Yuka Furukawa1, Toshifumi Takahashi2*, Ryota Suganuma1, Miki Ohara1, Kuniaki Ota2, Hyo Kyozuka1, Akiko Yamaguchi1, Shu Soeda1, Takafumi Watanabe1, Hiromi Komiya1, Hideki Mizunuma2 and Keiya Fujimori1
1 Department of Obstetrics and Gynecology, Fukushima Medical University, Fukushima 960-1295, Japan.
2 Fukushima Medical Center for Children and Women, Fukushima Medical University, Fukushima 960-1295, Japan.
Correspondence to: Toshifumi Takahashi. Fukushima Medical Center for
Children and Women, Fukushima Medical University, Fukushima 960-1295,
Japan. Tel: +81-247-1385, Fax: +81-247-1386 E-mail:
totakaha@fmu.ac.jp
Published: January 1, 2019
Received: September 14, 2019
Accepted: November 18, 2019
Mediterr J Hematol Infect Dis 2020, 12(1): e2020005 DOI
10.4084/MJHID.2020.005
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
A
35-year-old female patient with chronic myeloid leukemia (CML) wanted
to have a child. She had been treated with imatinib and had achieved
major molecular remission, after which imatinib was intentionally
discontinued, and interferon-α treatment was initiated. After three
failed cycles of artificial insemination with her husband's semen, the
patient underwent treatment with assisted reproductive technology.
After two cycles of in vitro fertilization, two embryos (8-cell stage
and blastocyst) were cryopreserved. The patient again had elevated
major BCR-ABL mRNA levels; thus, infertility treatment was
discontinued. After 18 months of dasatinib treatment, major molecular
remission was again observed, and the patient underwent
vitrified–warmed embryo transfer with a single blastocyst. After that,
she became pregnant. Discontinuation of tyrosine kinase inhibitors
combined with the timely initiation of infertility treatments,
including assisted reproductive technology, might thus be useful for
treating women with CML who wish to become pregnant.
|
Introduction
The
number of adolescent and young adult (AYA) cancer survivors is
increasing due to advances in cancer treatment. Male and female AYA
cancer survivors exhibit sequelae for future fertility and late-onset
complications. Many cancers that occur in the AYA population are
hematological diseases such as leukemia. Further, patients with acute
lymphocytic and myeloid leukemia generally undergo chemotherapy with
multiple anticancer drugs and radiotherapy, which can be gonadotoxic.
Tyrosine
kinase inhibitors (TKIs) can be used as a standard treatment for
chronic myeloid leukemia (CML) instead of chemotherapy with multiple
anticancer drugs. For example, imatinib improves the prognosis for
women with CML and preserves fertility, unlike conventional anticancer
drugs; however, it is contraindicated in women of childbearing age due
to its teratogenic effects. Moreover, intentional imatinib withdrawal
has been reported to restore the possibility of spontaneous pregnancy
in previous infertile women with CML;[1-7] however,
there have been no reports of successful pregnancies following assisted
reproductive technology (ART) treatment for infertile women with CML.
Here, we report a successful planned pregnancy through vitrified–warmed
embryo transfer in a woman with CML showing molecular remission.
