Chiara Mozzini1*, Giancarlo Pesce2, Alder Casadei3, Domenico Girelli1 and Maurizio Soresi4.
1 Department of Medicine, Section of Internal Medicine, University of Verona, Piazzale L.A. Scuro, 10, 37134 Verona, Italy.
2
Sorbonne Universitè INSERM UMR-S1136 Institut Pierre Louis d’
Epidemiologie et de Sanitè Publique, Team EPAR F75012, Paris, France.
3 Ultrasound Association of South-Tyrol, Bolzano Health District, Piazza W.A. Loew-Cadonna 12, 39100 Bolzano, Italy.
4
Department of Health Promotion Sciences Maternal and Infant Care,
Internal Medicine and Medical Specialities, University of Palermo, Via
del Vespro, 141-90127 Palermo, Italy.
Correspondence to: Chiara Mozzini. Department of Medicine, Section of
Internal Medicine, University of Verona, Piazzale L.A. Scuro, 10,37134
Verona, Italy. E-mail:
chiaramozzini@libero.it
Published: November 1, 2019
Received: September 25, 2019
Accepted: October 17, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019066 DOI
10.4084/MJHID.2019.066
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
This
review covers the role of ultrasonography as an essential non-invasive
diagnostic approach when facing patients with anaemia, a common
clinical problem. Abdomen ultrasound is well recognised as a first-line
examination in the setting of blood loss, both acute and chronic. Less
is clear about the additional opportunities, given by ultrasound in
anaemia, due to the many other possible causes.
Here we provide
information on the utility of ultrasound in different contexts and a
practical guide for clinicians facing anaemic patients.
|
Introduction
Anaemia
is the most common haematological disorder, affecting more than two
billions people worldwide, with iron deficiency being the prevalent
cause.[1] According to the World Health Organization,
anaemia is defined by haemoglobin levels less than 13 g/dL in adult
males, and less than 12 g/dL in adult females.[1]
Anaemia is classified in several ways, e.g., acute versus chronic
and/or according to the leading cause, as detailed elsewhere.[2-5]
Ultrasonography, a widely and increasingly used non-invasive diagnostic
approach, can be very useful also in patients with anaemia. The aim of
this paper is to review the role of ultrasound in different conditions,
eventually providing a practical guide for clinicians facing anaemic
patients. A large emphasis is given to the elderly, as well as to
patients with cancer or other chronic diseases, in whom anaemia is an
independent predictor of adverse outcomes, and non-invasive approaches
are often preferred because of their fragile conditions. The possible
applications of ultrasonography in anaemic patients are depicted in Figure 1.
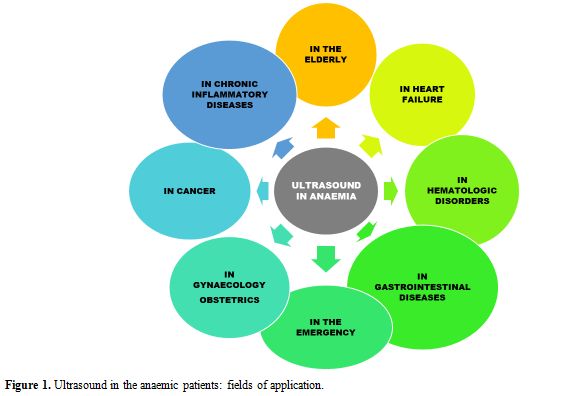 |
Figure
1. Ultrasound in the anaemic patients: fields of application. |
Abdomen Ultrasound: the First Line Examination in Different Clinical Settings
Abdomen ultrasound may be the first-line examination in different settings, as summarised in Figure 2.
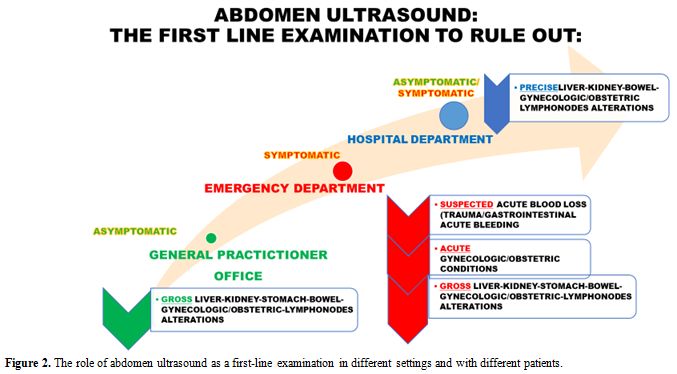 |
Figure 2. The role of abdomen ultrasound as a first-line examination in different settings and with different patients. |
General
Practitioners (GPs) usually represent the first contact with the
healthcare system for patients with chronic anaemia, especially when it
is due to iron-deficiency, which in turn tends to be underdiagnosed
and/or under-coded.[6-8] In this setting, an
appropriate first-line examination aimed not only at the diagnosis of
iron deficiency per se but also addressing its possible aetiology, is
crucial. Indeed, point-of-care abdomen ultrasound could detect gross
alterations (e.g. abdominal masses) guiding further investigation.
Anaemia due to acute blood loss in the Emergency Department.
