0001].
It is important to note
that among the 31 patients who received antifungal prophylaxis, 20
(65%) had a positive BAL GM, while only 13 (42%) had a positive serum
GM.
BAL Safety.
BAL-related complications were observed in 4/112 patients (3.5%); one
patient (0.9%) presented fever after the procedure, while 3 (2.6%)
developed a grade 2 hypoxia requiring intermittent supplemental oxygen.
All complications occurred within 4-6 hours after the procedure.
Regardless of the PLT count, no bleeding was observed.
Correlation of GM levels with radiological diagnosis of fungal infection.
To evaluate the diagnostic performance of GM levels in the serum and
BAL fluid, ROCs were generated as a tool to predict a chest HRCT
pattern that fulfilled the EORTC/MSG criteria of probable or possible
IFI.[17]
Median serum GM levels were 0.42 (range
0.1 – 0.5) in patients with a radiological pattern of a probable
infection, and 0.51 (range 0.43 – 0.62) in those with a radiological
pattern of possible infection or inconsistent with IFI in according
with EORTC/MSG criteria. The ROC curve analysis resulted in an area
under the curve of 0.51 (0.39 – 0.64, p=0.82) (Figure 1 A and B).
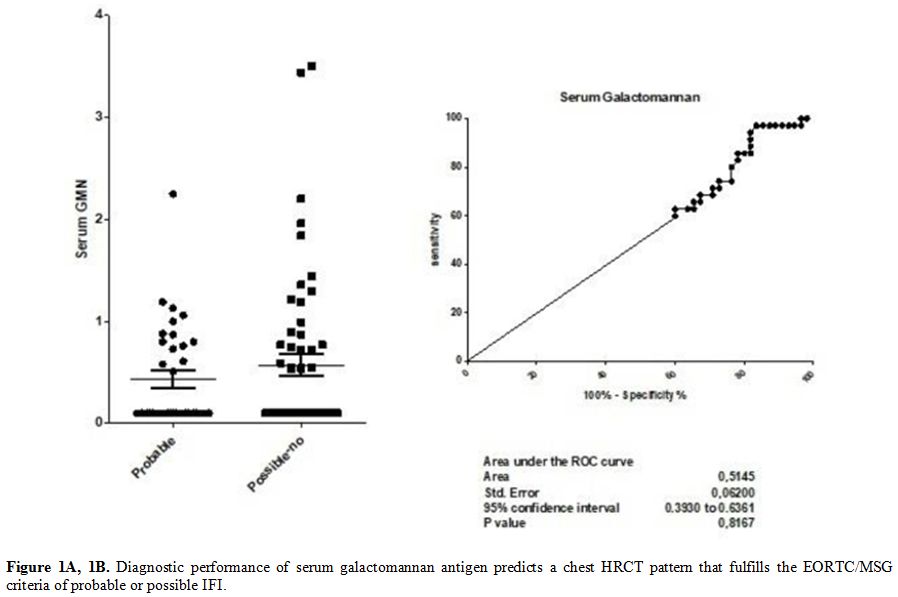 |
Figure 1A, 1B. Diagnostic
performance of serum galactomannan antigen predicts a chest HRCT
pattern that fulfills the EORTC/MSG criteria of probable or possible
IFI. |
Median
BAL GM levels were 2.12 (range 1.35 – 2.72) in patients with a
radiological pattern of probable infection, and 0.48 (range 0.43 –
1.03) in those with a radiological pattern of possible infection or
inconsistent with IFI, according with EORTC/MSG criteria. The ROC curve
analysis resulted in an area under the curve of 0.85 (0.79 – 0.92,
p<0.0001) (Figure 2 A and B). The sensibility and specificity of the different cut-offs in serum and BAL GM values are shown in Tables 2 and 3.