Case Report
When
the patient was 27 years old, she got married and was diagnosed with
CML in the chronic phase. Cytogenetic studies showed a (9;22) (q34;q11)
translocation in all 20 metaphase cells and BCR-ABL fusion gene signals
were observed in 95 % of the cells by fluorescent in-situ hybridization
and polymerase chain reaction amplifying major BCR-ABL (p210). She had
low-risk Sokal and Hasford scores, and her performance status was zero
at diagnosis. She immediately started to receive imatinib (Glivec®,
NOVARTIS, Tokyo, Japan) treatment at a daily dose of 400 mg and
achieved major molecular remission (MMR). At 35 years of age, the
patient was admitted to our hospital as she desired a child. At that
time, she had received imatinib for 96 months and had been in MMR for
more than 80 months. Imatinib treatment was discontinued and switched
to 3,000,000 IU interferon-α (IFN-α, Sumiferon®, Sumitomo Dainippon
Pharma, Tokyo, Japan) along with twice-weekly consultations with a
hematologist before infertility treatment. Additionally, both the
patient and her husband were screened to check for causes of
infertility. The patient’s menstrual period was regular, and her body
mass index was 27.6 kg/m2
(overweight). Although there were no abnormal findings based on
bimanual palpitation, transvaginal ultrasonography revealed a 3-cm
subserosal fibroid and polycystic ovary on the left side. On the fourth
day of the patient's menstrual cycle, the levels of luteinizing
hormone, follicle-stimulating hormone (FSH), prolactin, 17β-estradiol,
and free testosterone were 6.95 mIU/mL, 5.01 mIU/mL, 18.98 ng/mL, 33
pg/mL, and 0.6 pg/mL, respectively. On the nineteenth day of her
menstrual cycle, 17β-estradiol and progesterone levels were 126.1 pg/mL
and 12.6 ng/mL, respectively. Hysterosalpingography revealed bilateral
tubal patency. The husband’s semen findings were within normal ranges
according to World Health Organization criteria as follows: semen
volume, 2.0 mL; sperm concentration, 157 × 106/mL;
total motility, 68%. The patient’s peripheral blood showed a white
blood cell count of 4300/µL (47% lymphocytes, 39% neutrophils, 10%
monocytes, and 2% eosinophils), a red blood cell count of 4.23 × 106/µL, hemoglobin of 12.1 g/dL, hematocrit of 36.1%, and a platelet count of 26.7 × 104/µlL,
with a major BCR-ABL mRNA copy number of 8 per assay. After the
infertility workup, the patient’s doctor recommended and implemented an
initial treatment of artificial insemination with the husband’s semen
(AIH) with ovarian stimulation and clomiphene citrate (CC). After three
rounds of AIH treatment, the patient failed to become pregnant. By this
time, six months had passed since the start of infertility treatment,
and despite IFN-α treatment, her major BCR-ABL mRNA copy number and
ratio of BCR-ABL to ABL mRNA (converted to international
scale-normalized copy number [IS-NCN]) had increased. Under these
circumstances, the patient decided to undergo in vitro fertilization
(IVF) treatment, receiving controlled ovarian stimulation (COS) with a
gonadotropin-releasing hormone (GnRH) agonist-long protocol. Oocyte
retrieval was canceled during the first attempted IVF treatment cycle
due to the risk of ovarian hyperstimulation syndrome (OHSS). At this
time, the IFN-α treatment dose (3,000,000 IU) was increased from twice
to three times per week due to the increasing BCR-ABL levels. During
the second IVF treatment cycle, the patient underwent COS with CC and
recombinant FSH treatment, followed by triggering with a GnRH agonist
to prevent OHSS. One mature cumulus-oocyte complex was retrieved and
subjected to IVF. The fertilized oocyte developed to an eight
cell-stage cleavage embryo, which was vitrified and stored in liquid
nitrogen. During the third IVF treatment cycle, COS was performed using
the GnRH antagonist protocol, followed by triggering with a GnRH
agonist; one mature oocyte was retrieved. The fertilized oocyte
developed into a blastocyst-stage embryo, which was vitrified and
stored in liquid nitrogen. Therefore, a total of two embryos were
vitrified and stored. Since the IS-NCN level was 1.2847% during IFN-α
treatment, the hematologist suggested that it was necessary to
administer dasatinib (Suprycel®, Bristol-Myers Squibb, Tokyo, Japan) in
addition to IFN-α. Consequently, the patient received a daily dose of
100 mg of dasatinib in addition to IFN-α (3,000,000 IU) three times per
week and temporarily suspended infertility treatment. Five months
later, BCR-ABL levels became undetectable and were maintained at this
level for a further 12 months. The patient then stopped IFN-α and
dasatinib treatment and resumed infertility treatment three months
after the last dose, undergoing vitrified–warmed embryo transfer using
the 8 cell-stage embryo under a hormone replacement cycle. Two weeks
after embryo transfer, the patient was found to be pregnant, testing
positive for urinary human chorionic gonadotropin. Two weeks later, the
patient was confirmed to have one fetus with a heartbeat in her uterus.