In the setting of acute bleeding, ultrasound has a prominent role. For
example, in patients referring because of trauma, ultrasound represents
a useful complement to basic clinical evaluation, influencing bedside
decision making, and determining whether or not the patient requires
further procedural intervention. Of note, in as many as 50% of patients
with severe abdominal trauma, the initial physical examination can
appear normal, leading to dangerous reassurance. Similarly, unconscious
patients or those unable to provide a clear history of the trauma
whatever the reason, can be particularly difficult to manage. Thus,
physicians largely depend on diagnostic imaging, so that ultrasound
represents an essential tool in the trauma resuscitation area. In this
setting, the Focused Assessment with Sonography for Trauma (FAST)
evaluation can be of crucial help for the rapid identification of the
presence of free fluid suggestive for hemoperitoneum, hemothorax,
and/or hemopericardium.[9] The overarching assumption
of FAST is that all clinically significant abdominal injuries are
associated with hemoperitoneum. The traditional FAST approach includes
four basic sonographic views: pericardial, perihepatic, perisplenic and
pelvic. The detailed procedure is well described.[10]
Ultrasound can easily detect as little as 200 mL of fluid in the
Morrison pouch. This technique can be completed in less than one
minute. Nevertheless, FAST has a number of limitations, especially in
penetrating trauma, as well as in detecting small retroperitoneal
bleeding. Thus, computed tomography (CT) remains the gold-standard
technique.
Understanding the strengths and limitations of FAST is
essential to recognise when further testing is indicated. It has been
reported that FAST contributes to a decrease in abdominal CT use by
about 50%.[11]
The algorithm for FAST-oriented further investigations is described in Figure 3 and is influenced by patient’s hemodynamic status.
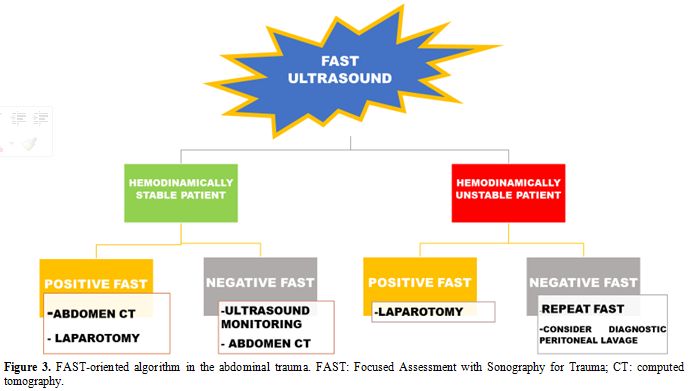 |
Figure 3. FAST-oriented
algorithm in the abdominal trauma. FAST: Focused Assessment with
Sonography for Trauma; CT: computed tomography. |
Ultrasound
has been suggested as a useful non-invasive tool for the early
detection of bleeding also in non-traumatic settings. Two well
recognised sonographic markers of hypovolemia are the diameter of the
inferior cave vein (DICV) and the thickness of the left ventricle.
Several studies[12,13] have highlighted the correlation between DICV and the need for blood transfusions in patients with acute bleeding.[14]
Moreover, serial changes in DICV reliably predict ongoing hemorrhagic shock, even better than arterial pressure or heart rate.[15]
Pseudo-hypertrophy of the left ventricle has been reported as another
possible non-invasive early marker for hemorrhagic shock in
experimental animal models.[13,16]
Gastrointestinal (GI) bleeding.
GI bleeding is a common problem in the Emergency Department.
Hemodynamic monitoring by ultrasound of the inferior cave vein
(inspiratory collapse) and the so-called “kissing sign” of the left
ventricle are useful markers of high risk in emergency GI bleeding.[17]
Acute
or overt GI bleeding can be easily recognised when hematemesis, melena,
or hematochezia are present, while chronic or occult GI bleeding often
leading to iron-deficiency anaemia can be detected by a positive faecal
occult blood test. Upper endoscopy and colonoscopy are the mainstays of
the investigations. Angiography, radionuclide imaging, capsule
endoscopy, and deep enteroscopy are further options to investigate
acute GI bleeding and obscure GI bleeding, respectively.[18]
In
the elderly, nutrient deficiency (in particular of iron, folate, and
B12 vitamin) accounts for at least one-third of all cases of anaemia.
Within this group, more than half is related to absolute iron
deficiency.[3,4] The so-called anaemia of chronic
diseases (in particular cardiovascular, kidney, and inflammatory
diseases including cancer), is present in near another third of the
anaemic elderly.[19,20] Nevertheless, a substantial
proportion of anaemia in the elderly remains apparently unexplained. In
hospitalised elderly patients, anaemia is present in up to near 50% and
is independently associated with increased length of in-hospital stay,
in-hospital readmission, and mortality.[21-23]
Ultrasound
has a minimal role in the diagnosis of gastric bleeding, where
endoscopy represents the gold standard. On the other hand, ultrasound
can be helpful in intestinal diseases. Conventional ultrasound can
provide quick information about bowel status and helps in the choice of
adequate further examinations. However, it is worthy of note that
negative findings do not exclude the presence of bowel disease. Two
types of probes with different ultrasound frequencies may be used
(3.5-5 MHz and 5-17 MHz) to obtain a panoramic view of the abdomen. The
five layers of the colonic wall may be clearly distinguishable as
concentric rings of alternating echogenicity. The measurement of wall
thickness (normal value < 3 mm) is essential.[24]
More
recent ultrasound techniques, as elastography, contrast-enhanced, and
Doppler ultrasound, rectal and trans-perineal ultrasonography allow
further examinations, in particular, to evaluate bowel vascularisation
abnormalities.[25]
Inflammatory bowel disease (IBD).