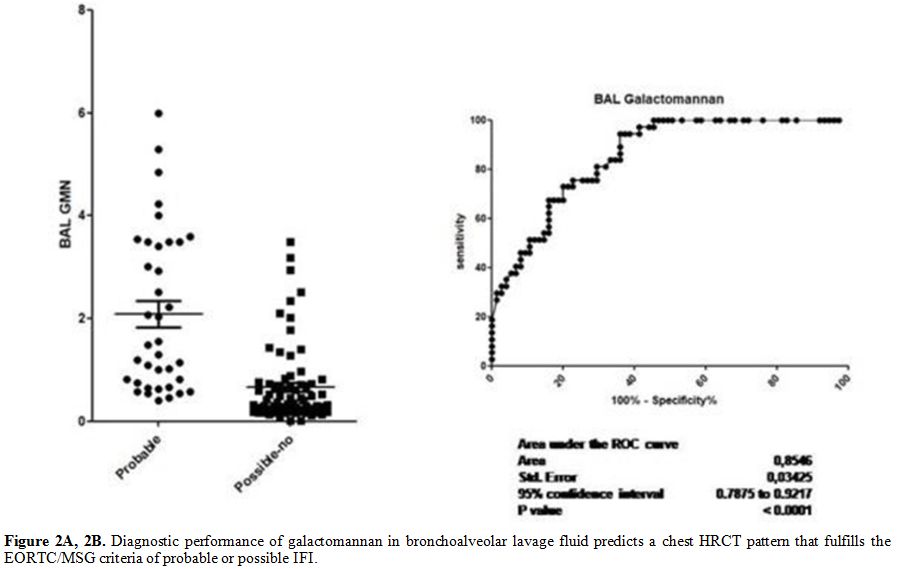 |
Figure 2A, 2B. Diagnostic performance of
galactomannan in bronchoalveolar lavage fluid predicts a chest HRCT
pattern that fulfills the EORTC/MSG criteria of probable or possible
IFI. |
 |
Table 2. Correlation between different cut
off values of serum galactomannan antigen and chest HRCT patterns that
fulfill the EORTC/MSG criteria of probable/possible or no fungal
infection. |
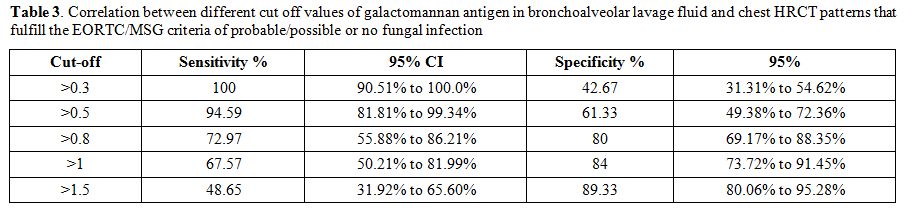 |
Table 3. Correlation
between different cut off values of galactomannan antigen in
bronchoalveolar lavage fluid and chest HRCT patterns that fulfill the
EORTC/MSG criteria of probable/possible or no fungal infection. |
Utility.
Based on the results of BAL GM, 57/64 (89%) patients with an ODI
>0.5 started anti-fungal therapy. Voriconazole or liposomal
amphotericin B (L-Amb) were used in 44 (77%) and 29 (51%) patients,
respectively. Voriconazole was used alone in 25 patients (57%),
combined with L-Amb in 18 patients (41%) or caspofungin in 1 patient
(2%). L-Amb was used alone in 10 patients (18%). Posaconazole and
itraconazole were the other reported options and were utilized in a
minority of patients.
Among the 57 patients who received a BAL
GM-driven antifungal therapy, the HRCT performed after 30 days of
treatment showed an improvement in 35 (61%) cases and stability in 12
(21%) cases. All 41 patients with BAL GM ODI > 0.8 received
antifungal therapy, which was voriconazole in 18 (44%), L-Amb in 10
(24%), and a combination of the two agents in 13 (32%). In this group
of patients, the HRCT after 30 days of treatment showed an improvement
in 21 (51%) cases and stability in 14 (34%) cases. All 35 patients with
BAL GM ODI > 1
received antifungal therapy, which was voriconazole in 16 (46%), L-Amb
in 7 (20%), and voriconazole plus L-Amb in 12 (34%). An improvement
upon radiological examination performed on day 30 was observed in 17
(48%) cases, stability 11 (31%). Finally, we have combined radiological
improvement and stability data assessed by HRCT on day 30 in patients
with a BAL GM value > 0.8, as compared to those with a BAL GM value
> 0.5 (85% vs 82%, p=ns) (Figure 3). On day 30, overall mortality rate was 14% (16/112), while the AMR rate was 9% (10/112).