In total, it took 34 months from the start of infertility treatment
until the pregnancy was achieved, at which point the patient was 38
years old. She underwent non-invasive prenatal testing (NIPT) after
genetic counseling at 12 weeks of gestation, the NIPT report for
trisomy 13, 18, and 21 being negative. The course of the pregnancy
was uneventful until 27 weeks of gestation when the patients' BCR-ABL
levels showed a slight increase (0.1059% IS-NCN); therefore, IFN-α
treatment (3,000,000 IU) was resumed three times per week. At 31 weeks
of gestation, ultrasonography showed fetal ventricular brain
enlargement and a mass in the sacral area, which was thought to be a
meningocele. The patient was admitted to the hospital and received a
tocolytic agent to prevent preterm labor as her cervix had shortened to
25 mm, and her amniotic fluid index had increased to 25 cm at 33 weeks
of gestation. The patient was scheduled to undergo an elective cesarean
section to prevent perforation of the meningocele, delivering a female
infant with an Apgar score of 8 and weighing 2634 g at 37 weeks of
gestation. After delivery, the infant was diagnosed with a meningocele
without other congenital anomalies, and the meningocele was repaired
the same day. The patient was discharged from the hospital seven days
after delivery without any complications. The clinical course of the
patient’s infertility treatment is shown in Figure 1.
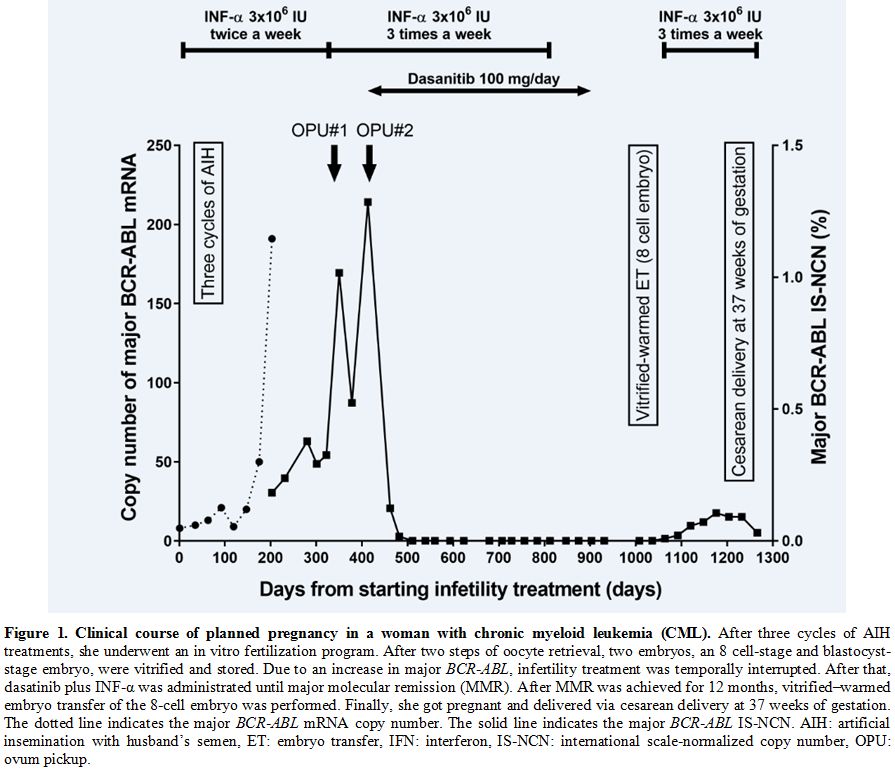 |
Figure
1. Clinical course of planned pregnancy in a woman with chronic myeloid leukemia (CML).
After three cycles of AIH treatments, she underwent an in vitro
fertilization program. After two steps of oocyte retrieval, two
embryos, an 8 cell-stage and blastocyst-stage embryo, were vitrified
and stored. Due to an increase in major BCR-ABL,
infertility treatment was temporally interrupted. After that, dasatinib
plus INF-α was administrated until major molecular remission (MMR).