Anaemia is among the most frequent manifestations of IBD. Its
prevalence is around 24% and can be due to chronic inflammation,
chronic blood loss, or both.[26] The European Crohn’s
and Colitis Organisation (ECCO) guidelines indicate intestinal
ultrasonography as the imaging technique of choice for screening
patients with clinically suspected Crohn's disease.[26]
Nevertheless, intestinal ultrasound is particularly important in the
follow-up of patients after the initial diagnosis, where fluctuations
in disease course require repeated examinations.[27] The ultrasonographic signs usually detected in Crohn’s disease are well characterised,[27]
and include thickening, decreased compressibility, and increased
vascularisation of the bowel wall. Pericolic fluid and lymph node
enlargement may be detected with high sensitivity and specificity.[28] Also abscesses narrowing the bowel lumen, fistulas in the intestinal loops, and cutaneous fistulas may be detected.[28]
The assessment of the disease activity and the precise overview of
Crohn’s extraluminal complications is beyond the scope of conventional
abdomen ultrasound. Contrast-enhanced magnetic resonance enterography
is the method of choice.[29,30] The use of colour
Doppler, contrast ultrasound and elastography increases the accuracy of
the conventional ultrasound, when magnetic resonance is not available.[31]
In particular, Doppler ultrasound may show bowel wall increased
vascularisation due to inflammation. Elastography techniques, such as
strain and shear wave elastography, have shown promising results,
because of their ability to differentiate active inflammation from
fibrosis. A comprehensive review of the current evidence supporting the
use of elastography techniques in intestinal disorders is reported
elsewhere.[32]
Cross-sectional imaging should be
considered where conventional ultrasound is inconclusive or
non-diagnostic or when it appears normal, but there is still high
clinical suspicion of disease.
Cancer: focus on colorectal cancer and gastro-intestinal lymphoma. Anaemia is frequent in cancer patients and often characterised by multifactorial pathophysiology.[20]
Blood losses (either by tumour mass or associated with surgery), and
inadequate nutrient intake due to cachexia or malnutrition are often
present, along with inflammation mainly due to the release of
cancer-associated pro-inflammatory cytokines. Such cytokines,
especially interleukin-6, increase in turn hepcidin synthesis in the
liver, eventually leading to iron sequestration into macrophages and
functional iron deficiency.[34,35] Anaemia in cancer
patients can also be due to decreased red cell survival, erythropoiesis
disorders, low erythropoietin levels and progressive erythropoietin
resistance of erythroid progenitors.[33]
Bone
marrow infiltration by neoplastic cells, myelosuppression due to chemo-
or radiotherapy, and possibly concomitant kidney disease also
contribute to anaemia in individual cancer patients.
The role of
conventional abdomen ultrasound is negligible as compared to endoscopy.
Nevertheless, it often represents the first-line examination, when the
disease is suspected, or endoscopy is not unfeasible.
Hypoechoic
bowel wall thickening with irregular contour, loss of stratification of
the wall layers, and the absence of normal peristalsis can be
suggestive of malignancy (the so-called “pseudo-kidney sign”), as well
as the detection of liver lesions suggesting metastasis from colorectal
cancer.[36] Martinez-Ares and colleagues[36]
evaluated the diagnostic performance of abdomen ultrasonography in 145
patients with suspected colorectal carcinoma who were admitted for
colonoscopy. They concluded that abdominal ultrasound is a technique
with high sensibility and specificity to detect colon cancer, in
accordance with other authors.[37] High specificity
and sensibility have been reported in the diagnosis of tumours located
above the recto-sigmoid junction, but small polypoid lesions can be
overlooked. Due to its non-invasiveness, abdominal ultrasound should be
considered as an alternative to the conventional radiological and
endoscopic examinations, especially in patients for which no
therapeutic option would be possible because of comorbidities or
advanced age. For other patients with suspected cancer, abdomen
ultrasound should be the first diagnostic examination that may justify
further endoscopic examinations also in the Emergency Department.
Gastrointestinal
lymphoma is the second more frequent extra-nodal lymphoproliferative
disorder. The clinical presentation is non-specific, including weight
loss, dyspepsia, abdominal pain, and also anaemia. With the exception
of relatively indolent gastric localisation, it usually has a high
degree of malignancy.[38]
Over 77% of GI lymphoma exceeds 5 cm in diameter, and the average length of the affected bowel is 12 cm.[38]
Abdominal ultrasound is the first-line examination to address the right
diagnosis, followed by CT and endoscopy. In small bowel lymphoma,
ultrasonography usually shows a bulky, lobulated, predominantly
hypoechoic mass with a central echogenic component. Enlarged mesenteric
and peri-aortic lymph nodes may also be detected.[38]
In IBD, especially in patients with Crohn's diseases on
immunosuppressive treatment, the worsening of anaemia may herald the
evolution towards a lymphoproliferative disease.[39]
In such cases, changes in the size, morphology, echogenicity or the
appearance of abdominal lymph nodes may be useful in confirming/ruling
out the suspected diagnosis.
Other causes of anaemia in the gastrointestinal tract.