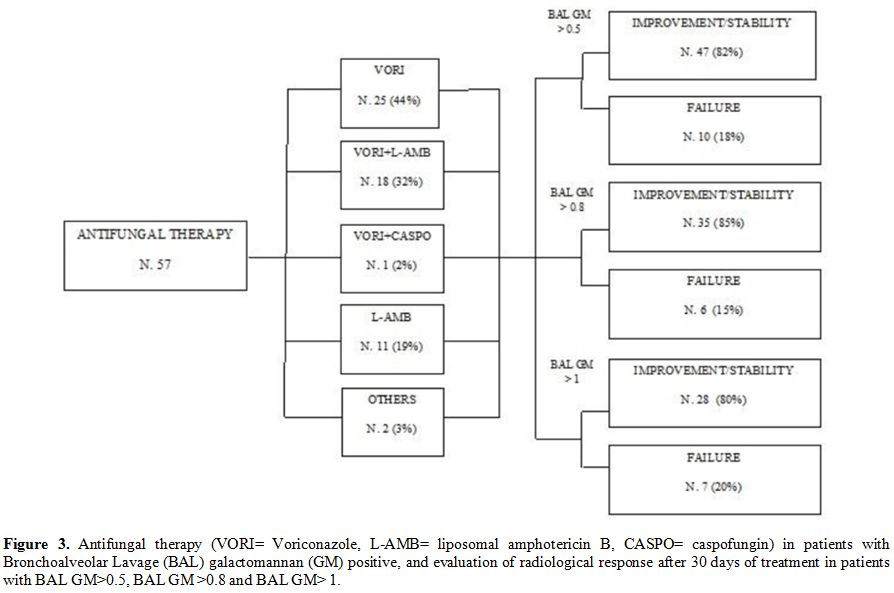 |
Figure 3. Antifungal
therapy (VORI= Voriconazole, L-AMB= liposomal amphotericin B, CASPO=
caspofungin) in patients with Bronchoalveolar Lavage (BAL)
galactomannan (GM) positive, and evaluation of radiological response
after 30 days of treatment in patients with BAL GM>0.5, BAL GM
>0.8 and BAL GM> 1. |
Discussion
This
retrospective study focused on the safety and utility of BAL in
patients with HM and on the diagnostic performance of BAL GM assay. In
a recent observational study of 1949 bronchoscopies performed in a
series of non-HM patients, mild adverse events were reported in 7.2% of
the cases, while moderate adverse events, such as hypoxemia and
bleeding, were described in 4.9% and in 2.1%, respectively.
Furthermore, the rate of severe adverse events requiring additional
intervention was 0.5% (pneumothorax, 0.4%, severe bleeding with patient
death, 0.1%).[11]
Our study included a small but
homogeneous set of cases characterized by the prevalence of patients
with thrombocytopenia. Similar to other reports,[1,22]
BAL was well tolerated and its complications, observed in 3.5% of
patients, were mild and manageable. In patients with PLTs count
<20x109/L, BAL was performed only
by expert operators after prophylactic PLTs transfusion to minimize the
risk of major bleedings and, as a result, no instances of bleeding were
observed.
Our results demonstrated an improved sensitivity of
the BAL GM assay compared to serum GM evaluation. We also observed a
statistically significant correlation between BAL GM values and
radiological patterns. Our findings are consistent with previously
reported results and confirm that BAL GM assay is a more helpful
diagnostic tool than serum GM assay, especially in patients with HM.[4,23-25]
BAL GM positivity was assumed applying the recommended cut-offs, ranging between 0.5 and 1.0.[5,26]
The data presented here suggests that an ODI value of >0.8 is the
best predictor of a positive IFI diagnosis (sensitivity 72.97% and
specificity 80%; Table 3).