After MMR was achieved for 12 months, vitrified–warmed embryo transfer
of the 8-cell embryo was performed. Finally, she got pregnant and
delivered via cesarean delivery at 37 weeks of gestation. The dotted
line indicates the major BCR-ABL mRNA copy number. The solid line indicates the major BCR-ABL
IS-NCN. AIH: artificial insemination with husband’s semen, ET: embryo
transfer, IFN: interferon, IS-NCN: international scale-normalized copy
number, OPU: ovum pickup. |
Discussion
We
report a successful planned pregnancy in a woman with CML showing MMR
via the vitrified–warmed transfer of an embryo derived from IVF. To the
best of our knowledge, this is the first report of a planned pregnancy
using ART in a female patient with CML and infertility. The ability of
TKIs to improve prognosis and preserve fertility has increased the
number of CML patients of reproductive age desiring children. Although
the median age of disease onset for CML is > 60 years, the
proportion of men and women of reproductive age is 30–40%.[8]
Although imatinib has been reported to affect testosterone production
in male CML patients, TKIs have little or no effect on male fertility.[9]
Conversely, TKIs can exhibit major teratogenicity in female CML
patients; therefore, female CML patients who desire children must
discontinue TKI therapy.
Recently, a guideline regarding TKI discontinuation in female CML patients who wish to have children was revised and published.[10]
The guideline recommends that such individuals should discontinue TKI
treatment before conceiving and maintain TKI discontinuation during
pregnancy. However, the major problem associated with this strategy is
CML relapse during TKI discontinuation. There have been several reports
on TKI discontinuation criteria and relapse rates following TKI
interruption in CML patients. According to reports from TKI
discontinuation trials, the recurrence rate is approximately 50–60% in
CML patients with complete MMR or a deep molecular response.[9] Moreover, if untreated after TKI discontinuation, recurrence is generally observed within six months.[9]
Therefore, female CML patients who wish to become pregnant must switch
from imatinib to another CML treatment and have a limited amount of
time to achieve pregnancy successfully.
Treatment options during TKI withdrawal or pregnancy include the administration of hydroxyurea and INF-α.[10-13]
Hydroxyurea is not a safe option due to observed teratogenic effects in
an animal model. However, INF-α is safe for women who wish to have
children or for pregnant women.[13] In this case,
INF-α was administered after TKI withdrawal and was continued during
infertility treatment. Due to prolonged infertility treatment,
interferon-α monotherapy was unable to suppress the CML disease state.
Therefore, another TKI, dasatinib, was administered for disease control.
There
are multiple ways to achieve pregnancy and, subsequently, delivery,
such as natural pregnancy and infertility treatment. Although natural
pregnancy is ideal, infertility treatment (particularly ART) is
effective in achieving pregnancy in a limited time. As shown in Table 1, 11 cases of planned pregnancy have been reported in female CML patients with TKI interruption, including this case.[1-7]
Besides one, all cases exhibited MMR at the time of TKI interruption.
IFN-α therapy was performed in three of the 11 cases, including ours,
after TKI discontinuation. Four of the cases conceived naturally, and
two underwent infertility treatment without ART. However, there have
been no previous reports of ART treatment for planned pregnancy in
female CML patients; therefore, our report might be the first case in
which pregnancy was achieved via vitrified–warmed embryo transfer.