In addition to acute/overt bleeding, anaemia in gastrointestinal
diseases may result from other causes: obscure bleeding, malabsorption,
and maldigestion (e.g. celiac disease, chronic pancreatitis), or
autoimmune disorder (e.g. pernicious anaemia).[40]
The role of intestinal ultrasound in this clinical setting is not
entirely defined. Recent guidelines issued by the Italian Association
of Hospital Gastroenterologists and Endoscopists (AIGO), and by the
Italian Society of Pediatric Gastroenterology Hepatology and Nutrition
(SIGENP) do not recommend intestinal ultrasound, suggesting other
imaging techniques like CT, enterography, magnetic resonance
enterography, capsule enteroscopy, and endoscopy.[40]
However,
abdominal ultrasound can still play a role in diseases of the small
bowel, as an initial and low-cost procedure to guide the successive
diagnostic approaches. It should be underlined that one of the reasons
for the controversies in including ultrasonography among the
recommended techniques for exploring the intestinal/abdominal causes of
anaemia may be related to its poor reproducibility. To overcome this
limitation, the European Federation of Societies for Ultrasound in
Medicine and Biology (EFSUMB) has recently published new guidelines
intending to standardise the examination technique and to facilitate
the correct training education of operators.[41]
Malabsorption and Maldigestion Syndromes. One of the most frequent signs of malabsorption syndromes is anaemia, in particular, iron deficiency anaemia.[40]
Indeed anaemia can be present in 12-69% of patients with celiac disease
(CD). CD diagnosis is usually pursued by serology including IgA
anti-transglutaminase (anti-tTG IgA) and IgG-anti-deamidated gliadin
peptides (anti-DGP IgG), and confirmed by histological documentation of
flattening of the villi.[40] In CD patients,
intestinal ultrasound examinations can reveal small bowel loops
thickening, enlarged mesenteric lymph nodes, and free fluid within the
bowel loops in 50-60% of cases.[42] The absence of
dilated and/or thickened loops has a negative predictive value of 98%
in excluding the diagnosis of CD by intestinal ultrasound.[43]
In
maldigestion due to pancreatic insufficiency, e.g., due to chronic
pancreatitis, anaemia can be related to decreased iron absorption,
chronic inflammation, and vitamin B12 deficiency. In patients with
anaemia and clinical signs of maldigestion, ultrasound may detect a
reduction in the size of the pancreas, irregular profiles, parenchymal
calcifications, or dilated Wirsung duct with stones. All these signs
lead to the diagnosis of chronic pancreatitis.[44]
Gynaecology/Obstetrics
Anaemia is commonly encountered in gynaecology/obstetrics practice.[45]
Bleeding caused by adenomyosis, uterine fibroids, or endometrial
hyperplasia frequently results in even severe iron-deficiency anaemia.
Ultrasound is the main diagnostic tool also to assess the location and
status of early pregnancy. Transvaginal ultrasound is the method of
choice. Nevertheless, in several situations, as for women who decline
transvaginal ultrasound, the trans-abdomen scan is the alternative
option. In particular, uterine disorders causing acute bleeding include
retained products of conception, uterine arterio-venous malformations,
and fibroids. Adnexal disorders may also cause bleeding, including
hemorrhagic ovarian cysts, and ectopic pregnancies.[46]
A detailed description of such diseases is beyond the scope of this
work. Comprehensive reviews on these topics can be found elsewhere.[47,48]
Focus on: Ultrasound for the Haematologist
Conventional
ultrasound is the recommended imaging method for lymph node evaluation,
with the advantages of high resolution, real-time evaluation, safety,
and low costs.[49] Nevertheless, recent advances in
ultrasound techniques, as contrast-enhanced ultrasound (CEUS),
contrast-enhanced endoscopic ultrasound (CE-EUS), and real-time
elastography, improves the evaluation accuracy for the differential
diagnosis between benign and malignant lymph nodes. CE-EUS is also used
for guiding fine needle aspiration. The differentiation of malignant
versus benign lymph nodes by ultrasound traditionally relies on size
and topographic distribution.[50] However, malignant
lymph node infiltration can occur in up to 30% lymph nodes of less than
5 mm. The evaluation of shape and borders does not allow a definitive
classification.[51] New ultrasound techniques provide
additional information; for example, CEUS can give information about
vascularisation and perfusion pattern. This technique identifies
changes in vascular architecture and avascular areas of malignant
infiltration. In summary, perfusion defects and centripetal
non-homogeneous enhancement suggest lymph node infiltration by
malignancy.[52] In lymphoma, CEUS patterns are highly variable, particularly regarding the vascular features.[52] Elastography is a non-invasive method in which the stiffness of the tissue is viewed as a colour map or shear wave velocity.[53] The details of these techniques are reported elsewhere.[53,54]
Abdomen
ultrasound and lymph nodes conventional ultrasound usually constitute
the initial diagnostic workup in haematological diseases as recently
reviewed.[55] Figure 4 represents the possible instrumental examination flow chart for suspected hematologic diseases.
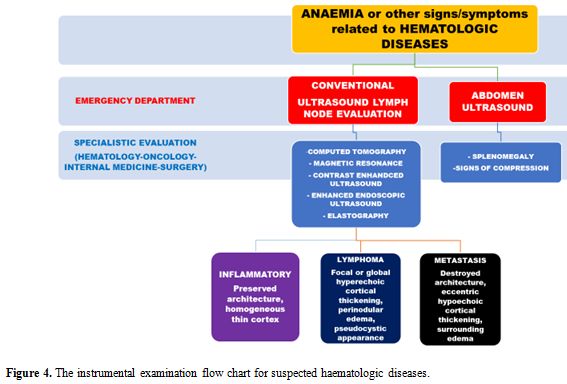 |
Figure 4. The instrumental examination flow chart for suspected haematologic diseases. |
Focus on: Anaemia and Cardiac Ultrasound (Heart Failure and the Oncologic Patient)
HF
is a clinical syndrome characterised by typical symptoms and signs
caused by structural or functional cardiac abnormalities, resulting in
a reduced cardiac output or elevated intra-cardiac pressures at rest or
during stress.[56] The prevalence of HF is
approximately 1–2% of the adult population in developed countries,
rising to 10% among people >70 years of age, where it represents the
leading cause of hospitalisation.[57] Anaemia is a common co-morbidity in HF patients, and it is associated with worse long-term outcomes.[58] In HF clinical trials and registries, the prevalence of anaemia ranges from 15% to 70% among hospitalised patients.[56,59]
The physiologic response to anaemia is a compensatory increase in
cardiac output in order to maintain adequate oxygen delivery, with a
decrease in myocardial contractility when the haemoglobin level is
below 7 g/dL.[60] Left ventricle hypertrophy and dilation have been observed in animal and human models of severe anaemia.[61]
The mechanisms by which anaemia worsens HF outcome are not fully
understood. They may be related to increased myocardial workload due to
hemodynamic, neurohormonal, and pro-inflammatory alterations finally
leading to left ventricle remodelling.[62]
Absolute or functional iron deficiency, inappropriate erythropoietin
production, and depressed bone marrow function are common cofactors
leading to anaemia in HF patients. Dysregulation of molecules involved
in these pathways is critical for the transition from adaptive cardiac
hypertrophy to cardiac remodelling, as represented in Figure 5.