Several
BAL procedures were performed in patients with AML before the start of
induction course of chemotherapy based on radiological picture
suggesting fungal infection. Among non-transplanted patients, those
with AML who underwent remission-induction therapy were at the highest
risk to develop IFI.[27] This risk was amplified in
presence of additional risk factors such as severe baseline
neutropenia, a low complete remission rate of haematological disease,
or an age greater than 65.[28] The European
Conference on Infections in Leukaemia (ECIL) recommendations considered
this category of patients for primary mold prophylaxis at an A1 level
of evidence.[29] However, the use of antifungals,
such as triazoles, in this setting is usually considered to
significantly decrease the sensitivity of serum GM assay.[30]
In this study, 31 (28%) patients undergoing BAL for GM detection
received antifungal prophylaxis. Of these, 20 (65%) patients showed BAL
GM positivity and 13 (42%) serum GM positivity.
The practical
utility of BAL GM testing is confirmed by the observation that the
majority of patients who started an antifungal treatment based on BAL
GM positivity showed radiological improvement or stability after 30
days from the start of treatment. Furthermore, a better, although not
significant, radiological result was observed in patients with BAL GM
value > 0.8 respect to those patients with BAL GM value > 0.5.
This observation seems to suggest a higher specificity of the BAL GM
value of 0.8 ODI compared to 0.5 ODI, although more significant sample
size is required to confirm this very preliminary result.
Conclusions
Our
data suggest that BAL can be safely utilized in HM patients with severe
thrombocytopenia and is able to identify IFI that is not otherwise
classifiable with EORTC/MSG criteria, in line with other experiences.[31-32] Moreover, a BAL GM ODI value of>0.8 may represent the most appropriate cut off in terms of sensibility and specificity.[25] Further prospective studies on larger series of patients with a longer follow up are needed to confirm these results.
References
- Svensson T, Lundström KL, Höglund M, Cherif H.
Utility of bronchoalveolar lavage in diagnosing respiratory tract
infections in patients with hematological malignancies: are invasive
diagnostics still needed? Ups J Med Sci. 2017;122(1):56-60. https://doi.org/10.1080/03009734.2016.1237595 PMid:27739337 PMCid:PMC5361433
- Baddley JW. Clinical risk factors for invasive aspergillosis. Med Mycol. 2011;49 Suppl1:S7-S12. https://doi.org/10.3109/13693786.2010.505204 PMid:20718606
- Eigl
S, Hoenigl M, Spiess B, HeldtS,Prattes J, Neumeister P, Wolfler A,
Rabensteiner J, Prueller F, Krause R, Reinwald M, Flick H, Buchheidt D,
Boch T. Galactomannan testing and Aspergillus PCR in same-day
bronchoalveolar lavage and blood samples for diagnosis of invasive
aspergillosis. Med Mycol. 2017;55(5):528-534. https://doi.org/10.1093/mmy/myw102 PMid:27744310
- Gupta
A, Capoor MR, Shende T, Sharma B, Mohindra R, Suri JC, Gupta DK.
Comparative evaluation of galactomannan test with bronchoalveolar
lavage and serum for the diagnosis of invasive aspergillosis in
patients with hematological malignancies. J Lab Physicians.