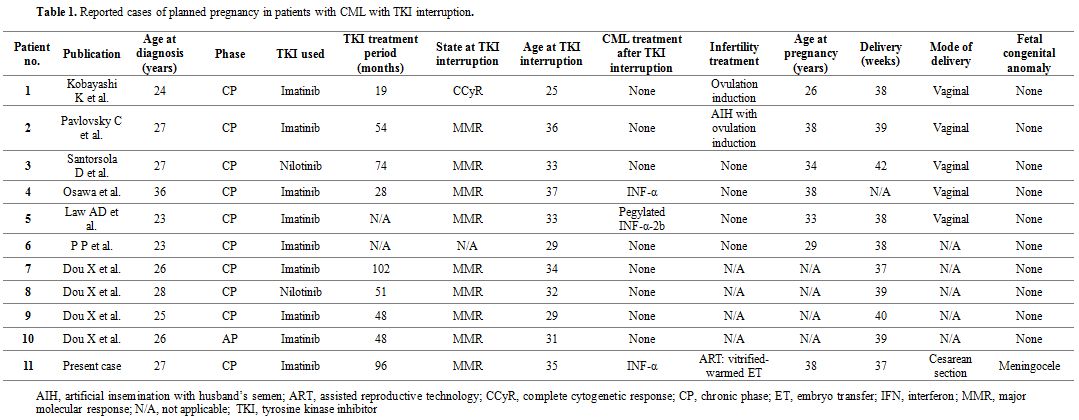 |
Table 1. Reported cases of planned pregnancy in patients with CML with TKI interruption. |
ART treatment with frozen embryos increases the chance of pregnancy in women with CML as well as other AYA cancer survivors.[14]
It might also be appropriate even if infertility treatment is
interrupted due to CML relapse. In this case, ART treatment was
administered after six months of non-ART infertility treatment. During
this time, levels of CML molecular markers started to increase, forcing
the infertility treatment to be interrupted when frozen embryos were
obtained after two cycles of ART treatment. The patient was then
treated with dasatinib, another TKI, in addition to IFN-α treatment.
After MMR had been confirmed after more than 12 months,
vitrified–warmed embryo transfer was scheduled, and pregnancy was
established. Thus, ART, particularly with frozen embryos, could be a
useful treatment option for female CML patients who have a limited
period to achieve pregnancy.
Given that there is currently
no effective strategy to prevent age-related fertility declines in
women, cryopreservation of eggs or ovarian tissue to preserve fertility
for women who wish to have children is an important issue.[15]
In this case, the patient was already 35 years old when she was
referred to our hospital, having been diagnosed with CML at 27 years
when she was already married to her partner. The patient might have
been able to undergo embryo cryopreservation by ART as soon as she was
judged to be in MMR. Recently, Gazdaru et al. reported successful
embryo cryopreservation for a TKI-resistant female CML patient who
changed from TKI to IFN-α treatment prior to conditioning chemotherapy
with hematopoietic stem cell transplantation.[16]
Accordingly, all female CML patients who wish to have children, even
those who are unmarried without a partner, should consider undergoing
embryo or oocyte cryopreservation to preserve their fertility.
Another critical issue to consider in such cases is the teratogenicity of treatment drugs during pregnancy in women with CML.[6,17]
In this case, we stopped dasatinib, a TKI, and IFN-α before the
scheduled vitrified–warmed embryo transfer; nonetheless, the child was
born with a meningocele despite the long drug-free period. There have
been previous reports of meningoceles occurring in the children of
female CML patients who became pregnant during imatinib treatment.[17,18]
Moreover, Cortes et al. reported that an infant with encephalocele, a
type of neural tube defect, was observed in a woman treated with
dasatinib.[19] In this case, since the pregnancy was
established more than three months after the discontinuation of
dasatinib administration, there might be almost no drug-related effects
on the fetus. In contrast, IFN-α has not been reported to exhibit
teratogenicity and can be used safely during pregnancy.[13]
Neural tube defects, such as meningoceles are associated with folate deficiency.[20] Generally, hematological malignancies, such as leukemia and pregnancy, require large amounts of folate for cell growth.[21,22]
Moreover, polymorphisms in the gene encoding methylenetetrahydrofolate
reductase, an enzyme involved in folate metabolism, have been
associated with CML in Asian patients.[23] In this case, additional folate supplementation might be required in addition to that generally recommended.
Conclusions
TKI
discontinuation and the timely initiation of infertility treatments
such as ART might be useful for treating women with CML who wish to
become pregnant.