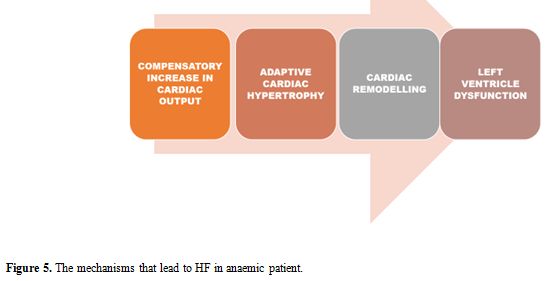 |
Figure 5. The mechanisms that lead to HF in anaemic patient. |
Echocardiographic alterations in anaemic patients have been studied, in particular, related to the left ventricle function.[63-66]
The presence of anaemia is associated with diastolic dysfunction
(alteration in peak mitral early diastolic, E velocity, and peak mitral
late diastolic, A velocity, E/A ratio, which reflects the increase of
left ventricular filling pressure), increased left ventricle mass index
and diameter, increased left atrium volume index, and higher systolic
pulmonary artery pressure estimated by tricuspid Doppler. The
correction of anaemia often results in the improvement of
echocardiographic parameters.[63-66]
Cardiac
imaging, particularly trans-thoracic echocardiography, plays an
essential role in the baseline assessment and serial follow- up of
oncologic patients, in which anaemia is a common feature. This
ultrasound technique is part of the relatively new discipline of
cardio-oncology, intending to prevent and monitor cardiovascular
complications resulting from cancer treatment.[67]
Cardiotoxicity is defined as a decrease in left ventricle ejection
fraction greater than 10% to a value of less than 53%, confirmed on
repeated imaging at 2-3 weeks from the initial evaluation.[68]
Current guidelines68 recommend a standardised cardio-oncology
echocardiographic protocol with the description of traditional approach
and the introduction of more advanced modalities, like the assessment
of global longitudinal strain as an early a marker of left ventricle
dysfunction.
The role of ultrasound in HF has been well established also in the routine assessment of non-oncologic patients.
Echocardiography
has a prominent role, but other ultrasound techniques have been
proposed for a complete evaluation, as recently proposed.[69]
A five-step ultrasound examination (“ABCDE”) to evaluate and monitor HF
patients may include the evaluations of the Ankle-brachial index (A),
B-lines (B), the Carotid intima-media thickness (C), the Diameters of
the abdominal aorta and of the inferior cave vein (D), and the
Echocardiographic assessment of the ejection fraction (E). All these
parameters may be helpful in bedside monitoring of recovery after acute
heart decompensation, as well as for a global cardiovascular assessment
tool in HF patients.[69]
Conclusions
Ultrasonography
is a safe and effective imaging tool that has to be considered when
facing patients with anaemia. Anaemia, especially in the elderly, is
often multifactorial,[22] and defining the prominent
cause(s) can be difficult. However, ultrasound may be a useful
diagnostic method to address the right diagnosis.Future
challenges include standardisation of the training process for
physicians (other than Radiologists) to ensure the appropriate use of
this technology, and a structuring policy to promote its effectiveness.Educational
strategies for increasing competency on the point of care ultrasound
(that is ultrasonography performed and interpreted by the clinician at
the bedside) represent the goal of several ultrasound societies
worldwide. Indeed, both EFSUMB and WFUMB (World Federation of
Ultrasound in Medicine and Biology) have developed periodically
up-dated guidelines on the minimum training requirements that should be
achieved at each level of practice in order to perform examinations
according to the operator’s capacity (from point of care/focused
ultrasound to conventional ultrasound and specialized application of
the technique).[70]
References
- World
Health Organization (WHO). Haemoglobin concentrations for the diagnosis
of anaemia and assessment of severity. Vitamin and mineral nutrition
system. Geneva WHO 2011
- Platt A, Eckman J Diagnosing anaemia. Rev Clin 2006;16(2):44-50
- Camaschella C Iron deficiency anaemia. N Engl J Med 2015; 372:1832-1843 https://doi.org/10.1056/NEJMra1401038 PMid:25946282
- Camaschella C Iron deficiency Blood 2019;133(1):30-39 https://doi.org/10.1182/blood-2018-05-815944 PMid:30401704
- Long B, Koyhman A Emergency medicine: evaluation and management of anaemia. Emerg Med Clin Am 2018;36:609-630 https://doi.org/10.1016/j.emc.2018.04.009 PMid:30037447
- Gikas
A, Triantafillidis JK The role of primary care physicians in early
diagnosis and treatment of chronic gastrointestinal diseases. Int J Gen
Med 2014; 7:159-173 https://doi.org/10.2147/IJGM.S58888 PMid:24648750 PMCid:PMC3958525
- Gandhi
SJ, Hagens I, Nathan K, Hunter K, Roy S Prevalence, comorbidity and
investigation of anaemia in the primary care office. J Clin Med Res
2017; 9:970-980 https://doi.org/10.14740/jocmr3221w PMid:29163729 PMCid:PMC5687900
- Levi
M, Rosselli M, Simonetti M, Brignoli O, Cancian M et al Epidemiology of
iron deficiency anaemia in four European countries: a population-based
study in primary care. Eur J Haematol 2016; 97:583-593 https://doi.org/10.1111/ejh.12776 PMid:27155295
- Scalea
TM, Rodriguez A, Chiu YC, Brenneman FD, Fallon WF et al Focused
assessment with sonography for trauma (FAST): results from an
international consensus conference. J Trauma 1999; 46:466-472 https://doi.org/10.1097/00005373-199903000-00022 PMid:10088853
- Rozycki GS, Newman PG Surgeon-performed ultrasound for the assessment of abdominal injuries. Adv Surg 1999; 33:243-259
- Stengel
D, Bauwens K, Rademaker G, Ekkernkamp A, Guthoff G Emergency
ultrasound-based algorithms for diagnosing blunt abdominal trauma.