2017;9(4):234-238. https://doi.org/10.4103/JLP.JLP_127_16 PMid:28966482 PMCid:PMC5607749
- Ullmann
AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K,
Lass-Flörl C, Lewis RE, Munoz P, Verweij PE, Warris A, Ader F, Akova M,
Arendrup MC, Barnes RA, Beigelman-Aubry C, Blot S, Bouza E, Brüggemann
RJM, Buchheidt D, Cadranel J, Castagnola E, Chakrabarti A,
Cuenca-Estrella M, Dimopoulos G, Fortun J, Gangneux JP, Garbino J,
Heinz WJ, Herbrecht R, Heussel CP, Kibbler CC, Klimko N, Kullberg BJ,
Lange C, Lehrnbecher T, Löffler J, Lortholary O, Maertens J, Marchetti
O, Meis JF, Pagano L, Ribaud P, Richardson M, Roilides E, Ruhnke M,
Sanguinetti M, Sheppard DC, Sinkó J, Skiada A, Vehreschild MJGT,
Viscoli C, Cornely OA. Diagnosis and management of Aspergillus
diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin
Microbiol Infect 2018; 24, e1-e38. https://doi.org/10.1016/j.cmi.2018.01.002 PMid:29544767
- Hoenigl
M, Seeber K, Koidl C, Buzina W, Wölfler A, Duettmann W, Wagner J,
Strenger V, Krause R. Sensitivity of galactomannan enzyme immunoassay
for diagnosing breakthrough invasive aspergillosis under antifungal
prophylaxis and empirical therapy.Mycoses. 2013;56(4):471-6. https://doi.org/10.1111/myc.12060 PMid:23432536
- Marr
KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases
sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin
Infect Dis. 2005;40(12):1762-9. https://doi.org/10.1086/429921 PMid:15909264
- Forslöw
U, Remberger M, Nordlander A, Mattsson J. The clinical importance of
bronchoalveolar lavage in allogeneic SCT patients with pneumonia. Bone
Marrow Transplant. 2010; 45(5):945-50. https://doi.org/10.1038/bmt.2009.268 PMid:19784077
- Shannon
VR, Andersson BS, Lei X, Champlin RE, Kontoyiannis DP. Utility of early
versus late fiberoptic bronchoscopy in the evaluation of new pulmonary
infiltrates following hematopoietic stem cell transplantation. Bone
Marrow Transplant. 2010;45(4):647-55. https://doi.org/10.1038/bmt.2009.203 PMid:19684637
- Hummel
M, Rudert S, Hof H, Hehlmann R, Buchheidt D. Diagnostic yield of
bronchoscopy with bronchoalveolar lavage in febrile patients with
hematologic malignancies and pulmonary infiltrates. Ann Hematol.
2008;87(4):291-7. https://doi.org/10.1007/s00277-007-0391-6 PMid:17932672
- Costa
ADS Jr, Scordamaglio PR, Suzuki I, Palomino ALM, Jacomelli M.
Indications, clinical outcomes and complications of 1,949 flexible
bronchoscopies. Einstein (Sao Paulo). 2018;16(4):eAO4380 https://doi.org/10.31744/einstein_journal/2018AO4380 PMid:30427487 PMCid:PMC6223942
- Elston
WJ, Whittaker AJ, Khan LN, Flood-Page P, Ramsay C, Jeffery PK, Barnes
NC. Safety of research bronchoscopy, biopsy and bronchoalveolar lavage
in asthma. Eur Respir J. 2004;24(3):375-7. https://doi.org/10.1183/09031936.04.00063003 PMid:15358694
- Hofmeister
CC, Czerlanis C, Forsythe S, Stiff PJ. Retrospective utility of
bronchoscopy after hematopoietic stem cell transplant. Bone Marrow
Transplant 2006;38:693-8. https://doi.org/10.1038/sj.bmt.1705505 PMid:16980989
- Feinstein
MB, Mokhtari M, Ferreiro R, Stover DE, Jakubowski A. Fiberoptic
bronchoscopy in allogeneic bone marrow transplantation: findings in the
era of serum cytomegalovirus antigen surveillance. Chest
2001;120:1094-100. https://doi.org/10.1378/chest.120.4.1094 PMid:11591544
- Jain
P, Sandur S, Meli Y, Arroliga AC, Stoller JK, Mehta AC. Role of flexible
bronchoscopy in immunocompromised patients with lung infiltrates. Chest
2004;125:712-22 https://doi.org/10.1378/chest.125.2.712 PMid:14769756
- Common Terminology Criteria for Adverse Events (CTCAE) v4.03: June 14, 2010
- De
Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T,
Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson
TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler
CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A,
Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR,
Zaoutis T, Bennett JE; European Organization for Research and Treatment
of Cancer/Invasive Fungal Infections Cooperative Group; National
Institute of Allergy and Infectious Diseases Mycoses Study Group
(EORTC/MSG) Consensus Group. Revised definitions of invasive fungal
disease from the European Organization for Research and Treatment of
Cancer/Invasive Fungal Infections Cooperative Group and the National
Institute of Allergy and Infectious Diseases Mycoses Study Group
(EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813-21. https://doi.org/10.1086/588660 PMid:18462102 PMCid:PMC2671227
- Tsitsikas
DA, Morin A, Araf S, Murtagh B, Johnson G, Vinnicombe S, Ellis S,
Suaris T, Wilks M, Doffman S, Agrawal SG. Impact of the revised (2008)
EORTC/MSG definitions for invasive fungal disease on the rates of
diagnosis of invasive aspergillosis. Med Mycol. 2012;50(5):538-42 https://doi.org/10.3109/13693786.2011.630040 PMid:22074309
- Du
Rand IA, Blaikley J, Booton R, Chaudhuri N, Gupta V, Khalid S, Mandal
S, Martin J, Mills J, Navani N, Rahman NM, Wrightson JM, Munavvar M;
British Thoracic Society Bronchoscopy Guideline Group. British Thoracic
Society guideline for diagnostic flexible bronchoscopy in adults:
accredited by NICE. Thorax. 2013;68 Suppl1:i1-i44. https://doi.org/10.1136/thoraxjnl-2013-203618 PMid:23860341
- Meyer
KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, Drent M,
Haslam PL, Kim DS, Nagai S, Rottoli P, Saltini C, Selman M, Strange C,
Wood B; American Thoracic Society Committee on BAL in Interstitial Lung
Disease. An official American Thoracic Society clinical practice
guideline: the clinical utility of bronchoalveolar lavage cellular
analysis in interstitial lung disease. Am J Respir Crit Care Med.
2012;185(9):1004-14. https://doi.org/10.1164/rccm.201202-0320ST PMid:22550210
- Wei
Zhou, Hongxing Li, Yan Zhang, Huang M, He Q, Li P, Zhang F, Shi Y, Su
X. Diagnostic Value of Galactomannan Antigen Test in Serum and
Bronchoalveolar Lavage Fluid Samples from Patients with Non neutropenic
Invasive Pulmonary Aspergillosis. J Clin
Microbiol.2017;55(7):2153-2161. https://doi.org/10.1128/JCM.00345-17 PMid:28446576 PMCid:PMC5483917
- Boersma
WG, Erjavec Z, van der Werf TS, de Vries-Hosper HG, Gouw AS, Manson WL.
Bronchoscopic diagnosis of pulmonary infiltrates in granulocytopenic
patients with hematologic malignancies: BAL versus PSB and PBAL. Respir
Med. 2007;101(2):317-25. https://doi.org/10.1016/j.rmed.2006.04.021 PMid:16774815
- Guo
YL, Chen YQ, Wang K, Qin SM, Wu C, Kong JL. Accuracy of BAL
galactomannan in diagnosing invasive aspergillosis: a bivariate
metaanalysis and systematic review. Chest 2010;138:817-24. https://doi.org/10.1378/chest.10-0488 PMid:20453070
- Maertens
J, Maertens V, Theunissen K, Meersseman W, Meersseman P, Meers S,
Verbeken E, Verhoef G, Van Eldere J, Lagrou K.. Bronchoalveolar lavage
fluid galactomannan for the diagnosis of invasive pulmonary
aspergillosis in patients with hematologic diseases. Clin Infect Dis.
2009;49(11):1688-93 https://doi.org/10.1086/647935 PMid:19886801
- D'Haese
J, Theunissen K, Vermeulen E, Schoemans H, De Vlieger G, Lammertijn L,
Meersseman P, Meersseman W, Lagrou K, Maertens J. Detection of
galactomannan in bronchoalveolar lavage fluid samples of patients at
risk for invasive pulmonary aspergillosis: analytical and clinical
validity. J Clin Microbiol. 2012;50(4):1258-63 https://doi.org/10.1086/647935 PMid:19886801
- Maertens
JA, Klont R, Masson C,Theunissen K, Meersseman W, Lagrou K, Heinen C,
Crépin B, Van Eldere J, Tabouret M, Donnelly JP, Verweij PE.