References
- Kobayashi K, Takebayashi C, Miyata S, Narimatsu H,
Kami M (2009) Successful delivery after planned discontinuation of
imatinib in a patient with chronic myeloid leukemia. Intern Med
48:369-371. https://doi.org/10.2169/internalmedicine.48.1687 PMid:19252364
- Pavlovsky
C, Giere I, Van Thillo G (2012) Planned pregnancy in a chronic myeloid
leukemia patient in molecular remission. Case Rep Hematol 2012:624590. https://doi.org/10.1155/2012/624590 PMid:22928126 PMCid:PMC3420611
- Santorsola
D, Abruzzese E (2015) Successful management of pregnancy and hepatic
toxicity in a CML female patient treated with nilotinib: a case report
and a review. Mediterr J Hematol Infect Dis 7:e2015020. https://doi.org/10.4084/mjhid.2015.020 PMid:25745547 PMCid:PMC4344172
- Dou
X, Qin Y, Huang X, Jiang Q (2019) Planned pregnancy in female patients
with chronic myeloid leukemia receiving tyrosine kinase inhibitor
therapy. Oncologist https://doi.org/10.1634/theoncologist.2019-0109 PMid:31186377
- Osawa
T, Takahashi T, Yasuda M, Umeda M, Nagaya K, Tachi T, Goto H, Kasahara
S, Teramachi H, Goto C (2015) [A female chronic myeloid leukemia
patient who gave birth after stopping imatinib intentionally but who
maintained a major molecular response with interferon]. Gan To Kagaku
Ryoho 42:477-479.
- Law
AD, Dong Hwan Kim D, Lipton JH (2017) Pregnancy: part of life in
chronic myelogenous leukemia. Leuk Lymphoma 58:280-87.
https://doi.org/10.1080/10428194.2016.1201571 PMid:27389567
- P P, Samal R, Ghose S (2019) Chronic myeloid leukaemia in pregnancy: call for guidelines. J Obstet Gynaecol 39:582-583. https://doi.org/10.1080/01443615.2018.1534815 PMid:30744447
- Hoffmann
VS, Baccarani M, Hasford J, Lindoerfer D, Burgstaller S, Sertic D,
Costeas P, Mayer J, Indrak K, Everaus H, Koskenvesa P, Guilhot J,
Schubert-Fritschle G, Castagnetti F, Di Raimondo F, Lejniece S,
Griskevicius L, Thielen N, Sacha T, Hellmann A, Turkina AG, Zaritskey
A, Bogdanovic A, Sninska Z, Zupan I, Steegmann JL, Simonsson B, Clark
RE, Covelli A, Guidi G, Hehlmann R (2015) The EUTOS population-based
registry: incidence and clinical characteristics of 2904 CML patients
in 20 European Countries. Leukemia 29:1336-1343. https://doi.org/10.1038/leu.2015.73 PMid:25783795
- Palera
A, Altman JK, Berman E, Abboud CN, Bhatnagar B, Curtin P, DeAngelo DJ,
Gotlib J, Hagelstrom RT, Hobbs G, Jagasia M, Kantarjian HM, Kropf P,
Metheny L, Moore JO, Ontiveros E, Purev E, Quiery A, Reddy VV, Rose MG,
Shah NP, Smith BD, Snyder DS, Sweet KL, Tibes R, Yang DT, Gregory K,
Sundar H, Deininger M, Radich JP (2016) NCCN guidelines insights:
Chronic myeloid leukemia, Version 1.2017. J Natl Compr Canc Netw
14:1505-12. https://doi.org/10.6004/jnccn.2016.0162 PMid:27956535
- Radich
JP, Deininger M, Abboud CN, Altman JK, Berman E, Bhatia R, Bhatnagar B,
Curtin P, DeAngelo DJ, Gotlib J, Hobbs G, Jagasia M, Kantarjian HM,
Maness L, Metheny L, Moore JO, Pallera A, Pancari P, Patnaik M, Purev
E, Rose MG, Shah NP, Smith BD, Snyder DS, Sweet KL, Talpaz M, Thompson