Cochrane Database Sys Rev 2013;7: CD004446 https://doi.org/10.1002/14651858.CD004446.pub3
- Lyon
M, Blaivas M, Brannam L Sonographic measurement of the inferior vena
cava as a marker of blood loss Am J Emerg Med 2005;23:45-50 https://doi.org/10.1016/j.ajem.2004.01.004 PMid:15672337
- Di
Segni E, Preisman S, Ohad DG Echocardiographic left ventricular
remodelling and pseudohypertrophy as markers of hypovolemia. An
experimental study on bleeding and volume repletion. J Am Soc
Echocardiogr 1997; 10:926-936 https://doi.org/10.1016/S0894-7317(97)80009-0
- YanagawaY,
Nishi K, Sakamoto T, Okada Y Early diagnosis of hypovolemic shock by
sonographic measurement of inferior vena cava in trauma patients. J
Trauma 2005; 58:825-829 https://doi.org/10.1097/01.TA.0000145085.42116.A7 PMid:15824662
- Yanagawa
Y, Sakamoto T, Okada Y Hypovolemic shock evaluated by sonographic
measurement of the inferior vena cava during resuscitation in trauma
patients J Trauma 2007;63:1245-1248 https://doi.org/10.1097/TA.0b013e318068d72b PMid:18212645
- Carr
BG, Dean AJ, Everett WW intensive bedside ultrasound (INBU) for volume
assessment in the intensive care unit: a pilot study. J Trauma 2007;
63:495-500 https://doi.org/10.1097/TA.0b013e31812e51e5 PMid:18073592
- Tung
Chen Y, Blancas Gomez-Casero R, Quintana Diaz M, Diaz HB Inspiratory
collapse of the inferior vena cava and the kissing ventricle sign:
markers of poor prognosis in emergency gastrointestinal bleeding.
Emergencias 2019;31:79-85
- Kim
BSM, Li BT, Engel A, Samza JS, Clarke S Diagnosis of gastrointestinal
bleeding: a practical guide for clinicians. World J Gastrointest
Pathophysiol 2014;15:467-478 https://doi.org/10.4291/wjgp.v5.i4.467 PMid:25400991 PMCid:PMC4231512
- Guralnik
JM, Eisenstaedt RS, Ferucci L, Klein HG, Woodman RC Prevalence of
anaemia in persons 65 years older in the United States: evidence for a
high rate of unexplained anaemia. Blood 2004:104:2263-2268 https://doi.org/10.1182/blood-2004-05-1812 PMid:15238427
- Busti
F, Marchi G, Ugolini S, Castagna A, Girelli D Anaemia and iron
deficiency in cancer patients: role of iron replacement therapy.
Pharmaceuticals 2018;11:94-108 https://doi.org/10.3390/ph11040094 PMid:30274354 PMCid:PMC6315653
- Nathavithrana
RL Anaemia is highly prevalent among unselected internal medicine
inpatients and is associated with increased mortality, earlier
readmission and more prolonged hospital stay: an observational
retrospective cohort study. Intern Med J 2014;42:683-691 https://doi.org/10.1111/j.1445-5994.2011.02566.x PMid:21790925
- Girelli D, Marchi G, Camaschella C Anemia in the elderly. HemaSphere 2018;2:pe40 https://doi.org/10.1097/HS9.0000000000000040
- Riva
E, Colombo R, Moreo G, Mandelli S, Franchi C et al Prognostic value of
degree and types of anaemia on clinical outcomes for hopitalized
patients. Arch Gerontol Geriatr 2017;69:21-30 https://doi.org/10.1016/j.archger.2016.11.005 PMid:27875713
- Muradali D, Goldberg DR US of gastrointestinal tract disease. Radiographics 2015;35:50-68 https://doi.org/10.1148/rg.351140003 PMid:25590387
- Havre R, Giljs OH, Elastography and strain rate imaging of the gastrointestinal tract. Eur J Radiol 2014;83:438-441 https://doi.org/10.1016/j.ejrad.2013.05.018 PMid:23769191
- Gomollón
F, Dignass A, Annese V 3rd European Evidence-based Consensus on the
Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and
Medical Management. J Crohn Colitis. 2017: 11:3-25 https://doi.org/10.1093/ecco-jcc/jjw168 PMid:27660341
- Conti
CB, Giunta M, Gridavilla D, Conte D, Fraquelli M Role of bowel
ultrasound in the diagnosis and follow-up of patients with Crohn's
disease. Ultrasound in Med and Biol 2017;43:725-734 https://doi.org/10.1016/j.ultrasmedbio.2016.12.014 PMid:28185694
- Migaleddu
V, Quaia E, Scano D, Virgilio G Inflammatory activity in Crohn's
disease: ultrasound findings. Abdom Imaging 2008;33:589-597 https://doi.org/10.1007/s00261-007-9340-z PMid:18172707
- Biernacka
CB, Baranska D, Grzelak P, Czkwianaianc E, Szabelska K Up-to-date
overview of imaging techniques in the diagnosis and management of
inflammatory bowel diseases. Gastroenterology Rev 2019;14:19-25 https://doi.org/10.5114/pg.2019.83423 PMid:30944674 PMCid:PMC6444107
- Imsirovic
B, Zarem E, Gusa E Djedovic M, Cengic A et al Comparison of
conventional ultrasound and contrast enhanced magnetic resonance (MR)
enterography in evaluation of patients with Cronh's disease. Acta