Optimization of the cutoff valueforthe Aspergillus double-sandwich
enzyme immunoassay. Clin Infect Dis 2007;44:1329-36. https://doi.org/10.1086/514349 PMid:17443470
- Pagano
L, Caira M, Candoni A, Offidani M, Martino B, Specchia G, Pastore D,
Stanzani M, Cattaneo C, Fanci R, Caramatti C, Rossini F, Luppi M,
Potenza L, Ferrara F, Mitra ME, Fadda RM, Invernizzi R, Aloisi T,
Picardi M, Bonini A, Vacca A, Chierichini A, Melillo L, de Waure C,
Fianchi L, Riva M, Leone G, Aversa F, Nosari A.. Invasive aspergillosis
in patientswith acute myeloid leukemia: a SEIFEM-2008 registry study.
Haematologica. 2010;95(4):644-50. https://doi.org/10.3324/haematol.2009.012054 PMid:19850903 PMCid:PMC2857195
- Nucci
M, Nouér SA, Cappone D, Anaissie E. Early diagnosis of invasive
pulmonary aspergillosis in hematologic patients: an opportunity to
improve the outcome. Haematologica. 2013;98(11):1657-60. https://doi.org/10.3324/haematol.2013.094359 PMid:24186309 PMCid:PMC3815162
- Maertens
JA, Girmenia C, Brüggemann RJ, Duarte RF, Kibbler CC, Ljungman P, Racil
Z, Ribaud P, Slavin MA, Cornely OA, Peter Donnelly J, Cordonnier C;
European Conference on Infections in Leukaemia (ECIL), a joint venture
of the European Group for Blood and Marrow Transplantation (EBMT), the
European Organization for Research and Treatment of Cancer(EORTC), the
Immunocompromised Host Society (ICHS) and; European Conference on
Infections in Leukaemia (ECIL), a joint venture of the European Group
for Blood and Marrow Transplantation (EBMT), the European Organization
for Research and Treatment of Cancer (EORTC), the Immunocompromised
Host Society (ICHS) and the European Leukemia Net (ELN). European
guidelines for primary antifungal prophylaxis in adult haematology
patients: summary of the updated recommendations from the European
Conference on Infections in Leukaemia. J Antimicrob Chemother.
2018;73(12):3221-3230. https://doi.org/10.1093/jac/dky286
- McCulloch
E, Ramage G, Rajendran R, Lappin DF, Jones B, Warn P, Shrief R,
Kirkpatrick WR, Patterson TF, Williams C. Antifungal treatment affects
the laboratory diagnosis of invasive aspergillosis. J Clin Pathol.
2012;65(1):83-6. https://doi.org/10.1136/jcp.2011.090464 PMid:22049217
- Maccioni
F, Vetere S, De Felici C, Al Ansari N, Micozzi A, Gentile G, Foà R,
Girmenia C. Pulmonary fungal infections in patients with acute myeloid
leukemia: is it the time to revise the radiological diagnostic
criteria? Mycoses. 2016; 59(6): 357-64. https://doi.org/10.1111/myc.12480 PMid:26865204
- Marchesi
F, Cattaneo C, Criscuolo M, Delia M, Dargenio M, Del Principe MI,
Spadea A, Fracchiolla NS, Melillo L, Perruccio K, Alati C, Russo D,
Garzia M, Brociner M, Cefalo M, Armiento D, Cesaro S, Decembrino N,
Mengarelli A, Tumbarello M, Busca A, Pagano L on behalf of the
Sorveglianza Epidemiologica Infezioni nelle Emopatie (SEIFEM) Group. A
bronchoalveolar lavage-driven antimicrobial treatment improves survival
in hematologic malignancy patients with detected lung infiltrates: A
prospective multicenter study of the SEIFEM group. Am J Hematol. 2019;
94(10): 1104-1112. https://doi.org/10.1002/ajh.25585 PMid:31321791