J, Yang DT, Gregory KM, Sundar H (2018) Chronic myeloid leukemia,
version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl
Compr Canc Netw 16:1108-1135. https://doi.org/10.6004/jnccn.2018.0071 PMid:30181422
- Berman
E, Druker BJ, Burwick R (2018) Chronic myelogenous leukemia: Pregnancy
in the era of stopping tyrosine kinase inhibitor Therapy. J Clin Oncol
36:1250-1256. https://doi.org/10.1200/JCO.2017.77.2574 PMid:29447062
- Abruzzese
E, Trawinska MM, de Fabritiis P, Baccarani M (2016) Management of
pregnant chronic myeloid leukemia patients. Expert Rev Hematol
9:781-791. https://doi.org/10.1080/17474086.2016.1205479 PMid:27352939
- Balsat
M, Etienne M, Elhamri M, Hayette S, Salles G, Thomas X (2018)
Successful pregnancies in patients with BCR-ABL-positive leukemias
treated with interferon-alpha therapy during the tyrosine kinase
inhibitors era. Eur J Haematol 101:774-780. doi: 10.1111/ejh.13167 https://doi.org/10.1111/ejh.13167 PMid:30179268
- Levine
J, Canada A, Stern CJ (2010) Fertility preservation in adolescents and
young adults with cancer. J Clin Oncol 28:4831-4841. https://doi.org/10.1200/JCO.2009.22.8312 PMid:20458029
- Stoop D, Cobo A, Silber S (2014) Fertility preservation for age-related fertility decline. Lancet 384:1311-1319. https://doi.org/10.1016/S0140-6736(14)61261-7
- Gazdaru
S, Perey L, Rosselet A, Mathevet P, Chalandon Y, Vulliemoz N (2018)
Successful ovarian stimulation for fertility preservation in a patient
with chronic myeloid leukemia: Switch from nilotinib to
interferon-alpha. Oncologist 23:719-721. https://doi.org/10.1634/theoncologist.2017-0381 PMid:29212733 PMCid:PMC6067946
- Pye
SM, Cortes J, Ault P, Hatfield A, Kantarjian H, Pilot R, Rosti G,
Apperley JF (2008) The effects of imatinib on pregnancy outcome. Blood
111:5505-5508. https://doi.org/10.1182/blood-2007-10-114900 PMid:18322153 PMCid:PMC4916938
- Choudhary
DR, Mishra P, Kumar R, Mahapatra M, Choudhry VP (2006) Pregnancy on
imatinib: fatal outcome with meningocele. Ann Oncol 17:178-179. https://doi.org/10.1093/annonc/mdj065 PMid:16291579
- Cortes
JE, Abruzzese E, Chelysheva E, Guha M, Wallis N, Apperley JF (2015) The
impact of dasatinib on pregnancy outcomes. Am J Hematol 90:1111-1115. https://doi.org/10.1002/ajh.24186 PMid:26348106 PMCid:PMC5115878
- Daly
LE, Kirke PN, Molloy A, Weir DG, Scott JM (1995) Folate levels and
neural tube defects. Implications for prevention. JAMA 274:1698-1702. https://doi.org/10.1001/jama.1995.03530210052030 PMid:7474275
- Stamm
RA, Houghton LA (2013) Nutrient intake values for folate during
pregnancy and lactation vary widely around the world. Nutrients
5:3920-3947. https://doi.org/10.3390/nu5103920 PMid:24084052 PMCid:PMC3820052
- Green R, Datta Mitra A (2017) Megaloblastic anemias: Nutritional and other causes. Med Clin North Am 101:297-317. https://doi.org/10.1016/j.mcna.2016.09.013 PMid:28189172
- Li
C, Yichao J, Jiaxin L, Yueting Z, Qin L, Tonghua Y (2015)
Methylenetetrahydrofolate reductase gene polymorphism and risk of
chronic myelogenous leukemia: a meta-analysis. J BUON 20:1534-1545.