Inform Med 2018;26:93-97 https://doi.org/10.5455/aim.2018.26.93-97 PMid:30061778 PMCid:PMC6029906
- Biernacka
CB, Baranska D, Grzelak P, Czkwianaianc E, Szabelska K Up-to-date
overview of imaging techniques in the diagnosis and management of
inflammatory bowel diseases. Gastroenterology Rev 2019;14:19-25 https://doi.org/10.5114/pg.2019.83423 PMid:30944674 PMCid:PMC6444107
- Branchi
F, Caprioli F, Orlando S, Conte D, Fraquelli M Non-invasive evaluation
of intestinal disorders: the role of elastographic techniques. World J
Gastroenterol 2017;23:2832-2840 https://doi.org/10.3748/wjg.v23.i16.2832 PMid:28522902 PMCid:PMC5413779
- Roy CN Anaemia of inflammation. Haematology Am Soc Hematol Educ Program 2010;276-280 https://doi.org/10.1182/asheducation-2010.1.276 PMid:21239806
- Kario K, Matsuo T, Nakao K Serum erythropoietin levels in the elderly. Gerontology 1991;37:345-348 https://doi.org/10.1159/000213283 PMid:1765284
- Bruunsgaard H, Pedersen BK Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am 2003;23:15-39 https://doi.org/10.1159/000213283 PMid:1765284
- Martinez-Ares
DS, Barrenechea I, Souto-Rouzo J, Yanez-LopezJ, Pallares Peral A,
Vazquez-Iglesias JL The value of abdominal ultrasound in the diagnosis
of colon cancer. Rev Exp Enferm Dig 2005;97:877-886 https://doi.org/10.4321/S1130-01082005001200004
- Rutgeerts
LJ, Vernbanck JJ, Carpe AW, Buyse BM, Ghillebert GL Detection of
colorectal cancer by routine ultrasound. J Belge Radiol 1991;74:11-13
- Chang ST, Menias CO Imaging of primary gastrointestinal lymphoma. Semin Ultrasound 2013;34:558-65 https://doi.org/10.1053/j.sult.2013.05.008 PMid:24332207
- Annese
V, Beaugerie L, Egan L et al. European Evidence-based Consensus:
Inflammatory Bowel Disease and Malignancies. J Crohns Colitis
2015;9:945-65 https://doi.org/10.1093/ecco-jcc/jjv141 PMid:26294789
- Elli
L, Norsa L, Zullo A et al Diagnosis of chronic anaemia in
gastrointestinal disorders: A guideline by the Italian Association of
Hospital Gastroenterologists and Endoscopists (AIGO) and the Italian
Society of Paediatric Gastroenterology Hepatology and Nutrition
(SIGENP). Dig Liver Dis 2019;51:471-483 https://doi.org/10.1016/j.dld.2019.01.022 PMid:30850345
- Nylund
K, Maconi G, Hollerweger A et al EFSUMB Recommendations and Guidelines
for Gastrointestinal Ultrasound - Part 1: Examination Techniques and
Normal Findings (Long version). Ultraschall in Med 2017;38: e1-e15 https://doi.org/10.1055/s-0042-115853 PMid:27604052
- Soresi
M, Mansueto P, Terranova A Abdominal Ultrasound Does Not Reveal
Significant Alterations in Patients With Nonceliac Wheat Sensitivity. J
Clin Gastroenterol. 2019;53:e31-e36 https://doi.org/10.1097/MCG.0000000000000969 PMid:29206754
- Soresi
M, Pirrone G, Giannitrapani L A key role for abdominal ultrasound
examination in "difficult" diagnoses of celiac disease. Ultraschall Med
2011;32:S53-61 https://doi.org/10.1055/s-0028-1110009 PMid:20235005
- Frulloni L, Falconi M, Gabbrielli A Italian consensus guidelines for chronic pancreatitis. Dig Liver Dis 2010;42:S381-406 https://doi.org/10.1016/S1590-8658(10)60682-2
- Breymann
C, Auerbarch M Iron deficiency in gynaecology and obstetrics: clinical
implications and management. Hematology 2017;1:152-159 https://doi.org/10.1182/asheducation-2017.1.152 PMid:29222250 PMCid:PMC6142528
- Murugandoss
N, Coyle N, Dotta S Ultrasound in obstetrics and gynaecology.
Obstetrics, Gynaecology and Reproductive Medicine 2018;29:42-50 https://doi.org/10.1016/j.ogrm.2018.12.009
- Woodfield CA The usefulness of ultrasound imaging in gynaecologic oncology. PET Clin 2018;13:143-163 https://doi.org/10.1016/j.cpet.2017.11.003 PMid:29482747
- Iraha Y, Okada M, Iraha R, Azama K, Yamashiro T CT and MR imaging of gynaecologic emergencies. Radiographics 2017;37:1569-1586 https://doi.org/10.1148/rg.2017160170 PMid:28753380
- Chiorean
L, Cui XW, Kleen SA Clinical value of imaging for lymph nodes
evaluation with particular emphasis on ultrasonography. Z Gastroenterol
2016;54:774-790 https://doi.org/10.1055/s-0042-108656 PMid:27529528
- Sharma
A, Fidias P, Hayman LA, Loonis SL, Taber KH et al Patterns of
lymphoadenopathy in thoracic malignancies. Radiographics
2004;24:419-434 https://doi.org/10.1148/rg.242035075 PMid:15026591
- Vassallo
P, Wernecke K, Roos N, Peters PE Differentiation of benign from
malignant superficial lymphadenopathy: the role of high resolution US.
Radiology 1992;183:215-220 https://doi.org/10.1148/radiology.183.1.1549675 PMid:1549675
- Yu
M, Liu Q, Song HP, Han ZH, Su HL et al Clinical application of contrast
enhanced ultrasonography in diagnosis of superficial lymphadenopathy. J
Ultrasound Med 2010;29:735-740 https://doi.org/10.7863/jum.2010.29.5.735 PMid:20427785
- Dietrich CF Elastography applications. Endo Heute 2011;24:177-212 https://doi.org/10.1055/s-0030-1262579
- Bachmann- Nielsen M, Saftoiou A Elastography, true or false? Ultraschall Med 2011;32:5-7 https://doi.org/10.1055/s-0029-1246008 PMid:21305435
- Trenker
C, Gorg C, Jenssen C, Klein S, Neubauer A et al The role of abdominal
ultrasound in haematological diseases. Z Gastroenterol
2018;56:1063-1076 https://doi.org/10.1055/a-0603-4240 PMid:30223283
- The
task force for the diagnosis and treatment of acute and chronic heart
failure of the European Society of Cardiology (ESC) 2016 ESC guidelines
for the diagnosis and treatment of acute and chronic heart failure. Eur
Heart J 2016; 37:2129-2200 https://doi.org/10.1093/eurheartj/ehw128 PMid:27206819
- Maggioni
AP EUR observational Research Programme. The heart failure pilot survey
(ESH-HF Pilot). Eur J Heart Fail 2010;12(10):1076-1084 https://doi.org/10.1093/eurjhf/hfq154 PMid:20805094
- Tang
YD, Katz SD The prevalence of anemia in chronic heart failure and its
impact on clinical outcomes. Heart Fail Rev 2008;13:387-392 https://doi.org/10.1007/s10741-008-9089-7 PMid:18246424
- Amand IS Anaemia and chronic heart failure. J Am Coll Cardiol 2008; 52:501-511 https://doi.org/10.1016/j.jacc.2008.04.044 PMid:18687241
- Hegde N, Rich MW, Gayomali C The cardiomiopathy of iron deficiency. Tex Heart Inst J 2006;33:340-344
- Datta
BN, Silver MD, Cardiomegaly in chronic anaemia in rats and man
experimental study including ultrastructural histometric and
stereologic observations. Lab Invest 1975;32:503-514 https://doi.org/10.1080/00033797500200421
- Yoo
RK Wook BP, Gil JS Relation of anaemia to echocardiographically
estimated left ventricular filling pressure in hypertensive patients
over 50 years old. J Cardiovasc Ultrasound 2010; 18:56-90 https://doi.org/10.4250/jcu.2010.18.3.86 PMid:20967155 PMCid:PMC2955338
- Tobili
JE, Di Gennaro F, Rivas C Changes in echocardiographic parameters in
iron deficiency patients with heart failure and chronic kidney disease
treated with intravenous iron. Heart Lung Circ 2015;24:686-695 https://doi.org/10.1016/j.hlc.2014.12.161 PMid:25666998
- Sutil-Vega
M, Rizzo M, Martinez-Rubio A. Anaemia and iron deficiency in heart
failure: a review of echocardiographic features. Echocardiography 2019 https://doi.org/10.1111/echo.14271 PMid:30693550
- Shen
J, Zhou Q, Liu Y, Runlan L, Li T Evaluation of left atrial function in
patients with iron-deficiency anaemia by two-dimensional speckle
tracking echocardiography. Cardiovasc Ultrasound 2016:14:34-43 https://doi.org/10.1186/s12947-016-0078-z PMid:27550185 PMCid:PMC4994319
- In-Jeong
C, Yeung CM, Ki HK, Gil JS Effect of anaemia correction on left
ventricular structure and filling pressure in anaemic patients without
overt heart disease. Korean J Intern Med 2014;29:445-453 https://doi.org/10.3904/kjim.2014.29.4.445 PMid:25045292 PMCid:PMC4101591
- Venneri
L, Zoppellaro G, Khattar RS Cardio-oncology: the role of advanced
echocardiography in cancer patients. Expert Rev of Cardiovasc Ther
2018;16:249-258 https://doi.org/10.1080/14779072.2018.1443394 PMid:29457984
- Plana
JC, Galderisi M, Barac A Expert consensus for multimodality imaging
evaluation of adult patients during and after cancer therapy. A report
form the American Society of Echocardiography and the European
association of Cardiovascular imaging. Eur Heart J Cardiovasc Imaging
2014;15:1063-1093 https://doi.org/10.1093/ehjci/jeu192 PMid:25239940 PMCid:PMC4402366
- Mozzini
C, Cominacini L, Casadei A, Schiavone C, Soresi M Ultrasonography in
heart failure: a story that matters. Curr Probl Cardiol 2019;44:116-136
https://doi.org/10.1016/j.cpcardiol.2018.05.003 PMid:30172551
- Dietrich
CF, Hoffmann B, Cantisani V et al Medical student ultrasound education:
A WFUMB position paper, Part I. Ultrasound Med Biol 2019;45:271-281 https://doi.org/10.1016/j.ultrasmedbio.2018.09.017 PMid:30497768





